Abstract
Limited data are available regarding the risk factors and outcome of polymicrobial prosthetic joint infection (PJIs) when compared with monomicrobial PJI. Between January 1998 and November 2006, we retrospectively identified 34 of 174 prosthetic joint infections (19%) were polymicrobial. The 2-year cumulative probability of success of treating polymicrobial and monomicrobial PJIs was 63.8% and 72.8%, respectively. Twenty-six percent, 38%, and 29% of PJIs were treated with two-stage exchange, débridement and retention, or resection arthroplasty, respectively, and the 2-year survival rate free of treatment failure in each group was 77.7% (95% confidence interval, 42.8%–94.2%), 52.7% (95% confidence interval, 28.4%–75.9%), and 64.2% (95% confidence interval, 28.7%–88.9%). Methicillin-resistant Staphylococcus aureus (26.4% versus 7.1%) and anaerobes (11.7% versus 2.8%) were more common in polymicrobial PJIs. Polymicrobial PJIs occurred in patients with a soft tissue defect/dehiscence (23.5% versus 2.8%), drainage (79.4% versus 39.2%), or prior local irradiation (8.8% versus 0.71%). We found the following factors associated with polymicrobial prosthetic joint infections: the presence of a soft tissue defect/wound dehiscence (odds ratio, 5.9), drainage (odds ratio, 5.0), and age 65 years or older (odds ratio, 2.8).
Level of Evidence: Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Prosthetic joint infections (PJIs) are an infrequent complication of total joint arthroplasty. Although the rate of infection in most centers now ranges between 0.3% and 1.3% for THA [6] and 1% to 2% for TKA [5, 10], the increase in numbers of total hip and knee procedures has led to a higher total number of infections. Prosthetic joint infections are costly and disabling (estimate of ∼50,000 $/infection) [16].
Polymicrobial PJIs account for 4% to 27% of all PJI infections [3, 17, 19]. A recent study reports a higher frequency of polymicrobial PJI of 37% [12]. In this study, polymicrobial PJIs occurred more frequently in the early postoperative period [12]. These studies do not focus on the specific risk factors or the outcome of such infections, but they are rather descriptive studies. One study considering risk factors for polymicrobial PJI reported four patients believed to have acquired their infections as a result of building construction [18].
Traditionally, polymicrobial infection was associated with failure and was considered a contraindication for one-stage reimplantation in the management of PJIs [7]. However, in one report of 54 patients with TKA PJI treated with two-stage exchange, reimplantation was successful in 71.4% of patients with polymicrobial PJIs [6]. These studies highlight the varied success rates of polymicrobial PJIs treated with certain surgical modalities. Identifying potential risk factors for polymicrobial PJIs will allow the treating physician to identify patients at risk of acquiring polymicrobial infection, and may help in selecting a broader empiric antimicrobial coverage following the surgery that can be further narrowed down when the offending microorganisms are known. Also, depending on the pathogen involved and the surgical modality performed, these data will serve as a guide in predicting the outcome of such infections.
We hypothesized (1) there is a difference in the 2 year survival free of treatment failure between the monomicrobial and polymicrobial PJIs; and (2) several risk factors may be associated with polymicrobial PJIs, particularly local factors.
Materials and Methods
We retrospectively reviewed the medical records of all 195 patients with THA or TKA PJIs between January 1998 and November 2006. Cases were identified from an electronic database using the International Classification of Diseases, 9th Revision code specific for PJI (996.66). We did not see any patients to acquire data for this study. Among the 195 patients, 174 had PJI (THA and TKA) caused by an identified microorganism; 21 had culture-negative PJIs and were excluded from further review or analysis. Thirty-four of the 174 PJI infections (20%) in 34 patients were polymicrobial. From the records we identified potential risk factors for a polymicrobial PJI and its outcome. Characteristics and outcomes of patients with PJIs resulting from polymicrobial organisms were compared with those resulting from monomicrobial organisms. We then stratified potential risk factors associated with a polymicrobial PJI. The median duration of followup for successfully treated monomicrobial PJI infections was 526 days (range, 4–3134 days) and 380 days (range, 21–1925 days) for polymicrobial PJI infections. This study was approved by the Institutional Review Board of Medical University of South Carolina (HR# 10935).
The minimum followup was 4 days (median 274 days; range, 4–334 days). The patient who had 4 days of followup was not seen further in our clinic and therefore the documentation of successful outcome was available at the time of last followup date. We did not initiate active measures for followup such as telephone calls or letters.
A polymicrobial PJI was defined as isolation of the same two or more microorganisms from at least two cultures of joint aspirates or intraoperative tissue specimens or isolation in at least one intraoperative culture of two or more microorganisms plus evidence of infection in a joint space (purulence, acute inflammation, sinus tract communicating with a joint space) [17]. Wound drainage was defined as any drainage occurring in the immediate period before the diagnosis of PJI, as recorded by the treating physician in the medical record as presenting symptom.
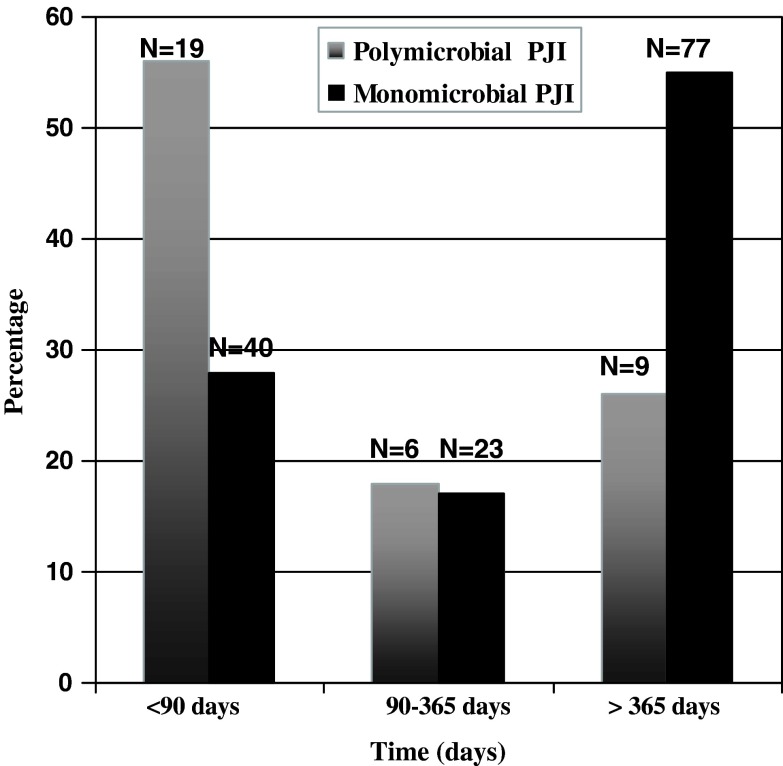
Enterococcus spp was isolated in 11 (32%), streptococci in two (6%), Pseudomonas aeruginosa in nine (26%), methicillin-resistant S. aureus in nine (26%), other Gram-negative bacilli (Escherichia coli, Klebsiella pneumoniae, Proteus spp, Citrobacter, Enterobacter spp, Acinetobacter) in 12 (35%), anaerobes (Bacteroides fragilis, anaerobes not otherwise identified, Propionibacterium acnes) in four (12%), and other microorganisms (coagulase-negative staphylococci, Corynebacterium spp, Bacillus spp) in 17 (50%) of the 34 polymicrobial infections. Candida spp (albicans, tropicalis) was present in two of 34 polymicrobial infections. Of the polymicrobial PJIs that contained enterococci, vancomycin-resistant enterococci were isolated only in one instance; the remaining enterococci were all penicillin-susceptible. There were no extended-spectrum beta-lactamase Gram-negative microorganisms isolated in the polymicrobial cultures. Nineteen of 34 (56%) of the polymicrobial PJIs occurred within 90 days from prosthesis implantation , whereas only 40 (29%) of the monomicrobial PJIs occurred within this timeframe. Nine of the polymicrobial (26%) and 77 (55%) of the monomicrobial PJI infections occurred more than 1 year after prosthesis implantation (Fig. 1). Only 6 monomicrobial PJI infections and 7 polymicrobial infections occurred within 21 days from prosthesis implantation. There were no major differences in terms of comorbidities, surgical therapy, or presenting symptoms among this subgroup of patients.
Fig. 1.
The graph shows earlier occurrence within 90 days from prosthesis implantation of polymicrobial prosthetic joint infections compared to monomicrobial prosthetic joint infections.
The primary outcome measure of treatment failure was defined by one of the following: (1) recurrence of a PJI with at least one of the original microorganisms present in culture (relapse); (2) different microorganisms than the original ones at any time after the surgical procedure (reinfection); (3) presence of acute inflammation (≥ 5 polymorphonuclear cells per high-power field) in the periprosthetic tissue on histopathologic examination; purulence in the joint space; (4) development of a sinus tract communicating with the joint prosthesis; death from prosthesis-related infection; (5) a new synovial fluid or periprosthetic tissue culture yielding a different microorganism than the original one while the patient is still receiving intravenous therapy (superinfection); or (6) indeterminate clinical failure (clinical, laboratory, or radiographic findings suggestive of PJI at any time after the surgical procedure). Outcome was determined at time of last documented clinic visit, clinical failure, or death.
Demographic characteristics, comorbid conditions, infection characteristics, surgical modality, and clinical presentation (Table 1) were abstracted from the medical records and entered into a FileMaker Pro database (FileMaker Inc, Santa Clara, CA, USA). Compared with the monomicrobial PJIs, polymicrobial PJIs occurred in patients 65 years of age and older (67% versus 49%, p = 0.05), presenting with a soft tissue defect/wound dehiscence (23.5% versus 2.8%, p = 0.0002), drainage (79.4% versus 39.2%, p < 0.0001), those who had prior local irradiation (8.8% versus 0.71%, p = 0.02), and less bacteremia (0% versus 14.2%, p = 0.01). None of the polymicrobial PJI infections were associated with endocarditis. Polymicrobial and monomicrobial PJIs did not considerably differ in gender, type of comorbidity, immunosuppressive therapy, median duration of intravenous antimicrobial therapy, prior joint revisions, prior two-stage exchange or débridement and retention of prosthesis, or type of surgery (two-stage exchange, one-stage revision, débridement and retention).
Table 1.
Characteristics among patients with monomicrobial and polymicrobial PJIs
| Variable | Polymicrobial PJI (n = 34) | Monomicrobial PJI (n = 140) | p Value |
|---|---|---|---|
| Age, median years (range) | 69.5 (32–93) | 63 (28–89) | 0.09 |
| Black | 8 (23.5%) | 44 (31.4%) | 0.41 |
| Female | 19 (55.8%) | 80 (57.1%) | 1.00 |
| TKA | 13 (38.2%) | 82 (58.5%) | 0.06 |
| Joint age, median days (range) | 50 (10 –9210) | 453 (14–10,132) | 0.004 |
| Comorbid conditions | |||
| Diabetes mellitus | 9 (26.4%) | 45 (32.1%) | 0.67 |
| Rheumatoid arthritis | 4 (11.7%) | 15 (10.7%) | 0.76 |
| Liver cirrhosis | 2 (5.8%) | 7 (5%) | 0.68 |
| Immunosuppressive medications* | 3 (8.8%) | 8 (5.7%) | 0.45 |
| Systemic malignancy† | 4 (11.7%) | 12 (8.5%) | 0.5 |
| Clinical presentation and local wound factors | |||
| Soft tissue defect/wound | 8 (23.5%) | 4 (2.8%) | 0.0002 |
| dehiscence | |||
| Drainage | 27 (79.4%) | 55 (39.2%) | < 0.0003 |
| Sinus tract | 9 (26.4%) | 25 (17.8%) | 0.33 |
| Prior local irradiation | 3 (8.82%) | 1 (0.71%) | 0.02 |
| Bacteremia | 0 (0%) | 20 (14.2%) | 0.01 |
| Endocarditis | 0 (0%) | 3 (2.14%) | 1.00 |
| Microbiology | |||
| Methicillin-resistant Staphylococcus aureus | 9 (26.7%) | 10 (7.1%) | 0.003 |
| Anaerobes | 4 (11.7%) | 4 (2.86%) | 0.04 |
| Intravenous antimicrobial therapy, median days (range) | 42 (12–66) | 42 (4–175) | 0.97 |
| Surgery and surgical factors | |||
| Two-stage exchange | 9 (26.4%) | 49 (35%) | 0.41 |
| Débridement and retention | 13 (38%) | 51 (36%) | 0.37 |
| Resection/Girdlestone | 10 (29.4%) | 20 (14.2%) | 0.04 |
| One-stage revision | 2 (5.8%) | 17 (12%) | 0.37 |
| Amputation | 2 (5.8%) | 3 (2.1%) | 0.25 |
| Antibiotic-impregnated spacer | 15 (44.1%) | 60 (42.8) | 1.00 |
| Joint aspiration | 0 (0%) | 2 (1.4%) | NS |
| Prior two-stage exchange | 2 (5.8%) | 16 (11.3%) | 0.53 |
| Prior revision surgery | 8 (23.5%) | 43 (30.7%) | 0.52 |
| Prior débridement/retention | 8 (23.5%) | 35 (25%) | 1.00 |
| No surgery (suppression only) | 0 (0%) | 1 (0.7%) | NS |
* Includes steroids, tumor necrosis factor-receptor antagonists, cyclosporine, methotrexate, or other immunosuppressive/immunomodulator medications; †includes colon, breast, lung, or malignant melanoma; PJI = prosthetic joint infection; NS = not significant.
The surgical and medical therapy for the treatment of PJI was left to the discretion of the orthopaedic and infectious disease physicians. Over the study period, the vast majority of surgical procedures were performed by the same two surgeons (HDS, HAD). Because of the retrospective nature of the study, we could not always discern the rationale for choosing a specific surgical procedure; duration of symptoms, timing of the infection (eg, early versus late postoperative period), implant stability, and patient preference were factors that were considered for surgical treatment. Débridement and retention of the prosthesis and exchange of the modular components were the surgical modalities used for the majority of polymicrobial PJIs and were performed in 13 (38.2%) infections. Ten infections were treated with resection arthroplasty (29.4%), nine infections (26.4%) were treated with two-stage exchange, and two (approximately 6%) with partial or total one-stage revision surgery. Polymicrobial PJI infections were treated more frequently with resection arthroplasty compared with monomicrobial infections (Table 1). Two of the polymicrobial infected patients and three of the polymicrobial PJI infected patients went on to have amputations. At the time of resection arthroplasty (infections intended to have a future reimplantation performed), an antibiotic-impregnated polymethylmethacrylate spacer with either tobramycin or vancomycin-tobramycin was used. Intravenous antimicrobial therapy was used in all but one episode of polymicrobial PJI for a median duration of 42 days (range, 12–66 days). The one infected patient who did not receive intravenous antimicrobial therapy was treated with a quinolone (highly bioavailable drug) and had a successful outcome. All but one patient with polymicrobial PJI received appropriate systemic antimicrobial therapy based on the antimicrobial susceptibility pattern of bacteria isolated from the intraoperative cultures. One patient who had vancomycin-resistant enterococci, penicillin-susceptible enterococcus, and Bacteroides fragilis isolated in polymicrobial culture was treated with resection arthroplasty, piperacillin-tazobactam, and gentamicin for an intended duration of 6 weeks of antimicrobial therapy. The length of followup for this patient was 21 days. Vancomycin-based combinations were used for the majority of cases (in combination with quinolones in 12 infections; trimethoprim-sulfamethoxazole in one episode; first-, third-, and fourth-generation cephalosporins in seven infections, rifampin in four, fluconazole in two, and imipenem-cilastatin in one). Vancomycin alone was used in eight cases of infection. Nafcillin-cefepime was used in one infection, cefazolin-ciprofloxacin in one infection, and ampicillin or piperacillin-tazobactam ± gentamicin were used in three infections. Four cases of infection (three treated with débridement and one with resection arthroplasty) received chronic oral suppression for a median of 146 days (range, 40–519 days). Doxycycline, trovafloxacin, clindamycin, or ampicillin combined with ciprofloxacin were the oral antimicrobials used for chronic suppression. Two patients developed Clostridium difficile colitis, one had a β-lactam-induced hypersensitivity reaction, one had rifampin-induced hepatitis, and one developed trimethoprim-sulfamethoxazole-induced hyperkalemia as a result of antimicrobial therapy.
We considered the following variables as potential risk factors for a polymicrobial infection: age, race, gender, comorbid conditions (immunocompromising conditions, chronic renal insufficiency or urinary tract infections, systemic malignancy), joint location, joint age, duration of symptoms, presence of bacteremia, surgical procedure and prior surgeries on the affected joint, local wound factors and clinical presentation (eg, wound dehiscence, soft tissue defect that required coverage, wound drainage, presence of a sinus tract, prior local irradiation on the affected joint), chronic oral administration (longer than 2 weeks) of prednisone (5 mg or greater daily), or other immunosuppressive or immunomodulator therapy (eg, tumor necrosis factor-receptor antagonists, cyclosporine, methotrexate).
We compared categorical variables between monomicrobial and polymicrobial PJI groups using chi square or Fisher’s exact test as appropriate. Continuous variables were compared with the Wilcoxon rank sum test. All tests were two-sided. Type I error (α) was set at 0.05. Risk factors we considered clinically important for a polymicrobial infection were included in a multivariable logistic regression model in a stepwise fashion (SAS 9.1, Cary, NC). Hosmer-Lemeshow test was performed to assess goodness-of-the-fit of the final model. We estimated survival free of treatment failure between polymicrobial and monomicrobial PJI groups by using the Kaplan-Meier method and log rank test.
Results
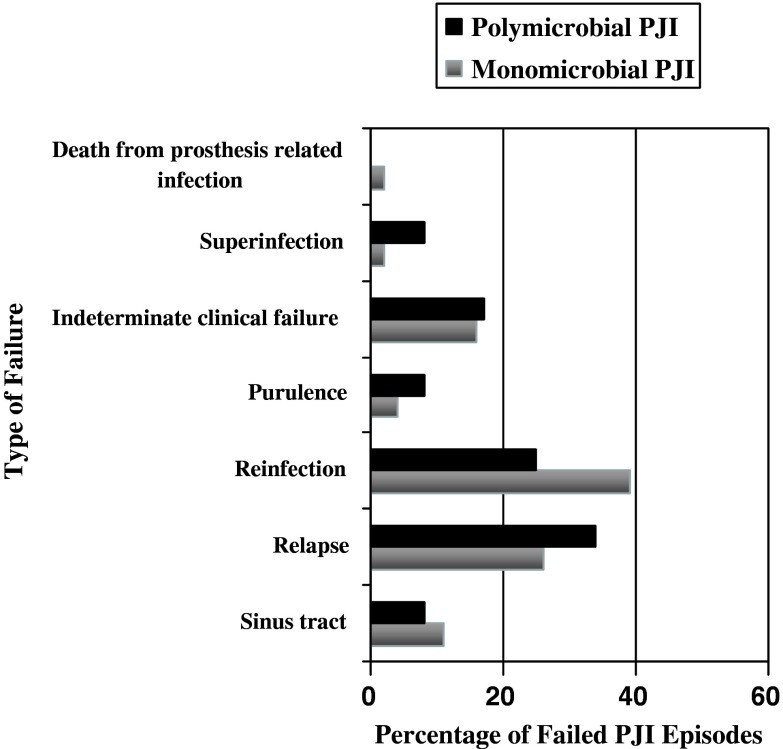
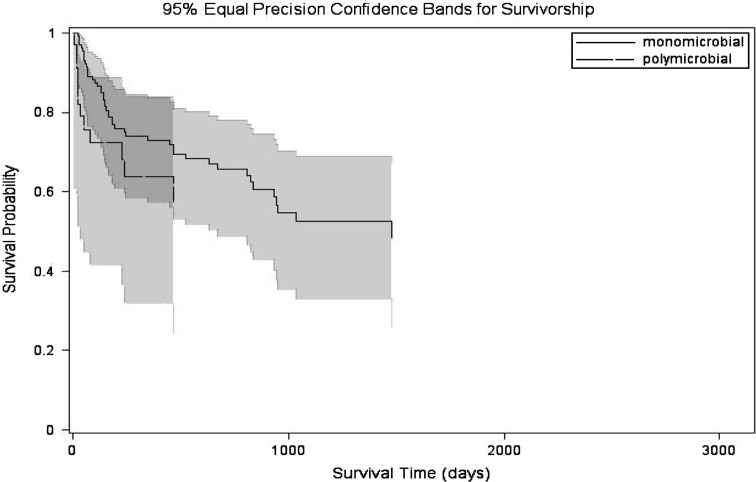
The outcome of polymicrobial PJI infections was similar (p = 0.24) to that of monomicrobial PJI infections. Twelve (35.3%) of the 34 infections of polymicrobial PJIs and 46 (32.8%) of the 140 monomicrobial PJI infections went on to develop treatment failure. Distribution of treatment failure between monomicrobial and polymicrobial PJIs included presence of a sinus tract, relapse, reinfection, purulence in the joint space, indeterminate clinical failure, superinfection, and death from prosthesis related infection (Fig. 2). Among the 21 reinfections (3 in the polymicrobial and 18 in the monomicrobial group), 8 went on to have débridement and retention, 3 had resection arthroplasty, 2 had two-stage exchange, 1 had amputation, 2 had arthrodesis, 1 had one-stage exchange, and only 4 had no additional surgical procedures. The 2-year cumulative probability of success of polymicrobial PJIs was 63.8% (95% confidence interval [CI], 43.8%–80.5%) and of monomicrobial PJIs was 72.8% (95% CI, 63%–80.9%) (Fig. 3). The 2-year survival free of treatment failure for polymicrobial PJIs treated with two-stage exchange, débridement, and retention of prosthesis and resection arthroplasty was 77.7%% (95% CI, 42.8%–94.2%), 52.7% (95% CI, 28.4%–75.9%), and 64.2% (95% CI, 28.7%–88.9%), respectively. The 2-year survival free of failure for monomicrobial PJIs treated with two-stage exchange and débridement and retention was higher, but not statistically significant different than of polymicrobial PJI treated with similar surgical modalities: 83.9% (95% CI, 67.4%–92.8%; p = 0.24), and 54% (95% CI, 37.9%–69.2%; p = 0.64), respectively.
Fig. 2.
Distribution of treatment failure between monomicrobial and polymicrobial prosthetic joint infections is illustrated, included presence of a sinus tract, relapse, reinfection, purulence in the joint space, indeterminate clinical failure, superinfection, and death from prosthesis related infection.
Fig. 3.
Kaplan-Meier estimate of survival free of treatment failure showing no difference between 2 year survival free of treatment failure between monomicrobial and polymicrobial prosthetic joint infections.
Presence of a soft tissue defect or wound dehiscence (odds ratio [OR], 5.9; 95% CI, 1.5–23.3), drainage (OR, 5.0; 95% CI, 1.9–12.9), and age 65 years or older (OR, 2.8; 95% CI, 1.1–6.8) were factors associated with polymicrobial PJIs (Table 2).
Table 2.
Multivariable analysis of risk factors associated with polymicrobial prosthetic joint infections
| Variable | Odds ratio | 95% Confidence interval |
|---|---|---|
| Presence of a soft tissue defect/wound dehiscence | 5.9 | 1.5–23.3 |
| Wound drainage | 5.0 | 1.9–12.9 |
| Age 65 years or older | 2.8 | 1.1–6.8 |
There were nine deaths in the monomicrobial PJI group (three related to the underlying malignancy, two resulting from myocardial infarction, one resulting from gastrointestinal hemorrhage, one resulting from hyperkalemia, one resulting from prosthesis-related infection, and one resulting from another infectious process). No deaths were recorded in the polymicrobial group.
Discussion
The purpose of this study was to assess potential risk factors associated with polymicrobial THA and TKA PJIs and their outcome at our institution. The available data from the literature regarding polymicrobial PJI are scarce and they do not address potential risk factors for polymicrobial infection. These studies highlight the varied success rates of polymicrobial PJIs treated with certain surgical modalities. Data provided from our study can serve as a guide in predicting the outcome of such infections and may help the treating physician to identify some of the risk factors associated with a polymicrobial PJI. It will also help with the selection of a broader empiric antimicrobial coverage following the surgery for a specific subset of patients who could potentially have risk factors for acquiring a polymicrobial PJI. We hypothesized there is a difference in the 2-year survival free of treatment failure between polymicrobial and monomicrobial PJIs; and there are risk factors associated with polymicrobial PJIs, particularly local factors.
Our study has several limitations. First, it is a single-center retrospective study with potential for uncontrolled selection biases among subgroups. Our institution is a tertiary care referral center that provides treatment for patients who might have had prior infections of PJI managed at a different institution. Second, the number of polymicrobial PJIs identified during the study period was relatively small, and thus the study may have lacked power to detect differences among subsets of patients. Furthermore, because of the relatively small number of outcome measures (eg, polymicrobial PJI), a limited number of covariates could be entered in the final multivariable logistic regression model. Therefore, it is possible additional risk factors for polymicrobial PJIs could have been found if the number of polymicrobial PJIs had been larger. Third, we were unable to use Musculoskeletal Infection Society Staging System (McPherson’s classification) [11], because we were unable to identify some of the local risk factors from the medical record given the retrospective nature of the study. The basis for Musculoskeletal Infection Society Staging System was clinical experience suggesting patients who are systemically ill are more difficult to treat and cure. Additionally, patients with more severe infections at the local site are more difficult to reconstruct and are doing functionally worse after reconstruction. This classification takes into account the acuteness of the symptoms, systemic host grade (comorbid conditions) and local extremity grade. However, the classification does not take into account the microbiology, which may be an important factor in determining the outcome of infection. This classification may be important to identify targeted treatment modalities for specific levels of medical and local wound compromise, and to provide an accurate prognosis to patients with PJIs. Finally, we could not analyze the difference in survival free of failure between monomicrobial or polymicrobial groups in patients treated with one-stage exchange as a result of the small number of patients treated with this method. The 2-year survival free of treatment failure for patients with polymicrobial PJIs treated with two-stage exchange (approximately 78%) was similar to that for patients with monomicrobial PJIs (approximately 84%), perhaps because of a lack of power to detect such difference. Because various treatment methods were used throughout the study period, it raises the possibility our conclusions were affected by this. However, we provided individualized 2-year estimates of the survival free of failure for infections of PJI treated with various surgical modalities.
We evaluated the outcome and factors potentially associated with polymicrobial PJIs. Our hypothesis was that the outcome of polymicrobial PJIs is in general favorable when compared with the PJIs resulting from the monomicrobial infection and there are additional risk factors associated with a polymicrobial PJIs, particularly local factors. The bacterial species strongly affects outcome. The most frequently recovered isolates in PJIs are S. aureus and Staphylococcus epidermidis [9]. We previously demonstrated methicillin-resistant S. aureus PJI resulted in higher risk of treatment failure [14]. Polymicrobial infection, infection with Gram-negative organisms, and methicillin-resistant microorganisms were considered factors associated with failure in PJIs treated with one-stage exchange [7]. Our results are similar to those reported in a previous series of 54 patients with TKA PJI treated with two-stage exchange when reimplantation was successful in 71.4% of patients with polymicrobial PJIs [6]. Nevertheless, we have not seen in our cohort higher rates of success of 90% with two-stage exchange reported in the literature, even for the monomicrobial PJI infections. The overall 2-year survival free of treatment failure for monomicrobial PJIs (54%) was similar to that observed for polymicrobial PJIs (52%). Relatively low 2-year survival free of treatment failure of 64% was also observed for polymicrobial PJIs treated with resection arthroplasty. Our polymicrobial PJI infections had a relatively large proportion of Gram-negative organisms and Pseudomonas aeruginosa isolated, although multidrug-resistant or extended-spectrum beta-lactamase-producing organisms were absent from this cohort. In the past, these bacteria were difficult to eradicate. With the introduction of extended-spectrum beta-lactamases and third- and fourth-generation of cephalosporins, quinolones, and carbapenems, recent clinical experience has demonstrated infection with Gram-negative bacilli can be treated as effectively as infection with Gram-positive microorganisms [20]. This may explain in part the lack of a considerable difference between the outcome of polymicrobial and monomicrobial PJIs. As in the study by Moran et al. [12], we found methicillin-resistant S. aureus was present at higher frequency in the polymicrobial group compared with the monomicrobial group. We believe the presence of methicillin-resistant S. aureus in polymicrobial culture may have been a more considerable risk factor for failure for such infections. In addition, and perhaps not surprisingly, we found a higher proportion of anaerobes in polymicrobial cultures.
Polymicrobial PJIs accounted for 10.5% to 19% of THA PJIs and 9% to 12.3% of TKA PJIs in previous studies [9]. A more recent study reports a higher frequency of polymicrobial PJI of 37% [12]. In this study, polymicrobial PJIs occurred more frequently in the early postoperative period [12]. We have observed a 19% frequency of polymicrobial infections in our cohort. Similarly, the majority of polymicrobial PJI infections in our cohort occurred within 90 days from the implantation of prosthesis.
Several risk factors for PJIs such as rheumatoid arthritis, diabetes mellitus, poor nutritional status, obesity, concurrent urinary tract infections, steroid therapy, malignancy, postoperative site infection, and National Nosocomial Infection Surveillance score greater than 0 were considered in primary joint arthroplasty. Many of these factors such as diabetes mellitus, rheumatoid arthritis, steroid therapy were noteworthy only in univariate, but not in multivariable analysis [1, 8, 13, 15, 21]. Conversely, prior joint surgery, prolonged operating room time, and preoperative infection were considered potential risk factors for infection after revision arthroplasty [9]. Postoperative wound healing complications, including “superficial” infection, hematoma, delayed healing, necrosis of the wound edge, and dehiscence, occur more commonly in patients who manifest deep infection and are important risk factors for subsequent deep wound infection [17]. In a large case-controlled study, Berbari et al. [1] reported the four most important risk factors predictive of PJI were postoperative surgical site infection, National Nosocomial Infection Surveillance score greater than 2, concurrent malignancy, and prior joint arthroplasty. We found the presence of a systemic malignancy, other major comorbid conditions, or prior joint surgeries were not associated with a polymicrobial PJI, perhaps as a result of relatively small numbers within the subgroups.
Periprosthetic infection classification proposed by McPherson et al. [11] takes into consideration infection type, systemic host grade, and local extremity grade. An intact soft tissue envelope is critical to eradicating the infection at the local site. We focused as well on some of the local factors and reported the presence of drainage, wound dehiscence, or a soft tissue defect was associated with the development of a polymicrobial infection. Prior local irradiation, another factor associated with impaired wound healing, was more frequently reported among the polymicrobial group as opposed to the monomicrobial group in our univariate analysis. However, prior local irradiation was not associated with a polymicrobial infection in the multivariable analysis. In the present study, older age was associated with polymicrobial PJIs. Gavet et al. [4] reported advanced age is a risk factor for septic arthritis and poor outcome. Older age may be a surrogate marker for poorer host status (e.g., a host that may have more obscure immunocompromising factors that were not captured in the current study). Extreme age (younger than 2 years and older than 70 years old) was considered part of the “B” host (systemic compromise) as suggested by Cierny et al. [2].
Our data suggest polymicrobial PJIs represent a substantial proportion of all PJI occurrences. Their frequency of 19% in the present study is in concordance with the reported frequency from the literature, ranging from 4–37% [3, 12, 17, 19]. As in the study by Moran et al. [12], we found that polymicrobial PJIs tend to occur more frequently in the early postoperative period. We also found polymicrobial PJIs have a relatively favorable outcome, especially when treated with two-stage exchange. In our study reimplantation was successfully performed in 77.7% of polymicrobial infections and is comparable with the previously reported result of 71.4% in patients with polymicrobial TKA infection [6]. In addition, our analysis identified several factors associated with polymicrobial PJIs that include presence of drainage, soft tissue defect or wound dehiscence, and age 65 years or older. Additional larger studies may be needed to identify other potential risk factors associated with polymicrobial PJIs.
Acknowledgments
We thank Drs. Harry A. Demos, MD and H. Del Schutte Jr, MD from the Department of Orthopedic Surgery at the Medical University of South Carolina for their surgical expertise.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case report and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 2.Cierny G, DiPasquale D. Periprosthetic total joint infections:staging, treatment and outcomes. Clin Orthop Relat Res. 2002;402:23–28. doi: 10.1097/00003086-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gallo J, Kolar M, Koukalova D, Sauer P, Loveckova Y, Dendis M, Kesselova M, Petrzelova J, Yapletalova J. Bacterial pathogens of periprosthetic infections and diagnostic possibilities [in Russian] Klinicka Mikrobiologie a Infekcni Lekarstvi. 2006;12:117–123. [PubMed] [Google Scholar]
- 4.Gavet F, Tournadre A, Soubrier M, Ristori JM, Dubost JJ. Septic arthritis in patients aged 80 and older: a comparison with younger adults. J Am Geriatr Soc. 2005;53:1210–1213. doi: 10.1111/j.1532-5415.2005.53373.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:11–22. [PubMed] [Google Scholar]
- 6.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 7.Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;381:101–105. doi: 10.1097/00003086-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470–475. doi: 10.1136/ard.56.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–1161. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 10.Lidgren L, Knutson K, Stefansdottir A. Infection and arthritis. Infection of prosthetic joints. Best Pract Res Clin Rheumatol. 2003;17:209–218. doi: 10.1016/S1521-6942(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 11.McPherson EJ, Tontz W, Jr., Patzakis M, Woodsome C, Holtom P, Norris L, Shufelt C. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop. 1999;28:161–165. [PubMed] [Google Scholar]
- 12.Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007;55:1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;182:117–126. [PubMed] [Google Scholar]
- 14.Salgado CD, Dash S, Robert Cantey J, Marculescu CE. Higher risk of treatment failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 15.Salvati EA, Robinson RP, Zeno SM, Koslin BL, Brause BD, Jr Wilson PD. Infection rates after 3175 total hip and total knee replacements performed with and without a horizontal unidirectional filtered air-flow system. J Bone Joint Surg Am. 1982;64:525–535. [PubMed] [Google Scholar]
- 16.Sculco TP. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18:871–873. [PubMed] [Google Scholar]
- 17.Steckelberg JM, Osmon DR. Prosthetic joint infections. In: Bisno AL, Waldwogel FA, eds. Infections of Indwelling Prosthetic Devices. Washington DC: ASM; 2000:173–209.
- 18.Tan ESL, Lennox HM, Doig GJ, Snyman RF. Polymicrobial deep joint replacement infection temporally associated with building construction: a case series. Inf Contr Hosp Epidemiol. 2005;26:430–432. doi: 10.1086/503513. [DOI] [PubMed] [Google Scholar]
- 19.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85(Suppl 1):S75–80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg. 1990;72:878–883. [PubMed] [Google Scholar]