Abstract
Thigh abscesses due to pyomyositis are uncommon. To guide empiric antibiotic therapy in diabetics we determined the rate of such infections due to oxacillin-resistant Staphylococcus aureus and Gram-negative organism infections, and whether the occurrence of oxacillin-resistant pathogens increased during the study period. We retrospectively reviewed 39 adult patients with diabetes mellitus treated for a deep thigh abscess. There were 29 men and 10 women; their mean age was 45 years. Comorbidities were present in 15 patients. S. aureus was the most common pathogen, present in 82% (32/39) of our patients. Gram-negative organisms were cultured in 14% (6/39) of patients and anaerobes in 10% (4/39). The infection was polymicrobial in 12 of 39 patients (31%). Oxacillin-resistant S. aureus comprised 25% (8/32) of infections due to S. aureus. Oxacillin-resistance increased during the last 3 years of this study from one of 18 S. aureus isolates from 1994 to 2004 to seven of 14 isolates from 2004 to 2006. In diabetic patients with thigh pyomyositis, empiric antibiotic therapy should provide broad spectrum coverage for oxacillin-resistant S. aureus, Gram-negative, as well as anaerobic organisms.
Level of Evidence: Level IV, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Diabetes mellitus is a common and major health problem, affecting 18 million people in the United States [2, 22]. Untreated diabetes leads to large and small vessel disease affecting many organ systems and to considerable morbidity and mortality; approximately 60% of patients with diabetes have one or more health complications, requiring approximately $23 billion in direct medical cost [2]. Diabetic patients are also predisposed to infectious complications because of impaired tissue perfusion and defective immune mechanisms, such as impaired chemotaxis, phagocytosis, and bactericidal activity of neutrophils [8, 18]. Infection and abscess formation in diabetic patients occurs frequently at the foot [16] and has been reported in most anatomic areas, including the liver [21], the kidney [30], the lung [10], the brain [14], and others [10, 11, 21, 26].
Pyomyositis—primary infection of skeletal muscle leading to abscess formation—was initially considered a tropical disease but has been increasingly recognized in temperate climates in patients with comorbidities such as diabetes mellitus or infection with HIV [4, 13, 15]. The literature on pyomyositis in diabetic patients is limited to case reports [1, 3, 5, 6, 9, 23, 25, 29, 34–37], a few series including among other patients one to 16 patient with diabetes [7, 20, 24, 27, 31, 38], and to reviews of the above studies [13, 19, 33], altogether amounting to 57 cases of pyomyositis in diabetic patients.
Staphylococcus aureus (S. aureus) is the most common pathogen in pyomyositis [4, 12, 13, 15] and in diabetic patients S. aureus has been isolated from 12 of 16 patients [38] to eight of nine patients [20]. Overall Gram-positive cocci (S. aureus or streptococcus) have been present in 91% (412/452) of patients with pyomyositis [4], and up to all (9/9) diabetic patients with pyomyositis [20]. Recommendations for empiric antibiotic therapy include a beta-lactamase-resistant penicillin [4, 12, 13, 29], and some authors advocate the addition of an aminoglycoside to achieve a synergistic effect on immunocompromised or septic patients [4, 13, 29].
However, Klebsiella pyomyositis has been reported in diabetic patients [34, 36], and a recent review of pyomyositis in the United States reported Gram-negative organisms were present in 14% (17/119) of HIV-negative patients with underlying medical conditions; the authors therefore recommended empiric coverage for both Gram-positive and Gram-negative organisms [15]. Recent studies also reported the emergence of oxacillin-resistant S. aureus as a pyomyositis pathogen [17, 28, 32], and suggested that empiric antibiotic therapy may need to include a glycopeptide in high-risk patients such as intravenous drug users [17].
To guide empiric antibiotic therapy we determined the rate of such infections due to oxacillin-resistant S. aureus and Gram-negative organism infections, and asked whether the occurrence of oxacillin-resistant pathogens increased during the study period.
Materials and Methods
We retrospectively reviewed the medical records of 39 adult patients (29 men and 10 women) with diabetes mellitus treated in our institution from 1994 to 2006 for a deep thigh abscess due to pyomyositis; no patients were seen in followup specifically for this study. Diabetes mellitus was defined as presence of fasting plasma glucose greater than 125 mg/dL on two separate occasions. Deep thigh abscess was defined as presence of pus deep to the thigh fascia and within the muscle compartments of the thigh. We excluded patients with previous surgery or penetrating trauma to the involved area or patients with subcutaneous abscesses located superficial to the fascia in order to study only patients with to primary infection of muscle (pyomyositis). Thirty-eight patients had unilateral involvement and one patient had bilateral thigh abscesses; therefore the total number of abscesses was 40, with 25 abscesses located at the left thigh and 15 at the right thigh. In all patients the diagnosis of a deep thigh abscess was confirmed with preoperative imaging studies and/or intraoperative findings of pus deep to the fascia and involving the muscle compartments of the thigh. The mean patient age was 45 years (range, 25–67 years). The mean blood glucose levels on admission were 278 mg/dL (range, 94–612 mg/dL). Thirty-five patients were aware of the diagnosis of diabetes mellitus but in four patients diabetes mellitus was diagnosed upon admission for the first time. One or more comorbidities were present in 15 patients, including smoking in nine patients, intravenous drug use in six, renal failure in three, hepatitis C with cirrhosis in two, HIV infection in one, and splenectomy in one patient. Prior institutional review board approval was obtained.
Upon admission, 29 of 39 (74%) patients were febrile. The mean systolic blood pressure was 121 mmHg (range, 62–181 mmHg), the mean diastolic blood pressure was 77 mmHg (range, 46–102 mm Hg), and the mean pulse rate was 102/min (range, 74–146/min). The mean C reactive protein was 95 mg/L (range, 34–210 mg/L), the mean ESR was 113 mm/h (range, 84–140 mm/h), and the mean WBC count was 13,200/mm3 (range, 5400–33,600/mm3). The diagnosis of a deep thigh abscess was confirmed by MRI in 18 patients (Fig. 1), by CT scan in four patients, and by ultrasound in one patient.
Fig. 1.
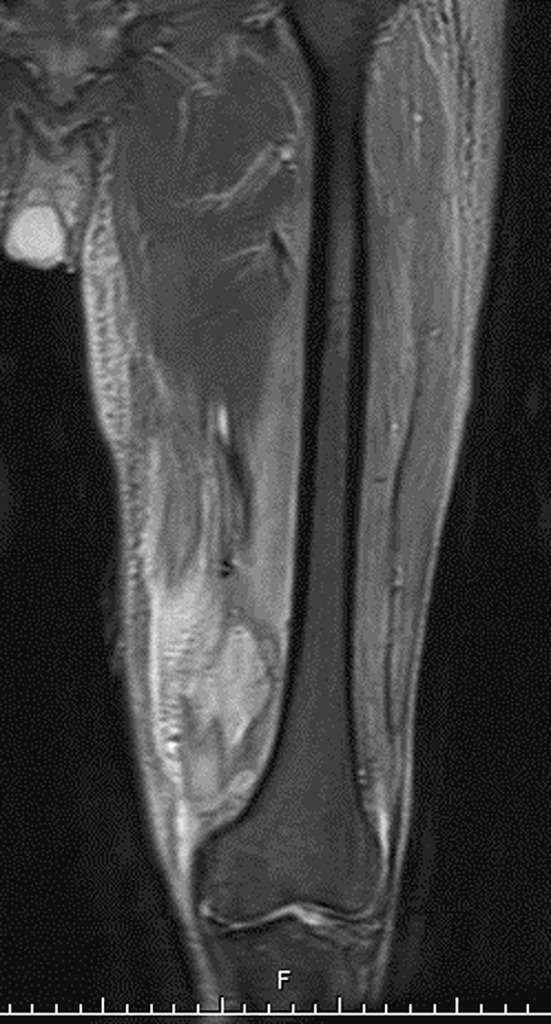
MRI of thigh abscess in the vastus medialis muscle is shown.
All abscesses were treated with drainage in the operating room, débridement, and irrigation. Necrotic muscle was present in 17 patients and was débrided. Irrigation was performed with 10 L of fluid, adding antibiotics in the last liter. The wound was left open or was partially closed. Twenty-three patients had a second-look débridement procedure. There was no perioperative mortality and there was one recurrence of infection that resolved following a repeat débridement procedure. Antibiotic therapy was administered for a median time of 4 weeks postoperatively (2 weeks intravenously and 2 weeks orally) based on culture and sensitivity results. No specific antibiotic protocol was used over the 13-year period. The most common pathogens were treated as follows: oxacillin-sensitive S. aureus was treated with oxacillin, cefazolin, clindamycin, or ampicillin/sulbactam; oxacillin-resistant S. aureus was treated with vancomycin; streptococcus was treated with cefazolin or ampicillin/sulbactam.
The proportions of S. aureus infections that were caused by oxacillin-resistant S. aureus were compared between the last 3 years of the study (2004 to 2006) and the previous ones using the Fisher’s exact test.
Results
S. aureus was the most common pathogen, present in 82% (32/39) of our patients (Table 1). Oxacillin-resistant S. aureus was present in 21% (8/39) of our patients and comprised 25% (8/32) of infections due to S. aureus.
Table 1.
Microbiology of deep thigh abscesses due to pyomyositis in diabetic patients (n = 39)
| Organisms Identified (n = 51*) | n | % |
|---|---|---|
| Oxacillin-sensitive Staphylococcus aureus | 24 | 62% |
| Oxacillin-resistant S. aureus | 8 | 21% |
| Streptococcus, beta-hemolytic | 5 | 13% |
| Other Gram-positive organisms | 4 | 10% |
| Coagulase-negative staphylococcus (2) | ||
| Enterococcus (1) | ||
| Diphtheroids (1) | ||
| Gram-negative organisms | 6 | 15% |
| E. coli (3) | ||
| Klebsiella (1) | ||
| Pseudomonas aeruginosa (1) | ||
| Aeromonas hydrophila (1) | ||
| Anaerobes | 4 | 10% |
| Microaerophilic streptococci (2) | ||
| Fusobacterium (1) | ||
| Peptostreptococcus (1) |
*12 of the 39 infections were polymicrobial with two organisms identified; therefore the total number of organisms was 51.
Gram-negative organisms were cultured in 14% (6/39) of patients; E. coli was identified in three patients, klebsiella in one, Pseudomonas aeruginosa in one, and Aeromonas hydrohila in one. Streptococci were present in 13% (5/39) of patients. Anaerobes were identified in four patients (10%). In 27 of 39 patients (69%) cultures were monomicrobial, whereas in 12 patients (31%) cultures identified two organisms (Table 1).
Pyomyositis due to oxacillin-resistant S. aureus increased (p = 0.01) during the course of the study (2004 to 2006): before 2004, oxacillin-resistant S. aureus comprised one of 18 S. aureus isolates, compared to seven of 14 S. aureus isolates in the years 2004 to 2006.
Discussion
Literature on pyomyositis points out that S. aureus is the most common organism [4, 12, 13, 15], however, recent studies have reported infections due to Gram-negative organisms [15, 34, 36], and oxacillin-resistant S. aureus [17, 28, 32]. We determined the rate of such infections due to oxacillin-resistant Staphylococcus aureus and Gram-negative organism infections, and asked whether the occurrence of oxacillin-resistant pathogens increased during the study period.
Our retrospective study has several limitations. Data were accumulated through a review of medical records, therefore some patients with pyomyositis may not have been identified. Prior penetrating trauma to the thigh may have not been recorded; some of our patients were intravenous drug users and the possibility of drug injection into the thigh and secondary infection can not be ruled out. All of our patients were started on antibiotic therapy prior to obtaining cultures, which may have altered the results in some cases. A prospective study would be needed to define more accurately the spectrum of organisms of these infections. However, to our knowledge, our study is the largest series of diabetic patients with thigh abscesses due to pyomyositis coming from a single institution.
Staphylococcus aureus was the most common pathogen in our series, isolated in 32 of 39 patients (82%). This is consistent with the existing reports on pyomyositis in diabetic patients, in which S. aureus was identified as the pathogen in 40 of the combined 57 patients (70%) [1, 3, 5–7, 9, 13, 19, 20, 23–25, 27, 29, 31, 33–38].
However, eight of our 32 S. aureus isolates (25%) were oxacillin-resistant and in the last 3 years of the study the proportion of oxacillin-resistance increased substantially from one of 18 to seven of 14 S. aureus isolates. This is in contrast with the earlier literature in which only two of 40 (5%) staphylococcal pyomyositis infections in diabetics were from oxacillin-resistant organisms. Eight patients with oxacillin-resistant S. aureus pyomyositis did not have prior hospitalization and only two of eight had risk factors such as intravenous drug use. Therefore, our study demonstrates that valid concerns were raised by recent studies reporting the emergence of community-acquired oxacillin-resistant S. aureus as an important cause of pyomyositis [17, 28, 32]. Empiric antibiotic therapy should provide coverage for oxacillin-resistant S. aureus.
Gram-negative and anaerobic organisms were present in a notable proportion of thigh abscesses in diabetics, 15% (6/39) and 10% (4/39), respectively. These rates in our study are similar to the recently reported 14% (17/119) Gram-negative infection rate and the 7% (8/119) anaerobic infection rate in HIV-negative patients with underlying medical conditions and pyomyositis [15]. We observed a higher proportion of polymicrobial infections than compared to previous reports: approximately 1/3 of our infections (12 of 39, 31%) were polymicrobial. Crum reported polymicrobial infections in 4% (5/119) of HIV-negative patients with underlying medical conditions and pyomyositis [15], and in the combined 57 diabetic patients with pyomyositis in the literature there were four polymicrobial infections (7%) [1, 3, 5–7, 9, 13, 19, 20, 23–25, 27, 29, 31, 33–38]. Based on our findings, it appears that coverage for Gram-positive cocci with a beta-lactamase-resistant penicillin may not be adequate as was previously suggested [4, 12, 13, 29] and empiric antibiotics should also cover for Gram-negative and anaerobic organisms.
In diabetic patients with thigh abscesses due to pyomyositis, empiric antibiotic therapy should provide broad spectrum coverage to include oxacillin-resistant S. aureus, Gram-negative, as well as anaerobic organisms.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akman I, Ostrov B, Varma BK, Keenan G. Pyomyositis: report of three patients and review of the literature. Clin Pediatr (Phila) 1996;35:397–401. doi: 10.1177/000992289603500803. [DOI] [PubMed] [Google Scholar]
- 2.American Association of Clinical Endocrinologists Web site. State of diabetes complications in America. Available at: http://www.aace.com/newsroom/press/2007/images/DiabetesComplicationsReport_FINAL.pdf. Accessed August 30, 2007.
- 3.Armstrong DG, D’Amato CR, Strong ML. Three cases of staphylococcal pyomyositis in adolescence, including one patient with neurologic compromise. J Pediatr Orthop. 1993;13:452–455. doi: 10.1097/01241398-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bickels J, Ben-Sira L, Kessler A, Wientroub S. Primary pyomyositis. J Bone Joint Surg Am. 2002;84:2277–2286. doi: 10.2106/00004623-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Breen JD, Karchmer AW. Staphylococcus aureus infections in diabetic patients. Infect Dis Clin North Am. 1995;9:11–24. [PubMed] [Google Scholar]
- 6.Brennessel DJ, Robbins N, Hindman S. Pyomyositis caused by Yersinia enterocolitica. J Clin Microbiol. 1984;20:293–294. doi: 10.1128/jcm.20.2.293-294.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JD, Wheeler B. Pyomyositis. Report of 18 cases in Hawaii. Arch Intern Med. 1984;144:1749–1751. doi: 10.1001/archinte.144.9.1749. [DOI] [PubMed] [Google Scholar]
- 8.Bybee JD, Rogers DE. The phagocytic activity of polymorphonuclear leukocytes obtained from patients with diabetes mellitus. J Lab Clin Med. 1964;64:1–13. [PubMed] [Google Scholar]
- 9.Caldwell DS, Kernodle GW, Jr., Seigler HF. Pectoralis pyomyositis: an unusual cause of chest wall pain in a patient with diabetes mellitus and rheumatoid arthritis. J Rheumatol. 1986;13:434–436. [PubMed] [Google Scholar]
- 10.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151:1557–1559. doi: 10.1001/archinte.151.8.1557. [DOI] [PubMed] [Google Scholar]
- 11.Chi CY, Fung CP, Liu CY. Aspergillus flavus epidural abscess and osteomyelitis in a diabetic patient. J Microbiol Immunol Infect. 2003;36:145–148. [PubMed] [Google Scholar]
- 12.Chiedozi LC. Pyomyositis. Review of 205 cases in 112 patients. Am J Surg. 1979;137:255–259. doi: 10.1016/0002-9610(79)90158-2. [DOI] [PubMed] [Google Scholar]
- 13.Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. 1992;15:668–677. doi: 10.1093/clind/15.4.668. [DOI] [PubMed] [Google Scholar]
- 14.Colombo AL, Rosas RC. Successful treatment of an Aspergillus brain abscess with caspofungin: case report of a diabetic patient intolerant of amphotericin B. Eur J Clin Microbiol Infect Dis. 2003;22:575–576. doi: 10.1007/s10096-003-0991-6. [DOI] [PubMed] [Google Scholar]
- 15.Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117:420–428. doi: 10.1016/j.amjmed.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Faglia E, Clerici G, Caminiti M, Quarantiello A, Gino M, Morabito A. The role of early surgical debridement and revascularization in patients with diabetes and deep foot space abscess: retrospective review of 106 patients with diabetes. J Foot Ankle Surg. 2006;45:220–226. doi: 10.1053/j.jfas.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Fowler A, Mackay A. Community-acquired methicillin-resistant Staphylococcus aureus pyomyositis in an intravenous drug user. J Med Microbiol. 2006;55:123–125. doi: 10.1099/jmm.0.46271-0. [DOI] [PubMed] [Google Scholar]
- 18.Gallacher SJ, Thomson G, Fraser WD, Fisher BM, Gemmell CG, MacCuish AC. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. 1995;12:916–920. doi: 10.1111/j.1464-5491.1995.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Reino JJ, Aznar JJ, Pablos JL, Diaz-Gonzalez F, Laffon A. Nontropical pyomyositis in adults. Semin Arthritis Rheum. 1994;23:396–405. doi: 10.1016/0049-0172(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Gordon BA, Martinez S, Collins AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology. 1995;197:279–286. doi: 10.1148/radiology.197.1.7568838. [DOI] [PubMed] [Google Scholar]
- 21.Han SH. Review of hepatic abscess from Klebsiella pneumoniae. An association with diabetes mellitus and septic endophthalmitis. West J Med. 1995;162:220–224. [PMC free article] [PubMed] [Google Scholar]
- 22.Herman WH. Diabetes epidemiology: guiding clinical and public health practice: the Kelly West Award Lecture, 2006. Diabetes Care. 2007;30:1912–1919. doi: 10.2337/dc07-9924. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez Rodriguez I, Fernandez-Martin J. Primary pyomyositis. Two more cases with atypical presentation in diabetic hosts. Br J Rheumatol. 1995;34:482–483. doi: 10.1093/rheumatology/34.5.482-a. [DOI] [PubMed] [Google Scholar]
- 24.Hossain A, Reis ED, Soundararajan K, Kerstein MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg. 2000;66:1064–1066. [PubMed] [Google Scholar]
- 25.Karaca Z, Tanriverdi F, Alp E, Abravici NO, Ozturk M, Unluhizarci K, Kelestimur F. A rare cause of uncontrolled hyperglycaemia: bacterial pyomyositis in two patients with diabetes mellitus. Diabet Med. 2007;24:1305–1306. doi: 10.1111/j.1464-5491.2007.02291.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee TY, Ko SF, Cheng YF, Wang YL, Chien WY. Primary gas-containing mediastinal abscess in a diabetic patient. Am J Emerg Med. 1995;13:427–429. doi: 10.1016/0735-6757(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 27.Patel SR, Olenginski TP, Perruquet JL, Harrington TM. Pyomyositis: clinical features and predisposing conditions. J Rheumatol. 1997;24:1734–1738. [PubMed] [Google Scholar]
- 28.Ruiz ME, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2005;352:1488–1489. doi: 10.1056/NEJM200504073521417. [DOI] [PubMed] [Google Scholar]
- 29.Seah MY, Anavekar SN, Savige JA, Burrell LM. Diabetic pyomyositis: an uncommon cause of a painful leg. Diabetes Care. 2004;27:1743–1744. doi: 10.2337/diacare.27.7.1743. [DOI] [PubMed] [Google Scholar]
- 30.Simpkins KC, Barraclough NC. Renal cortical abscess, perinephritis and perinephric abscess in diabetes. Br J Radiol. 1973;46:433–436. doi: 10.1259/0007-1285-46-546-433. [DOI] [PubMed] [Google Scholar]
- 31.Skoutelis A, Andonopoulos A, Panagiotopoulos E, Bassaris H. Non-tropical pyomyositis in adults: report of four cases and literature review. Eur J Clin Microbiol Infect Dis. 1993;12:769–772. doi: 10.1007/BF02098466. [DOI] [PubMed] [Google Scholar]
- 32.Sokolov KM, Kreye E, Miller LG, Choi C, Tang AW. Postpartum iliopsoas pyomyositis due to community-acquired methicillin-resistant Staphylococcus aureus. Obstet Gynecol. 2007;110:535–538. doi: 10.1097/01.AOG.0000269142.19323.88. [DOI] [PubMed] [Google Scholar]
- 33.Walling DM, Kaelin WG., Jr Pyomyositis in patients with diabetes mellitus. Rev Infect Dis. 1991;13:797–802. doi: 10.1093/clinids/13.5.797. [DOI] [PubMed] [Google Scholar]
- 34.Wang TK, Wong SS, Woo PC. Two cases of pyomyositis caused by Klebsiella pneumoniae and review of the literature. Eur J Clin Microbiol Infect Dis. 2001;20:576–580. doi: 10.1007/s100960100556. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe Y, Ohashi H, Asahina T, Watanabe K, Atsumi Y, Kitahara M, Matsuoka K. Pneumococcal paraspinal pyomyositis in a diabetic man: a case report. Diabetes Obes Metab. 2000;2:385–386. doi: 10.1046/j.1463-1326.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- 36.Yahalom G, Guranda L, Meltzer E. Internal obturator muscle abscess caused by Klebsiella pneumoniae. J Infect. 2007;54:e157–160. doi: 10.1016/j.jinf.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Yoneda M, Oda K. Type 2 diabetes complicated by multiple pyomyositis. Intern Med. 2003;42:174–177. doi: 10.2169/internalmedicine.42.174. [DOI] [PubMed] [Google Scholar]
- 38.Yu CW, Hsiao JK, Hsu CY, Shih TT. Bacterial pyomyositis: MRI and clinical correlation. Magn Reson Imaging. 2004;22:1233–1241. doi: 10.1016/j.mri.2004.08.005. [DOI] [PubMed] [Google Scholar]


