Abstract
Historically, osteosarcoma has been a problematic metastatic disease, with 40–80% of patients developing pulmonary metastasis after primary tumor resection. Recent treatment advancements have reduced the occurrence of metastatic lesions to less than 30%. Using attenuated Salmonella typhimurium, we previously demonstrated regression in tumor burden in murine solid tumor and metastatic models. We established a murine model for metastatic osteosarcoma to determine the effect of treatment with a single oral dose of attenuated S. typhimurium with (SalpIL2) and without (Sal-NG) a gene for a truncated human interleukin-2. Female balb/c mice were administered 2 × 105 (ATCC K7M2) osteosarcoma cells via tail vein injection from culture and treated by oral gavage of Salmonella species 3 days later. Mice were harvested for splenic lymphocytes and tumor enumeration by intratracheal injection with India ink 21 days after injection. Treatment with attenuated SalpIL2 reduced pulmonary metastases in number and volume compared to saline controls. Furthermore, splenic natural killer cell populations were increased 93% with SalpIL2 and 114% with Sal-NG compared to nontreated groups. This pulmonary metastasis model demonstrates attenuated Salmonella typhimurium with human interleukin-2 reduced metastatic osteosarcoma in mice and confirm the need for further investigation into the immunologic properties of SalpIL2 as a possible treatment for metastatic osteosarcoma.
Introduction
Osteosarcoma is the most common primary bone cancer, with approximately 900 new cases annually in the United States [14]. There is a peak incidence in early adolescence correlated with pubertal bone growth and a second peak after age 50 [5]. Primary tumors develop in the distal femur and proximal tibia and humerus. Current management of primary osteosarcoma involves surgical resection with wide margins or limb amputation in conjunction with pre- and postoperative neoadjuvant chemotherapy. Survival from local disease has improved from 20% in 1970 [10] to approximately 70% at 3 years [16, 18] with the advent of current treatment with high-dose methotrexate, cisplatin, ifosfamide, and doxorubicin [20]. Despite the dramatic enhancement in patients’ event free survival, toxicity affects nearly all patients treated with these therapies [20]. However, in patients who present with metastatic disease detectable by CT, less than 30% disease-free survival has been achieved [9, 21]. In some cases, intravenous interleukin-2 treatment has resulted in complete regression of the primary tumor, though severe side effects have been noted, including fever, nausea, capillary leak syndrome, and death [25, 26].
We recently demonstrated a single oral dose of an attenuated Salmonella typhimurium genetically engineered with a gene for a truncated human interleukin-2 (SalpIL2) substantially reduces unresectable adenocarcinoma metastases to the liver in experimental treatment and prophylactic mouse models [7, 8, 27, 29, 33]. In addition, SalpIL2 reduces the volume and mass of retroperitoneal neuroblastoma tumors in an experimental murine treatment model [1]. Interestingly, the Salmonella species of bacteria also have a unique propensity to colonize tumor cells [2]. In vitro experiments have demonstrated the ability of SalpIL2 to invade and divide preferentially within K7M2 osteosarcoma cells with respect to primary murine hepatocytes [34]. Thus SalpIL2 may be able to persist for long periods in malignant tissues providing a prolonged antigen presentation state and enhanced immune response in the region.
Based on our previous observations, we hypothesize SalpIL2 would substantially reduce osteosarcoma pulmonary metastases by increasing splenic and local NK cell populations in this newly developed experimental model.
Materials and Methods
In triplicate experiments, 45 balb/c mice were administered murine K7M2 osteosarcoma cells by tail vein injection. Three days later, animals were orally gavaged saline or attenuated Salmonella species; they were then euthanized on day 21 for tumor enumeration, volume, and assessment of systemic NK and T cell populations. In an additional experiment, animals were harvested for pulmonary lymphocyte analysis.
Attenuated S. typhimurium χ4550 and plasmid pYA292 were a gift from Dr. Roy Curtiss III, Washington University, St. Louis, MO. χ4550 was attenuated by Tn10 transposon mutagenesis to remove adenylate cyclase (cya), cyclin adenosine monophosphate receptor protein (crp), and aspartate semialdehyde dehydrogenase (asd) genes from the bacterial genome [21]. These mutants have virulence factors removed, but retain immunogenic properties [4]. Plasmid constructs with and without a truncated gene for human interleukin-2 were electroporated into χ4550 using well-described techniques and renamed SalpIL2 and Sal-NG [28]. Standardized glycerol stocks of approximately 108 CFU/mL were prepared by creating growth curves for overnight cultures in Luria broth (Difco Laboratories, Detroit, MI) and freezing aliquots with an O.D.600 of 0.160 in liquid nitrogen. For experiments, cryovials were thawed to room temperature, serially diluted, and plated on MacConkey agar plates to verify CFU concentration. Use of S. typhimurium with a gene for a truncated human interleukin-2 was approved by the University of Minnesota Institutional Biosafety Committee (numbers 541 and 542).
Female balb/c mice 6 to 8 weeks old were acquired from Harlan Sprague Dawley (Indianapolis, IN) and housed in microisolator cages, fed standard mouse chow and water ad libitum, and given 12 hours light/dark cycles under the strict care of the University of Minnesota Research Animal Resources.
The murine osteosarcoma cell line K7M2 was acquired from the American Type Culture Collection and maintained in 25 mL DMEM, 10% fetal bovine serum, 1% penicillin, streptomycin, and L-glutamine (Sigma Chemical, St. Louis, MO) at 37°C at 5% CO2. Media was changed twice weekly and cells were not allowed to become confluent. Tumor cells were incubated with 0.3% trypsin EDTA (Invitrogen, Carlsbad, CA) at 37°C at 5% CO2 for 3 minutes or until nonadherent. Cells were serially washed in Hanks’ balanced salt solution (HBSS, Invitrogen) before enumeration via trypan blue exclusion (Sigma Chemical) on a phase contrast hemocytometer (Hausser Scientific, Horsham, PA). The suspension was diluted to a concentration of 2 × 106 cells per mL and placed on ice prior to injection. All tumor preparations were more than 90% viable and used within 1 hour of preparation.
We developed a model for pulmonary metastases for these experiments. Similar techniques have been implemented for quantifying the metastatic potential of the K7M2 cell line [12]. In triplicate experiments, animals were anesthetized by intraperitoneal injection of 2:1 xylazine 20 mg/mL (Phoenix Pharmaceuticals, St. Joseph, MO) and ketamine 100 mg/mL (Abbot Laboratories, North Chicago, IL). The animals’ eyes were swabbed with Betadine ophthalmic eye ointment (Purdue Pharma LP, Stamford, CT) and the animals were placed in a Broome Rodent Holder (Kent Scientific, Torrington, CT). Tails were incubated for one minute in 47°C Betadine solution (Purdue Pharma LP) to allow for vasodilatation of the left lateral tail vein and scrubbed with a 70% ethanol swab before 200,000 K7M2 cells were injected into the left lateral tail vein. Mice were placed at random in cages with microisolators and placed on a warming pad for 2 hours or until animals were walking. On Day 3, mice were orally gavaged with their respective treatments (n = 5), 200 μL HBSS for controls or 3 × 107 CFU of either Sal-NG or SalpIL2. In all experiments the mice were evaluated for presence of metastases 3 weeks after injection by euthanasia followed by an intratracheal injection of 1.5 mL of 15% India-ink solution via a blunt-ended needle. The stained lungs were carefully resected and rinsed in Fekete’s solution (300 mL 70% ethanol, 30 mL 37% formaldehyde, 5 mL glacial acetic acid) and then placed in fresh Fekete’s solution overnight in a 60 × 15 mm tissue culture dish. Tumors were enumerated, their diameters were measured and volume was calculated by 4/3πr3, assuming the metastases were spherical. Spleens were aseptically removed and placed in 60 × 15 mm culture dishes for FACS analysis of splenic lymphocytes. Due to inability to collect pulmonary lymphocytes or perform histopathological analysis from Fekete stained lungs, two additional experiments with 25 mice were conducted. Lungs for histopathological analysis were aseptically removed and placed in 10% formalin and sent to the University of Minnesota’s Histopathological Core for slide preparation.
Spleens and lungs for FACS analysis were incubated with 37°C DMEM containing 10% fetal goat serum and crushed with sterile glass stoppers. Homogenates were filtered through a 150-μm nitex mesh (Sefar American, Kansas City, MO) and transferred onto 5-mL lymphocyte separation medium (Ficol, Mediatech Inc., Hendon, VA). The cell suspension was centrifuged for 1 hour and the lymphocyte layer was carefully collected. Cells were serially washed with PBS with 1% bovine serum albumin (BSA) and 0.1% NaN3 (Sigma Chemical) and split for monoclonal antibody staining. Cells were stained with DX5/CD 49a PE and CD 3 FITC for NK cell analysis and CD 8 PerCp and CD4 FITC (Pharminogen, San Diego, CA) for T lymphocyte populations. Cells were cold incubated for 30 minutes at 4°C before a final wash with PSB/BSA/NaN3 and stored under foil at 4°C until FACS analysis.
Splenic and pulmonary lymphocytes collected from experimental mice were analyzed with a FACScalibur (Becton Dickenson, Grenoble, France) and analyzed with Cell Quest Pro Software (Becton Dickenson, San Jose, CA). Lymphocyte populations were identified using forward-scatter versus side-scatter profiles and gated for mononuclear lymphocytes. Natural killer cell populations then were identified by DX5/CD 49b+/Cd 3−, TH and TC cells by single positive populations based on 10,000 gated events.
Number of tumors, volume, and lymphocyte populations were entered for each mouse at the experimental endpoint to calculate the total mean values for each treatment group. All differences between two groups were determined by Fisher’s exact test. Graphs were constructed using Microsoft Excel (Microsoft, Redmond, WA). Statistical tests were performed using StatView software v. 5.0.1 (SAS Institute, Cary, NC).
Results
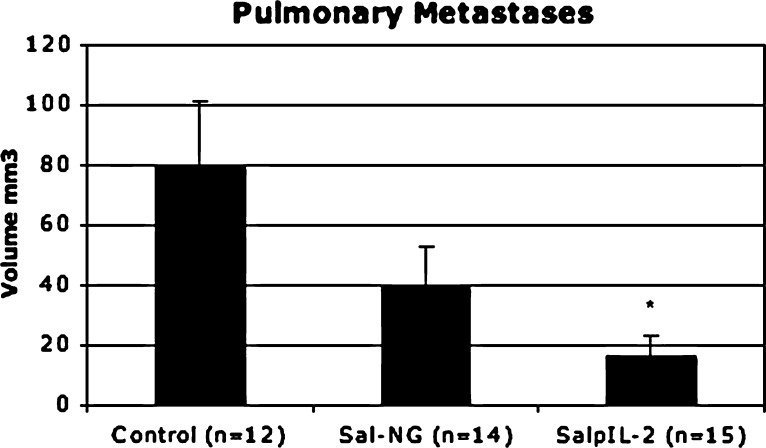
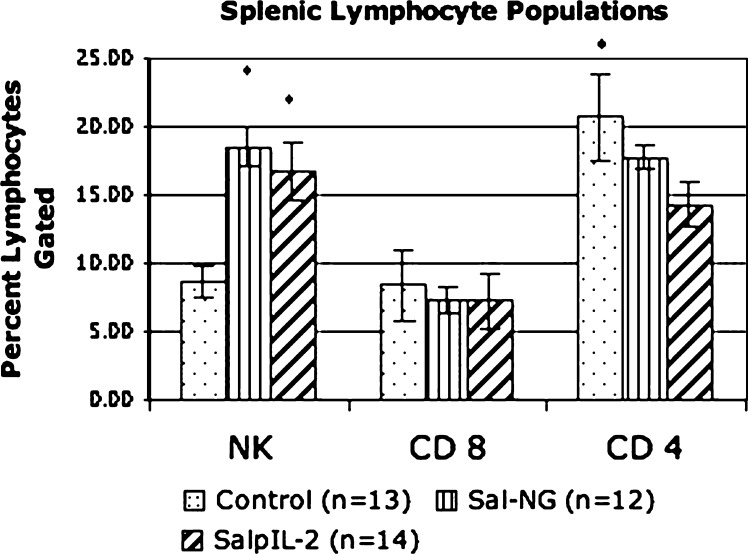
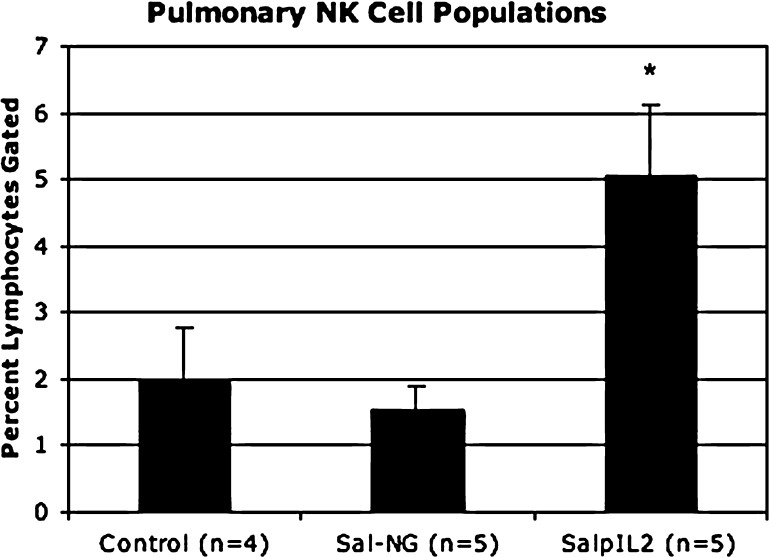
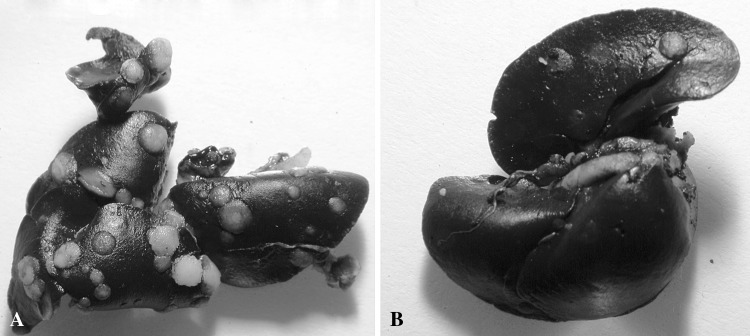
Attenuated S. typhimurium with and without a gene for human interleukin-2 had fewer total tumors (20.93 and 33, respectively; p < 0.0175 and 0.0006, respectively) compared to saline controls (58.42). SalpIL2 reduced (p < 0.0037) overall volume of pulmonary metastatic nodules by 78% with respect to saline controls (Fig. 1). There was no discernable difference in the reduction of tumor number and volume between the two Salmonella treatments. NK cell populations increased (p < 0.0163 and p < 0.0407, respectively) in Sal-NG- and SalpIL2-treated groups (18.5% and 16.8%, respectively) with respect to saline controls (8.6) (Fig. 2). Cytotoxic T lymphocyte populations were not noticeably affected by oral administration of Sal-NG and SalpIL2 (p = 0.270 and p = 0.237) compared to saline controls. T helper cell populations were reduced in the SalpIL2 group (14.3%; p < 0.0077) compared to saline controls(20.7%) (Fig. 2). Local pulmonary lymphocytes collected were elevated in SalpIL2 compared to control and Sal-NG treated animals (p < 0.0196 and p < 0.0070 respectively) (Fig. 3). Gross examination of the harvested pulmonary tissues demonstrate the reduction in the mean number of metastatic tumors by SalpIL2 with respect to saline controls (Fig. 4). Histological analysis of the tissues treated with SalpIL2 show a decreased invasion of the metastases into the subpleural space and an increase of mononuclear cells in the area (Fig. 5).
Fig. 1.
A single oral dose of SalpIL2 decreased pulmonary tumor volume by 78% with respect to saline controls (17.07 and 79.76 mm3 respectively; p < 0.0037). Sal-NG tended (p = 0.25) to reduce pulmonary metastatic tumor volume 50% from a mean of 80.0 in control animals to 39.8 mm3. * Indicates statistically significance.
Fig. 2.
SalpIL2 (right column) and Sal-NG (middle column) considerably increase natural killer cells by 93% and 113% (p = 0.0407 and p = 0.0163) with respect to saline-treated (left column) animals. CD 8+ T lymphocytes were not substantially increased by a single oral dose of Salmonella spp. CD4+ T lymphocytes were decreased (p < 0.0077) by 34% following SalpIL2 treatment. * Indicates statistically significance.
Fig. 3.
In an additional experiment, pulmonary lymphocytes populations in animals treated with SalpIL2 increased local NK cell populations 154% and 224% as compared to control and Sal-NG groups (p < 0.0196 and p < 0.0070, respectively). CD 8+ and CD 4+ T cell populations were not sufficiently detectable for analysis by flow cytometry. * Indicates statistically significance.
Fig. 4A–B.
The photographs represent mean samples from (A) control and (B) SalpIL2 orally treated mice. SalpIL2 substantially reduced the number and volume of surface detectable pulmonary metastases in the treatment model.
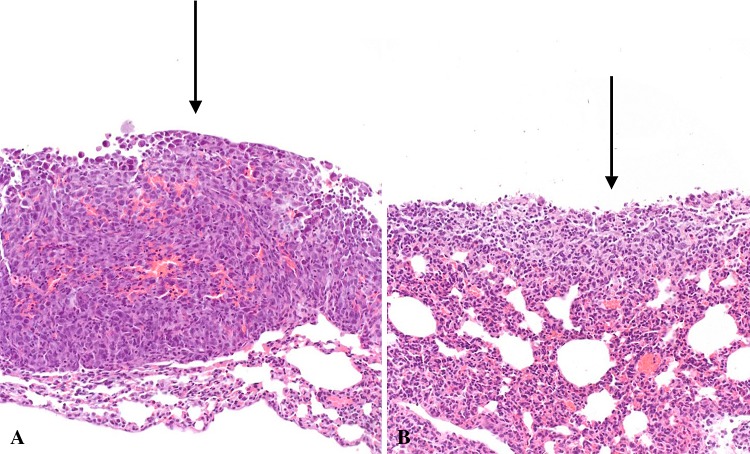
Fig. 5A–B.
Histopathological analysis of representative samples displays increased mononuclear infiltrate and decreased structure of tumor. (A) Histological representation of 20x control lung tissues displaying subpleural nodules (arrows) and linear aggregates and (B) 20x SalpIL2 treated tissues. Considerably less invasion into the subpleural space by osteosarcoma metastases may be observed in SalpIL2-treated lungs.
Discussion
While the prognosis for primary osteosarcoma has improved in the last three decades, no such survival advantage has been conferred upon patients with metastatic disease. Survival for patients diagnosed with osteosarcoma dramatically improved after the implementation of neoadjuvant chemotherapy for primary tumors nearly three decades ago. However, the benefits of such treatments have seemingly reached a plateau over the last decade [13, 19]. Individual drugs have been ineffective in treating pulmonary metastases [31], and combined therapy has not substantially altered the course of disease in patients who present with detectable pulmonary osteosarcoma metastases, as the 5-year survival rate continues to hover around 30% [9, 11, 37]. Our previous research demonstrates an antitumor effect in a variety of animal cancer models associated with oral dosing of attenuated Salmonella typhimurium with and without a truncated gene for human IL-2 [1, 7, 8, 27, 29, 33]. This project represents the early assessment of S. typhimurium in an osteosarcoma pulmonary metastatic model. We hypothesized SalpIL2 may substantially reduce osteosarcoma pulmonary metastases and increase splenic and local NK cell populations in a newly developed experimental model.
Our model’s most notable limitation is the absence of a primary tumor. By directly injecting highly metastatic tumor cells into the venous system, we are not allowing for the usual selective pressures normally exhibited on primary tumors in which only truly metastatic cells metastasize. Our model allows for a more timely investigation of an antitumor agent against pulmonary metastatic osteosarcoma. Despite this shortcoming, tumors in this model are grossly and microscopically similar to tumors produced by intratibial injection metastases models and mimic tumors produced in spontaneous orthotopic models [12]. Other limitations include our evaluation of the combined effect of two immunogenic particles (Salmonella and a truncated human IL2) simultaneously within a single species. In addition, we are evaluating tumor responses in an immunocompetent mouse strain, adding to the complexity of the data from which we draw conclusions, but also allowing for extrapolative interpretation for fully functioning biologic systems. The current plasmid construct of SalpIL2 produces a truncated IL-2 within the Salmonella spp. and exports recombinant peptide from the intracellular environment by an unknown mechanism. We do not currently know the fate of the truncated recombinant IL-2 after its synthesis; it may form inclusion bodies or be released upon bacterial lysis. Also, we do not fully understand whether the observed antitumor response is due to the biologic activity of the truncated IL-2 or its ability to initiate an antigenic response within our animal models. Further optimization of Salmonella as a delivery vector will involve utilizing prokaryotic excretion systems for localization of human and mouse homologs of truncated and full-length IL-2 into the local tumor environment.
Interleukin-2, a 15-kDa cytokine produced by activated CD4+ T cells and other immune cells, is involved in the activation of T lymphocytes and NK cells. Intravenous injections of liposomes containing IL-2 cDNA are effective in treating pulmonary metastases in canine spontaneous osteosarcomas, and have produced complete remission in some pediatric cases [6, 30, 32]. However, the highly toxic effects of intravenous IL-2 therapy has required investigators to alter the frequency, method of delivery and dosing, limiting the benefits of and response to the therapy [22, 30, 32, 36]. Intravenous interleukin-2 therapy requires multiple injections for results similar to those seen with a single oral dose of attenuated SalpIL2. Other investigators have used other cytokines in S. typhimurium in murine experimental models of pulmonary metastases. In those experiments S. typhimurium was able to reduce breast carcinoma metastases, however treatments required multiple intravenous injections and tissues were evaluated after 50 days [15]. Conversely, the intravenous administration of 2 × 105 K7M2 cells results in mortality of the animal subject by day 28 (data not shown). Other Salmonella strains using secretion mechanisms to deliver tumor specific antigens have been fairly successful when administered orally. However, these constructs were only effective when the tumor also produced the antigen, because tumors without the antigen were resistant to oral administration of S. typhimurium [23].
SalpIL2 appears a safe and effective delivery system that has similar antitumor efficacy compared to other methods of IL2 treatment, but without creating systemic toxicity. S. typhimurium is a facultative intracellular organism that preferentially tracks to and divides within osteosarcoma cells in vitro as well as reduces tumor burden in vivo in our treatment model of osteosarcoma pulmonary metastasis. In an attempt to establish a local delivery system for IL-2 that may diminish or prevent side effects associated with intravenous delivery, an attenuated strain of S. typhimurium was genetically engineered to carry a gene for a truncated human IL-2. Various strains of attenuated S. typhimurium have been investigated by other researchers as biologic vectors for antitumor therapy with limited success when given intravenously [2, 3, 17, 24, 35, 38]. By implementing an oral delivery system, SalpIL2 is able to colonize the Peyer’s patches, liver, lung, and spleen [28], and thus may be used to target primary and metastatic tumor in these tissues.
Attenuated S. typhimurium with a gene for a truncated human IL-2 limits the metastatic tumor burden in experimental models of neuroblastoma and metastatic colorectal adenocarcinoma. In these studies, NK cell populations appear largely responsible for the decrease in tumor burden in salmonella-treated animals [7, 8, 27, 29, 33]. We sought to determine whether previous antitumor mechanisms of SalpIL2 could be applied to an experimental model of metastatic osteosarcoma.
Our data suggest both strains of attenuated S. typhimurium elicited an antitumor response through proliferation of NK cells peripherally as demonstrated by our surrogate measure of splenic lymphocytes. In addition, mice gavaged with SalpIL2 also exhibited local expansion of NK cells in pulmonary tissues likely secondary to the locally produced truncated IL-2 carried by the bacterium. Other subtypes of lymphocytes were also evaluated. Interestingly, CD 8+ and CD 4+ T cells were either not involved in the local or systemic response or their expansion was suppressed by concomitant salmonella infection. Despite their absence, tumor growth was inhibited. This suggests nonspecific immune killing of tumor is sufficient to reduce metastatic disease associated with osteosarcoma.
Other experimental metastasis models utilizing SalpIL2 demonstrate NK expansion and involvement [7, 8, 27, 29, 33], implicating NK cell receptor involvement in the antitumor mechanism. Future studies within this model are focusing on the prophylactic use of SalpIL2 in metastatic osteosarcoma, local tumor/NK cell interaction, and the systemic cytokine response to tumor and SalpIL2. Further in vitro studies will focus on protein expression of metastatic determinants and endotoxin effects as seen with other S. typhimurium mutants in endothelial cells.
Based on the experimental results, SalpIL2 is effective at eliciting local and systemic NK cell proliferation and reducing murine pulmonary metastases. The addition of SalpIL2 to current treatment regimens may potentially result in improved prognosis and disease-free survival for osteosarcoma patients in the future.
Acknowledgments
We thank the Flow Cytometry Core Facility of the University of Minnesota Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598. We also thank Dr. Robert Acton, Dr. Denis Clohisy, Dr. Robert Bulander, Dr. Karen Wasiluk, Karen McCulloch and Jerry Vincent for their support of this project. We also thank the University of Minnesota’s Cancer Center’s Histopathology Core for their slide preparation of histological samples. All experiments were approved by the University of Minnesota Institutional Animal Care and Uses Committee (number 0409A63728).
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This project was paid for by the Arnold S. Leonard Cancer Research Fund and/or Department Chair.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Barnett SJ, Soto LJ, 3rd, Sorenson BS, Nelson BW, Leonard AS, Saltzman DA. Attenuated salmonella typhimurium invades and decreases tumor burden in neuroblastoma. J Pediatr Surg. 2005;40:993–997. doi: 10.1016/j.jpedsurg.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Bermudes D, Low B, Pawelek J. Tumor-targeted salmonella. highly selective delivery vectors. Adv Exp Med Biol. 2000;465:57–63. doi: 10.1007/0-306-46817-4_6. [DOI] [PubMed] [Google Scholar]
- 3.Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, Li Z, Luo X, King I, Zheng LM. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 4.Curtiss R, 3rd, Kelly SM. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75:203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::AID-CNCR2820751308>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Dow S, Elmslie R, Kurzman I, MacEwen G, Pericle F, Liggitt D. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum Gene Ther. 2005;16:937–946. doi: 10.1089/hum.2005.16.937. [DOI] [PubMed] [Google Scholar]
- 7.Feltis BA, Miller JS, Sahar DA, Kim AS, Saltzman DA, Leonard AS, Wells CL, Sielaff TD. Liver and circulating NK1.1(+)CD3(-) cells are increased in infection with attenuated salmonella typhimurium and are associated with reduced tumor in murine liver cancer. J Surg Res. 2002;107:101–107. doi: 10.1006/jsre.2002.6428. [DOI] [PubMed] [Google Scholar]
- 8.Feltis BA, Sahar DA, Kim AS, Saltzman DA, Leonard AS, Sielaff TD. Cyclooxygenase-2 inhibition augments the hepatic antitumor effect of oral salmonella typhimurium in a model of mouse metastatic colon cancer. Dis Colon Rectum. 2002;45:1023–1028. doi: 10.1007/s10350-004-6354-2. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292–315. doi: 10.1081/CNV-100102557. [DOI] [PubMed] [Google Scholar]
- 10.Foster L, Dall GF, Reid R, Wallace WH, Porter DE. Twentieth-century survival from osteosarcoma in childhood: Trends from 1933 to 2004. J Bone Joint Surg Br. 2007;89:1234–1238. doi: 10.1302/0301-620X.89B9.19255. [DOI] [PubMed] [Google Scholar]
- 11.Harting MT, Blakely ML, Jaffe N, Cox CS, Jr, Hayes-Jordan A, Benjamin RS, Raymond AK, Andrassy RJ, Lally KP. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41:194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 12.Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–271. doi: 10.1023/A:1006767007547. [DOI] [PubMed] [Google Scholar]
- 13.Lewis VO. What’s new in musculoskeletal oncology. J Bone Joint Surg Am. 2007;89:1399–1407. doi: 10.2106/JBJS.G.00075. [DOI] [PubMed] [Google Scholar]
- 14.Link MP, Gebhardt MC, Meyers PA, Pizzo PA, Poplack DG. Principles and Practice of Pediatric Oncology. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. Osteosarcoma; pp. 1051–1089. [Google Scholar]
- 15.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Attenuated salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci USA. 2007;104:12879–12883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Zhou H, Mizutani M, Mizutani N, Reisfeld RA, Xiang R. Transcription factor fos-related antigen 1 is an effective target for a breast cancer vaccine. Proc Natl Acad Sci USA. 2003;100:8850–8855. doi: 10.1073/pnas.1033132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 19.Meyers PA. High-dose therapy with autologous stem cell rescue for pediatric sarcomas. Curr Opin Oncol. 2004;16:120–125. doi: 10.1097/00001622-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K., Kelly S.M., Curtiss R., III Construction of an ASD+ expression cloning vector: Stable maintenance and high level expression of cloned genes in a salmonella vaccine strain. Nature Biotechnology. 1988;6:693–697. doi: 10.1038/nbt0688-693. [DOI] [Google Scholar]
- 22.Niethammer AG, Xiang R, Ruehlmann JM, Lode HN, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin 2 therapy enhances protective immunity induced by an autologous oral DNA vaccine against murine melanoma. Cancer Res. 2001;61:6178–6184. [PubMed] [Google Scholar]
- 23.Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, Ritter G, Jager E, Nomura H, Kondo S, Tawara I, Kato T, Shiku H, Old LJ, Galan JE, Gnjatic S. In vivo antigen delivery by a salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawelek JM, Low KB, Bermudes D. Tumor-targeted salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 25.Rosenberg SA. The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg. 1988;208:121–135. doi: 10.1097/00000658-198808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–84. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saltzman DA, Heise CP, Hasz DE, Zebede M, Kelly SM, Curtiss R, 3rd, Leonard AS, Anderson PM. Attenuated salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm. 1996;11:145–153. doi: 10.1089/cbr.1996.11.145. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman DA, Katsanis E, Heise CP, Hasz DE, Kelly SM, Curtiss R, 3rd, Leonard AS, Anderson PM. Patterns of hepatic and splenic colonization by an attenuated strain of salmonella typhimurium containing the gene for human interleukin-2: a novel anti-tumor agent. Cancer Biother Radiopharm. 1997;12:37–45. doi: 10.1089/cbr.1997.12.37. [DOI] [PubMed] [Google Scholar]
- 29.Saltzman DA, Katsanis E, Heise CP, Hasz DE, Vigdorovich V, Kelly SM, Curtiss R, 3rd, Leonard AS, Anderson PM. Antitumor mechanisms of attenuated salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent? J Pediatr Surg. 1997;32:301–306. doi: 10.1016/S0022-3468(97)90198-6. [DOI] [PubMed] [Google Scholar]
- 30.Schwinger W, Klass V, Benesch M, Lackner H, Dornbusch HJ, Sovinz P, Moser A, Schwantzer G, Urban C. Feasibility of high-dose interleukin-2 in heavily pretreated pediatric cancer patients. Ann Oncol. 2005;16:1199–1206. doi: 10.1093/annonc/mdi226. [DOI] [PubMed] [Google Scholar]
- 31.Seibel NL, Krailo M, Chen Z, Healey J, Breitfeld PP, Drachtman R, Greffe B, Nachman J, Nadel H, Sato JK, Meyers PA, Reaman GH. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: Children’s cancer group (CCG) 7943. Cancer. 2007;109:1646–1653. doi: 10.1002/cncr.22553. [DOI] [PubMed] [Google Scholar]
- 32.Smith KA. Lowest dose interleukin-2 immunotherapy. Blood. 1993;81:1414–1423. [PubMed] [Google Scholar]
- 33.Soto LJ, 3rd, Sorenson BS, Kim AS, Feltis BA, Leonard AS, Saltzman DA. Attenuated salmonella typhimurium prevents the establishment of unresectable hepatic metastases and improves survival in a murine model. J Pediatr Surg. 2003;38:1075–1079. doi: 10.1016/S0022-3468(03)00196-9. [DOI] [PubMed] [Google Scholar]
- 34.Soto LJ, 3rd, Sorenson BS, Nelson BW, Solis SJ, Leonard AS, Saltzman DA. Preferential proliferation of attenuated salmonella typhimurium within neuroblastoma. J Pediatr Surg. 2004;39:937–40. doi: 10.1016/j.jpedsurg.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 35.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA. Phase I study of the intravenous administration of attenuated salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trudel S, Trachtenberg J, Toi A, Sweet J, Li ZH, Jewett M, Tshilias J, Zhuang LH, Hitt M, Wan Y, Gauldie J, Graham FL, Dancey J, Stewart AK. A phase I trial of adenovector-mediated delivery of interleukin-2 (AdIL-2) in high-risk localized prostate cancer. Cancer Gene Ther. 2003;10:755–763. doi: 10.1038/sj.cgt.7700626. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchiya H, Kanazawa Y, Abdel-Wanis ME, Asada N, Abe S, Isu K, Sugita T, Tomita K. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: The japanese musculoskeletal oncology group study. J Clin Oncol. 2002;20:3470–3477. doi: 10.1200/JCO.2002.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Xu C, Li ZS, Du YQ, Gong YF, Yang H, Sun B, Jin J. Construction of recombinant attenuated salmonella typhimurium DNA vaccine expressing H pylori ureB and IL-2. World J Gastroenterol. 2007;13:939–944. doi: 10.3748/wjg.v13.i6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]