Abstract
While low-grade juxtacortical and low-grade intramedullary osteogenic sarcomas are histologically indistinguishable, they have been studied as separate entities. We retrospectively reviewed the clinical, radiographic, histologic features and treatment of 59 patients treated surgically to compare the rate of local recurrence, grade progression, and survival between low-grade intramedullary and low-grade juxtacortical osteogenic sarcoma. Forty-five (76%) patients were treated for low-grade juxtacortical osteogenic sarcoma and 14 (24%) were treated for low-grade intramedullary osteogenic sarcoma. Local recurrence rates of 7% were similar for both groups studied. The rate of distant metastases was also similar for both groups. . The rate of dedifferentiation for the entire group was 29%. Dedifferentiated lesions were treated with adjuvant chemotherapy in 16 of 17 cases. Recurrence preceded dedifferentiation in four cases. Five-year survival was over 90% in both groups. Low-grade intramedullary and low-grade juxtacortical osteogenic sarcoma were clinically indistinguishable with identical rates of local recurrence, distant metastases, dedifferentiation, and survival.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Low-grade osteogenic sarcoma comprises less than 5% of all osteogenic sarcomas and is typically divided into two main subgroups based on location relative to the cortex. Both low-grade intramedullary and low-grade juxtacortical osteogenic sarcomas are amenable to surgical resection without chemotherapy [3–5, 12]. They share histologic features including a bland fibroblastic stroma intermixed with well-developed bony trabeculae [1], and they have similar genetic profiles, with gains in chromosome 12 observed in both [10, 11]. Despite such similarities, the literature contains no direct comparison of their clinical behavior.
We therefore asked whether low-grade intramedullary and low-grade juxtacortical osteogenic sarcoma differed with regard to rate of local recurrence, metastasis, dedifferentiation, and survival.
Materials and Methods
We retrospectively reviewed the medical records of a consecutive series of patients diagnosed and treated for low-grade osteogenic sarcoma between January 1971 and November 2004. We included patients who were treated elsewhere first and then referred to our institution for recurrence. We identified 59 patients. Forty-five (76%) of the 59 patients were diagnosed with juxtacortical osteogenic sarcoma and fourteen (24%) were diagnosed with low-grade intramedullary osteogenic sarcoma. We obtained prior IRB approval.
The diagnosis was confirmed by histologic analysis with radiographic correlation in each case. In each case, the radiographic diagnosis of juxtacortical osteogenic sarcomas included a lobulated, opaque mass adherent to the cortex in the metaphysis of long bones (Fig. 1), and for low-grade intramedullary osteogenic sarcoma a sclerotic intramedullary metaphyseal lesion (Fig. 2). The histologic features of low-grade osteogenic sarcoma included well-delineated, long, bony trabeculae separated by spindled fibrous stroma (Fig. 3). The stroma had to be devoid of cytologic atypia to be considered low-grade.
Fig. 1.
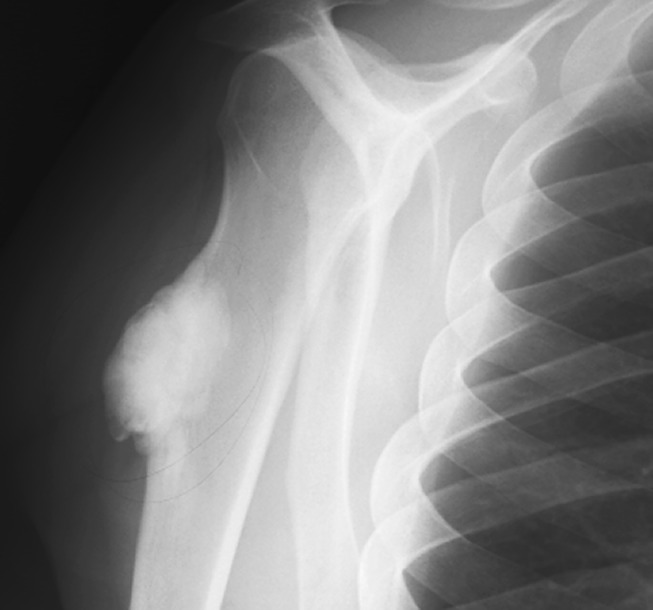
The figure shows the typical radiographic appearance of low-grade juxtacortical osteogenic sarcoma demonstrating a lobulated ossific mass on the surface of the bone.
Fig. 2.
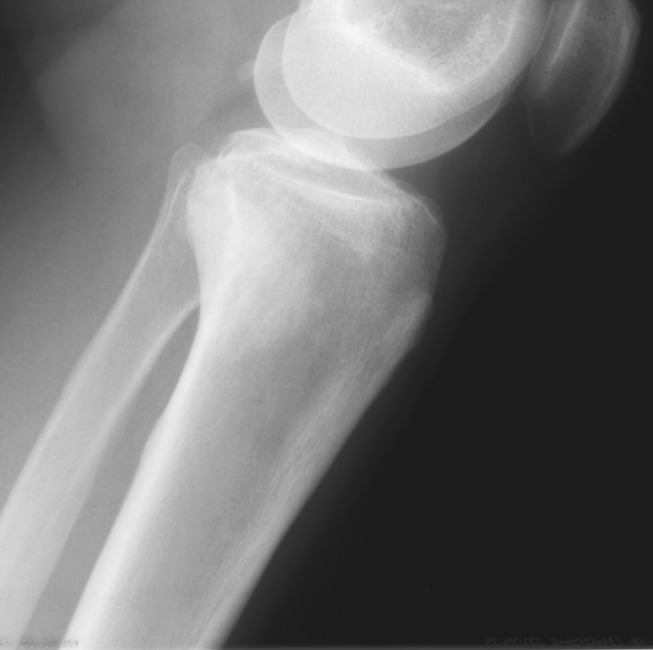
The typical radiographic appearance of low-grade intramedullary osteogenic sarcoma demonstrating vague sclerosis confined to the intramedullary cavity is shown.
Fig. 3.
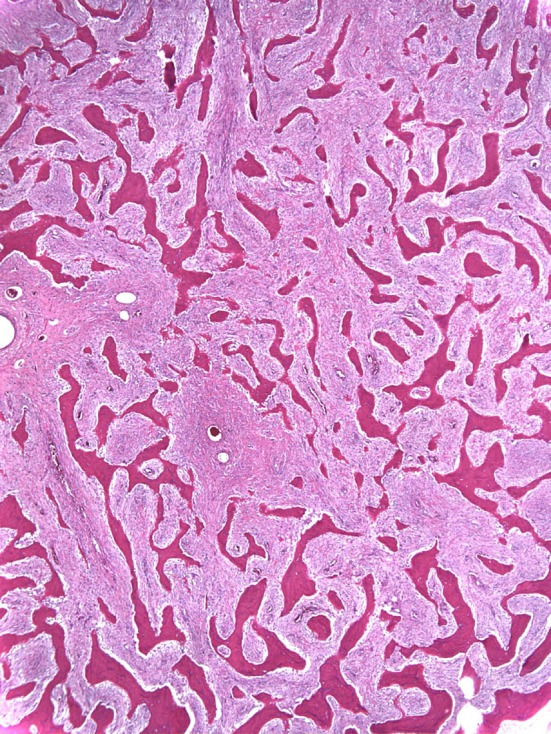
The histologic appearance of low-grade osteogenic sarcoma is shown.
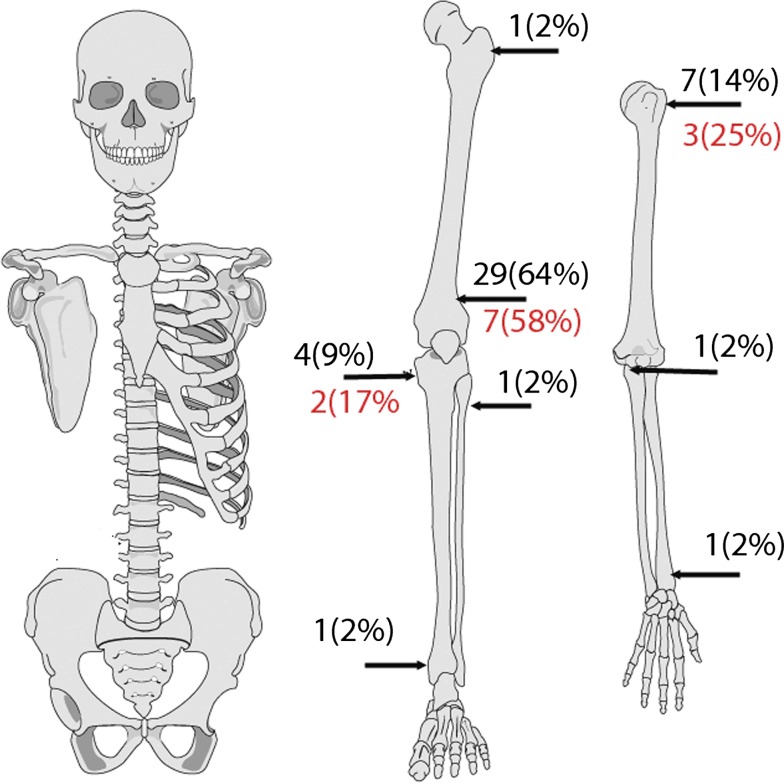
Forty-five (76%) patients with an average age of 31 years (range, 11–75 years) were juxtacortical. Twenty-four (53%) patients were male and 21 (47%) were female. Twelve patients had surgery for their disease before presenting to our institution. In nine cases the initial surgery was biopsy. Three patients presented to us with local recurrence after surgical treatment elsewhere; of these, two recurred following marginal resection and one with intralesional resection. The time to local recurrence was 20 months, 8.1 years, and 11 months, respectively. The anatomic distribution of the surface tumors was as follows: 29 in the distal femur (64%), seven in the proximal humerus (16%) and four in the proximal tibia (9%) (Fig. 4). The olecranon, distal radius, distal tibia, proximal fibula, and proximal femur each had one. Forty-two (93%) of the 45 patients with low-grade juxtacortical osteogenic sarcoma were treated with wide surgical margins. Three patients had a marginal excision; two of these patients had their margins extended with cryosurgery. Twelve (27%) of 45 patients had a high-grade “dedifferentiated” component to their tumors. All 12 patients were treated with wide surgical margins and multiagent adjuvant chemotherapy. The minimum followup was 1 year (median, 5.5 years; range, 1 to 29 years).
Fig. 4.
The anatomic distribution of low-grade juxtacortical osteogenic sarcoma demonstrating a propensity for the distal femur is shown. The second number below the arrow indicates sites of dedifferentiation.
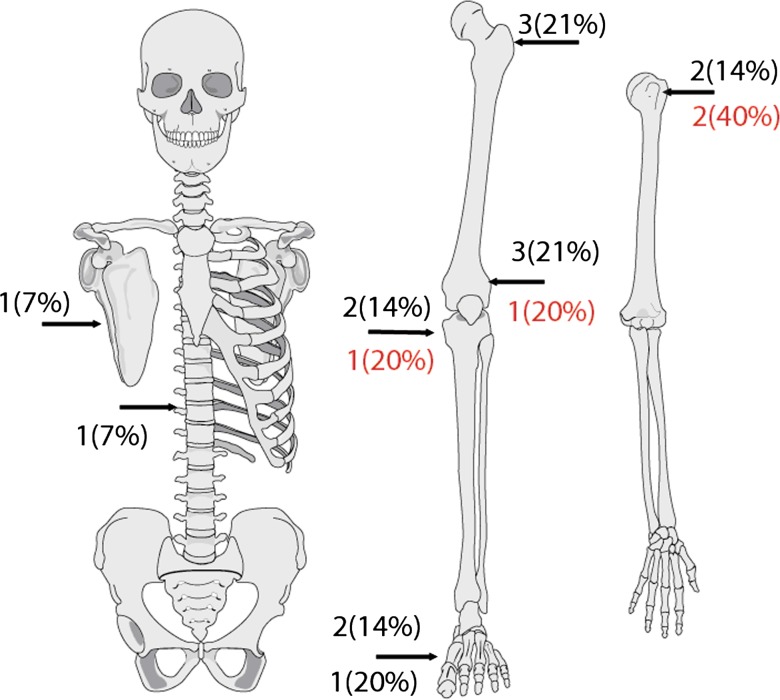
Fourteen (24%) of 59 patients with an average age of 39 years (range, 14–62 years) had low-grade intramedullary osteogenic sarcoma. Four patients were male and 10 were female. Seven patients had surgery elsewhere before presenting to our institution; four patients had an open biopsy and three patients had definitive surgery with subsequent local recurrence. The definitive surgeries included two intralesional excisions and one wide resection with time to local recurrence 1 year, 6 years, and 3.5 years respectively. The anatomic location of the intramedullary tumors was as follows: three low in the distal femur and three in the proximal femur (Fig. 5). The proximal humerus, proximal tibia and metatarsals each had 2. The thoracic spine and scapula each had one. Eleven patients with low-grade intramedullary OGS were treated with wide margins. Three patients were treated with intralesional margins, one of which was supplemented with liquid nitrogen. Five of 14 patients with low-grade intramedullary OGS had high-grade dedifferentiated components. The dedifferentiated pathology was high-grade osteogenic sarcoma in two cases, fibrosarcoma in two, and malignant fibrous histiocytoma in one. All five patients had their tumors widely resected. Four of the five received adjuvant multiagent chemotherapy. One patient whose lesion was in her fourth metatarsal refused chemotherapy. She had a ray amputation and remains free of disease.
Fig. 5.
The anatomic distribution of low-grade intramedullary osteogenic sarcoma is shown. The second number below the arrow indicates sites of dedifferentiation.
We used Fisher’s exact test to compare the rate of local recurrence, distant metastasis, and dedifferentiation between the two groups. Survival was determined using methods described by Kaplan-Meier. Analysis was performed with SPSS software. (SPSS, version 14, Chicago, IL).
Results
Thirteen of 14 patients with low-grade intramedullary osteogenic sarcoma were alive without evidence of disease after median follow up of 5 years and 4 months. One patient of 14 with low-grade intramedullary osteogenic sarcoma developed local recurrence 29 months after wide resection of her tumor. The recurrent lesion had a high-grade fibrosarcoma component. She was treated with wide resection followed by high-dose methotrexate, doxorubicin, and cisplatin. She subsequently developed acute myelogenous leukemia which was treated with cytarabine and asparaginase. She currently has no evidence of disease. One patient with low grade intramedullary OGS developed metastases to both her brain and lungs 27 months after having the primary tumor in her proximal tibia widely excised. Her primary tumor contained a focus of dedifferentiated sarcoma at presentation. She was 73 at the time of her primary diagnosis. She succumbed to her disease 42 months after her primary surgery.
Forty-two of 45 patients (93%) with low-grade juxtacortical osteogenic sarcoma remained alive without evidence of disease with a median follow up of 5 years and 3 months. Three (7%) local recurrences occurred in these 45 patients at 15 months, 4.2 years, and 22.7 years after primary wide resection. All three recurrences were associated with tumor grade progression to high-grade osteogenic sarcoma. Two of the three patients with recurrences developed metastatic disease and eventually died from their disease. Four (9%) of these 45 patients developed lung metastases, and one of the four also had brain metastasis. Lung metastasis occurred 6 months, 17 months, 10.6 years, and 22.5 years after the primary tumor excision. The two patients with the longer intervals between their primary excision and metastatic disease experienced prior local recurrence. Three of the four patients with metastatic disease died from their disease. One patient had bilateral pulmonary metastasectomy followed by the MSKCC T12 protocol. She remains alive with no evidence of disease.
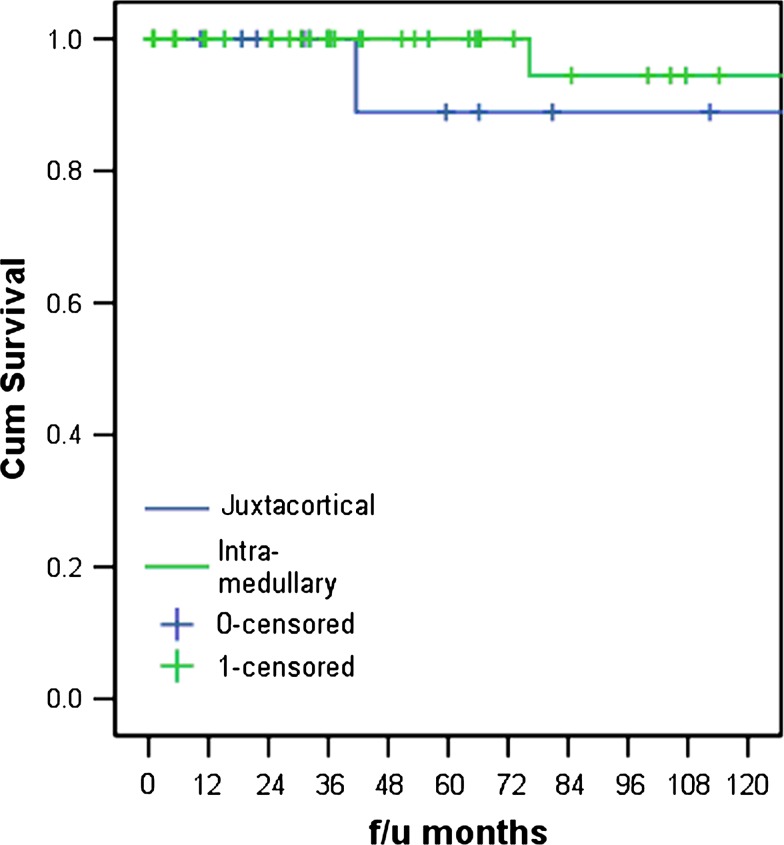
The disease-specific survival between low-grade intramedullary and low-grade juxtacortical osteogenic sarcoma was similar (Fig. 6). The two groups were similar with regard to local recurrence, rate of metastasis, or dedifferentiation.
Fig. 6.
The disease-free survival for patients with low-grade osteogenic sarcoma is greater than 90% for both the intramedullary and juxtacortical variants.
Discussion
Historically, low grade intramedullary and low grade juxtacortical osteogenic sarcomas are considered distinct clinical entities. A direct comparison of the two diseases has not been previously reported. We sought to determine the extent of clinical overlap between the two groups.
Our study is limited by the small numbers of patients available. These sorts of lesions are variable in presentation and behavior and it would require very large numbers to perform a formal statistical analysis including subgroup analyses. However, they are relatively uncommon and large numbers cannot be readily accumulated. Even if accumulated over time (ours were accumulated from 1971 to 2004, over three decades), diagnostic modalities and treatments change, rendering some comparisons difficult. Nonetheless, we believe the comparisons useful since overall the lesions behaved similarly.
Our data suggest low-grade intramedullary and juxtacortical osteogenic sarcomas are distinguished only by their location relative to the cortex. We found the rates of local recurrence for low-grade intramedullary (7%) and juxtacortical (7%) osteogenic sarcoma were similar. Previous reports have documented the association between inadequate surgical resection, local recurrence, and progression of tumor grade [4, 13]. Nearly 80% of intramedullary lesions and over 90% of juxtacortical lesions in this study were resected with wide margins. The local recurrences occurred an average of 7.6 years after their primary surgical resection (range, 1.3–22.7 years). All four local recurrences in our series occurred after wide resection and all were associated with progression to a higher grade. Two of those four patients eventually succumbed to their disease.
The rates of distant metastasis for low-grade intramedullary (7%) and juxtacortical (9%) osteogenic sarcoma were also similar. Others have reported distant metastatic rates of up to 20% [4]. All five patients had progression of tumor grade or initial dedifferentiation status. Four of the five patients who developed distant metastasis died from their disease. The lone survivor from distant metastasis was treated with thoracotomy for pulmonary disease followed by the multiagent chemotherapy.
Dedifferentiation occurred slightly more often in the intramedullary group (36%) compared to the juxtacortical group (27%). These rates fall within the reported rates of dedifferentiation in juxtacortical osteogenic sarcomas which range from 16% to 43% [2, 8, 9, 13]. The largest reported series of low-grade intramedullary osteogenic sarcoma had a rate of dedifferentiation of 25% [4]. While surgery alone is adequate for low-grade tumors, the addition of chemotherapy is prudent in the management of patients with areas of histologic dedifferentiation.
We consider the dedifferentiated component of low-grade osteogenic sarcoma as a separate entity from conventional high-grade osteogenic sarcoma. While this distinction is controversial and not uniformly accepted, it appears low-grade tumors with areas of focal dedifferentiation behave differently than their conventional high-grade counterparts. The patients with dedifferentiation in our series had a median age of 35 years and experienced 82% long-term survival (> 5 years). This is superior to the 5-year survival for both adult patients (58%) [6] and pediatric patients (65%–75%) [7] with localized high grade disease. Furthermore, both local and distant recurrence is a late phenomenon in the dedifferentiated group on the order of several years whereas conventional high-grade tumors usually experience recurrence in the first few years. Certainly all tumors with any dedifferentiated component should be treated as a high-grade tumor incorporating systemic treatment. Unfortunately there are no guidelines, such as percentage of the overall tumor with a high-grade component, to categorize these tumors as typical high-grade osteogenic sarcomas.
Low-grade intramedullary and low-grade juxtacortical osteogenic sarcomas behave similarly with regard to local recurrence, metastasis, dedifferentiation, and survival. While the anatomic distinction implied in their names is useful, their histologic appearance and clinical behavior are indistinguishable. We recommend wide surgical excision in primary lesions, and reserve adjuvant chemotherapy for dedifferentiated lesions.
Footnotes
Each author certifies that he or she has no commercial associations that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Antonescu CR, Huvos AG. Low-grade osteogenic sarcoma arising in medullary and surface osseous locations. Am J Clin Pathol. 2000;114 Suppl:S90–103. doi: 10.1093/ppr/114.1.s90. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni F, Bacchini P, Staals EL, Davidovitz P. Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer. 2005;103:2373–2382. doi: 10.1002/cncr.21039. [DOI] [PubMed] [Google Scholar]
- 3.Campanacci M, Picci P, Gherlinzoni F, Guerra A, Bertoni F, Neff JR. Parosteal osteosarcoma. J Bone Joint Surg Br. 1984;66:313–321. doi: 10.1302/0301-620X.66B3.6586725. [DOI] [PubMed] [Google Scholar]
- 4.Choong PF, Pritchard DJ, Rock MG, Sim FH, McLeod RA, Unni KK. Low grade central osteogenic sarcoma. A long-term followup of 20 patients. Clin Orthop Relat Res. 1996;322:198–206. doi: 10.1097/00003086-199601000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh TG, Cannon SR, Pringle J, Stoker DJ, Kemp HB. Parosteal osteosarcoma. Treatment by wide resection and prosthetic replacement. J Bone Joint Surg Br. 1990;72:959–965. doi: 10.1302/0301-620X.72B6.2246298. [DOI] [PubMed] [Google Scholar]
- 6.Manoso MW, Healey JH, Boland PJ, Athanasian EA, Maki RG, Huvos AG, Morris CD. De novo osteogenic sarcoma in patients older than forty: benefit of multimodality therapy. Clin Orthop Relat Res. 2005;438:110–115. doi: 10.1097/01.blo.0000179587.42350.4d. [DOI] [PubMed] [Google Scholar]
- 7.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Okada K, Frassica FJ, Sim FH, Beabout JW, Bond JR, Unni KK. Parosteal osteosarcoma. A clinicopathological study. J Bone Joint Surg Am. 1994;76:366–378. doi: 10.2106/00004623-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Sheth DS, Yasko AW, Raymond AK, et al. Conventional and dedifferentiated parosteal osteosarcoma. Diagnosis, treatment, and outcome. Cancer. 1996;78:2136–2145. doi: 10.1002/(SICI)1097-0142(19961115)78:10<2136::AID-CNCR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Szymanska J, Mandahl N, Mertens F, Tarkkanen M, Karaharju E, Knuutila S. Ring chromosomes in parosteal osteosarcoma contain sequences from 12q13–15: a combined cytogenetic and comparative genomic hybridization study. Genes Chromosomes Cancer. 1996;16:31–34. doi: 10.1002/(SICI)1098-2264(199605)16:1<31::AID-GCC4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Tarkkanen M, Böhling T, Gamberi G, Ragazzini P, Benassi MS, Kivioja A, Kallio P, Elomaa I, Picci P, Knuutila S. Comparative genomic hybridization of low-grade central osteosarcoma. Mod Pathol. 1998;11:421–426. [PubMed] [Google Scholar]
- 12.Temple HT, Scully SP, O’Keefe RJ, Katapurum S, Mankin HJ. Clinical outcome of 38 patients with juxtacortical osteosarcoma. Clin Orthop Relat Res. 2000;373:208–217. doi: 10.1097/00003086-200004000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Wold LE, Unni KK, Beabout JW, Sim FH, Dahlin DC. Dedifferentiated parosteal osteosarcoma. J Bone Joint Surg Am. 1984;66:53–59. [PubMed] [Google Scholar]