Abstract
The emergence of resistant strains of Gram-positive organisms in osteomyelitis creates treatment challenges. Daptomycin is an antibiotic that shows promise for treating some resistant strains of Gram-positive infections; however, it has not been widely used clinically for the treatment of osteomyelitis. We determined whether daptomycin eluted from calcium sulfate—a local delivery vehicle used for the treatment of osteomyelitis—retained activity against Gram-positive bacteria. Daptomycin was mixed with calcium sulfate hemihydrate, with both laboratory powder and a commercial kit, to form a hardened pellet. Daptomycin was eluted from calcium sulfate and retained its ability to inhibit bacterial growth of Staphylococcus aureus and Staphylococcus epidermidis for eluates gathered up to 28 days. Our preliminary data demonstrates sterilized pellets with daptomycin retained their ability to inhibit bacterial growth of certain strains of Gram-positive organisms.
Introduction
Daptomycin is an antibiotic that has activity against resistant Gram-positive organisms. This antibiotic has potential in musculoskeletal use and could be useful for local delivery to help combat infections, especially those due to resistant strains of bacteria. Infected bone can suffer bone loss and require bone grafting for defect restoration. Calcium sulfate has been used as a bone void filler for many years with good clinical outcomes [12, 13, 17]. In addition to calcium sulfate’s osteoconductive properties, this biomaterial can be used to deliver antibiotics or growth factors locally [3, 4]. Local delivery of antibiotics for the treatment of osteomyelitis can be advantageous over systemic delivery in cases where vasculature is compromised, because local delivery can safely provide higher concentrations of antibiotics at the infection site [10]. Further, daptomycin elutes from calcium sulfate in a manner similar to tobramycin in vitro [14].
Calcium sulfate benefits include moderate mechanical strength for space filling, radioopacity, and the ability to be administered in a clinical setting as a pellet or paste [17]. The compressive strength of calcium sulfate is between that of cancellous and cortical bone [2, 6, 7]. Studies in 1957 and 1959 by Peltier showed calcium sulfate in bone voids is absorbed by surrounding tissue, and might accelerate bone healing [12, 13]. More recent canine models demonstrate calcium sulfate used to treat bone defects is replaced by new bone, and that new bone is similar in volume, measured by area fraction, to that seen with use of autograft [18]. Further, calcium sulfate has been used as a delivery agent for antibiotics, such as tobramycin, vancomycin, and gentamicin [1, 5, 9, 16].
Daptomycin is currently indicated for complicated skin and skin structure infection (cSSSI) and right-sided endocarditis due to Staphylococcus aureus [14]. Calcium sulfate pellet mixed with daptomycin can elute daptomycin during the pellet degradation process [14]. The delivery system with calcium sulfate could also be advantageous, because this antibiotic is not bactericidal without free calcium ions [15]. The recommended level of calcium for the activation of daptomycin is approximately 50 μg/mL, which is approximately the calcium ion concentration in the blood of a healthy adult [8]. In areas with compromised circulation, these calcium levels may be varied depending on the vascular system status in the bone defect [11]. The requirement of calcium ions for daptomycin’s antibiotic mechanism of action and the potential for local delivery within a calcium-rich material could be clinically advantageous.
We asked the following questions: (1) Is daptomycin released from reagent grade calcium sulfate pellets active against bacteria commonly found in osteomyelitis, namely Staphylococcus aureus and S. epidermidis? (2) Can a similar daptomycin elution profile be achieved using a commercially available calcium sulfate bone graft substitute kit? and (3) Does gamma sterilization affect the elution profile of daptomycin or effectiveness of eluted daptomycin in the inhibition of growth of Gram+ organisms?
Materials and Methods
Two groups of calcium sulfate (CaSO4) pellets containing the antibiotic daptomycin were created through two techniques we term “laboratory pellets” (nonsterilized) and “clinical pellets”; a third group consisted of the gamma-sterilized laboratory pellets. The methods mimic the production of such pellets in a manufacturing-type scheme (casting in advance of use) and a clinical-type scheme (aseptic casting of sterile components immediately prior to use). We used nine vials of eight pellets each for the nonsterilized laboratory pellet experiments, three vials of eight pellets each for the clinical pellet experiments, and three vials of eight pellets each for the gamma-sterilized laboratory pellet experiments. The two groups of laboratory pellets were created to address concerns about gamma radiation-induced degradation of the antibiotic or its elution. The clinical pellets were created to confirm similar implant materials could be made with commercially available medical-grade products.
To create laboratory pellets, we mixed reagent-grade CaSO4 hemihydrate powder with a potassium sulfate (K2SO4) solution at 4 wt%. The mixing ratio was one part K2SO4 solution to four parts CaSO4 (by weight). After mixing the CaSO4 and K2SO4 solution for 2 minutes, lyophilized daptomycin powder was added, at one part daptomycin (Cubicin®; Cubist Pharmaceuticals, Lexington, MA) to 20 parts CaSO4. Pellets were cast in a silicone elastomer mold (4.8 mm diameter × 3.3 mm height) and allowed to cure for approximately 1 hour before demolding. Three groups of pellets were allowed to set for 24 hours before testing. One additional group was mixed, allowed to set for 24 hours, gamma sterilized, then evaluated.
Clinical pellets were made to simulate formulation in a clinical theatre. We used CaSO4 from commercially available Osteoset® (Wright Medical Technology, Arlington, TN) kits. CaSO4 from the kit was mixed with 7.2 g of a 3%wt K2SO4 solution (substituted for the CaSO4 kit mix solution) for 1 minute followed by one minute of undisturbed curing. Daptomycin was added at a ratio of two vials per kit, followed by one minute of mixing. The pellets were cast, demolded at 15 minutes, and testing began at 30 minutes.
Elution characteristics were determined for each group of pellets. Three elution samples per group were tested. Each sample consisted of eight pellets in 20 mL of phosphate buffered saline (PBS) kept at 37°C for the duration of the test. Aliquots of the eluent (1 mL) were collected for each laboratory pellet sample on days 1, 2, 5, 7, 10, 14, 21, and 28. The commercial kit pellet samples were collected at days 1, 2, 3, 4, 5, 7, 11, 15, 21, and 28. PBS solution was completely refreshed at each time interval, and the pellets were returned to the elution vessels at 37°C. Aliquots were frozen at −8°C prior to testing. The daptomycin levels in the sample eluents were determined by high performance liquid chromatography (HPLC). A method of measuring daptomycin concentrations with HPLC was previously reported by Lai and Brodeur [5] and Richelsoph et al. [14].
Two sets of vials were prepared with 1.8 mL of Mueller Hinton II (MHII) broth, supplemented with CaCl2 to obtain 50 μg/mL. Aliquots of 200 μL from each sample of the laboratory pellet were added to the broth. Eight vials of 200 μL of a known daptomycin concentration in PBS (0.15, 0.31, 0.62, 1.25, 2, 5, 10, and 20 μg/mL) and one vial with no daptomycin (200 μL of PBS) served as controls. Vials were inoculated with 20 μL (CFU ∼104) of S. aureus (daptomycin MIC approximately 0.15 μg/mL) or S. epidermidis (daptomycin MIC approximately 0.31 μg/mL), incubated at 37°C for 24 hours, and the absorbance at 530 nm (A530) was recorded. The spectrophotometer was adjusted to zero using a blank consisting of 1.8 mL MHII broth and 200 μL of PBS.
We used the Mann-Whitney rank sum test to determine differences between the elution of clinical and laboratory pellets, the elution of sterilized and nonsterilized pellets, and the ability of eluted daptomycin to inhibit bacterial growth, eluted from both sterilized and nonsterilized calcium sulfate pellets. GraphPad Prism (GraphPad Software, Inc. San Diego, CA) was used for all tests.
Results
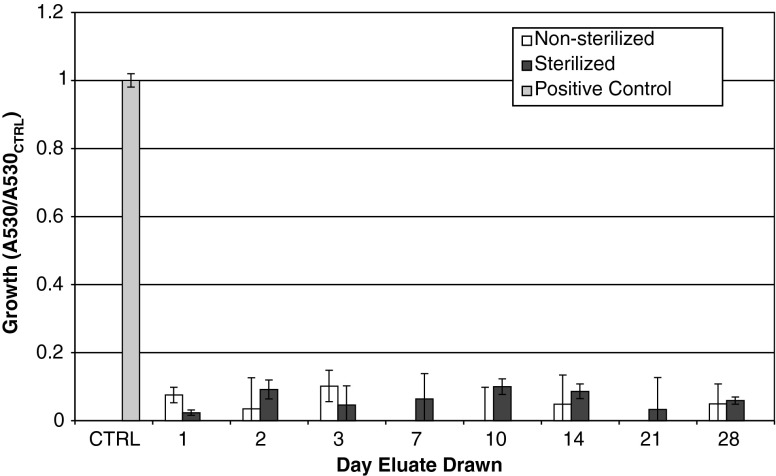
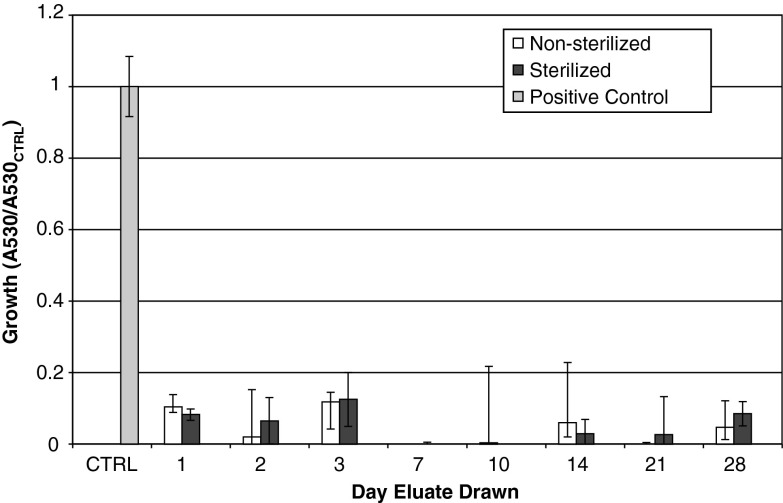
The eluates from the laboratory pellets drawn at all days inhibited growth of both S. aureus (Fig. 1) and S. epidermidis (Fig. 2) (Tables 1, 2). For days 10 and 14, one sample of the three had substantial growth (∼50% compared to control). The sample from Day 10 had an unknown contamination in the storage vial.
Fig. 1.
The activity of Staphylococcus aureus exposed to eluates is shown. All groups were pellets manufactured with 4% daptomycin per total powder weight, with a 4% K2SO4 solution. Ratio of growth was calculated as (A530 of sample)/(A530 of positive control). The group labeled “sterile” was gamma sterilized after pellet manufacture.
Fig. 2.
The activity of Staphylococcus epidermidis exposed to eluates is shown. All groups were pellets manufactured with 4% daptomycin per total powder weight, with a 4% K2SO4 solution. Ratio of growth was calculated as (A530 of sample)/(A530 of positive control). The group labeled “sterile” was gamma sterilized after pellet manufacture.
Table 1.
Average growth compared to positive control
| Staphylococcus aureus | ||||
|---|---|---|---|---|
| Day | Nonsterilized | Sterilized | ||
| 1 | 7.5% | (2.3) | 5.9% | (0.76) |
| 2 | 3.4% | (9.1) | 3.1% | (2.76) |
| 3 | 10.1% | (4.6) | 10.3% | (5.60) |
| 7 | −6.6% | (6.4) | −9.2% | (7.40) |
| 10 | −0.2% | (9.9) | −12.5% | (2.28) |
| 14 | 4.8% | (8.6) | 0.0% | (2.15) |
| 21 | −1.6% | (3.3) | 1.9% | (9.39) |
| 28 | 4.9% | (5.8) | 4.2% | (1.06) |
Parentheses indicate standard deviations.
Table 2.
Average growth compared to positive control
| Staphylococcus epidermidis | ||||
|---|---|---|---|---|
| Day | Nonsterilized | Sterilized | ||
| 1 | 10.4% | (3.4) | 8.2% | (1.59) |
| 2 | 1.9% | (13.2) | 6.5% | (6.50) |
| 3 | 11.8% | (2.7) | 12.5% | (7.56) |
| 7 | −13.6% | (8.9) | −14.5% | (14.96) |
| 10 | 0.4% | (21.4) | −19.2% | (4.06) |
| 14 | 6.0% | (16.8) | 2.8% | (4.00) |
| 21 | −4.0% | (4.3) | 2.6% | (10.68) |
| 28 | 4.6% | (7.5) | 8.5% | (3.40) |
Parentheses indicate standard deviations.
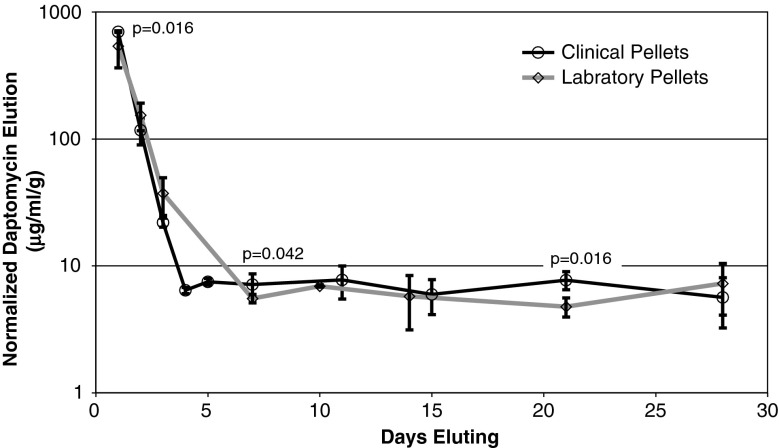
The elution trends for the clinical pellets and nonsterilized laboratory pellets were similar (Fig. 3). Daptomycin recovery for both the laboratory pellets and the clinical pellets was similar (35% and 30%, respectively). All nonsterilized laboratory samples had a concentration of at minimum 4 μg/mL of daptomycin or greater (Table 3); the clinical pellets had similar concentrations (Table 3).
Fig. 3.
The illustration shows clinical pellets manufactured from a commercial kit eluted similarly to laboratory pellets manufactured with laboratory-grade calcium sulfate hemihydrate. 3% K2SO4 was used with the commercial kit pellets, and 4% K2SO4 was used with the laboratory pellets. We observed small statistically different, but likely unimportant, elution on day 1 (p = 0.016), day 7 (p = 0.042), and day 21 (p = 0.016).
Table 3.
Daptomycin eluted from calcium sulfate pellets†
| μg/ml/ginit | ||||
|---|---|---|---|---|
| Days | Nonsterilized, laboratory | Std Dev | Sterilized, laboratory | Std Dev |
| 1 | 537.2 | (148.4) | 303.5 | (37.2) |
| 2 | 153.5 | (39.1) | 153.6 | (13.8) |
| 3 | 37.2 | (15.6) | 43.9 | (23.2) |
| 7 | 5.5 | (1.7) | 4.6 | (1.3) |
| 10 | 6.9 | (1.5) | 4.1 | (1.1) |
| 14 | 5.8 | (3.9) | 3.9 | (1.6) |
| 21 | 4.8 | (1.5) | 4.8 | (0.8) |
| 28 | 7.2 | (4.7) | 4.5 | (1.7) |
| Days | Clinical | Std Dev | ||
| 1 | 697.4 | (7.1) | ||
| 2 | 116.8 | (27.0) | ||
| 3 | 21.9 | (1.8) | ||
| 4 | 6.4 | (0.3) | ||
| 5 | 7.5 | (0.4) | ||
| 7 | 7.1 | (1.5) | ||
| 11 | 7.7 | (2.2) | ||
| 15 | 6.0 | (1.8) | ||
| 21 | 7.7 | (1.2) | ||
| 28 | 5.6 | (2.4) | ||
†Note: All concentrations have been divided by initial pellet weight, in grams.
Daptomycin elution of the gamma-sterilized and nonsterilized pellets was similar, with small differences on days 1 (p = 0.027) and 10 (p = 0.027) (Table 3). The contaminate observed on the elution vial drawn on day 10 on a nonsterilized pellet aliquot may have contributed to this difference. Gamma-sterilized pellets inhibited bacterial growth in a similar amount to the nonsterilized pellets for both S. aureus and S. epidermidis except for day 10 when for the S. aureus when the contamination may have contributed to the difference.
Discussion
The emergence of resistant strains of Gram-positive organisms in osteomyelitis has resulted in infections that cannot be readily treated with the usual antibiotics. Daptomycin shows promise for treating some of these resistant strains of Gram-positive infections. The purpose of this study was therefore threefold: (1) to verify that daptomycin eluted from calcium sulfate would still retain its ability to inhibit growth; (2) to compare the elution of daptomycin when added to calcium sulfate under conditions similar to the clinical theatre to daptomycin/calcium sulfate pellets prepared in the laboratory; and (3) to examine the behavior of gamma radiation (a commonly used sterilization technique) on the ability of daptomycin to inhibit bacterial growth of the selected strains of Staphylococcus.
One limitation exists for all in vitro elution studies. A set volume of fluid exchanged at predetermined intervals may not be an accurate model of a wound or defect in bone, which may have a varying volume, and have vascular supply that may change elution rate, dissolution rate, or local drug concentrations. The data collected is useful when compared to other materials tested with the same experimental conditions. In addition, only two strains of Staphylococcus were selected. These two strains may not be the causative organisms in some cases of osteomyelitis.
Rouse et al. [15] demonstrated daptomycin had similar activity and release from PMMA as vancomycin when used in a rat MRSA osteomyelitis model and suggesting daptomycin may be a useful antibiotic for local treatment of resistant bacterial strains causing osteomyelitis. Mader and Adams [8] showed that given the same concentrations of antibiotics in serum, less daptomycin will be present in infected bone than vancomycin. Therefore, daptomycin may be more appropriately delivered locally, versus systemically, for treatment of infection in bone. The use of a degradable local delivery vehicle may be beneficial compared to a nondegradable delivery material, as synthetic materials in the body may potentially serve as a substrate for bacterial biofilm attachment. A case observed by Neut et al. [11] demonstrated gentamicin PMMA bead implanted formed a layer of gentamicin-resistant coagulase-negative Staphylococci 5 years after implantation of the beads. These three observations give support to the use of a degradable local delivery material for the treatment of osteomyelitis with daptomycin.
Our data suggest the eluted daptomycin from a calcium sulfate pellet retains the ability to inhibit bacterial growth. Gamma irradiation did not appear to affect the elution or ability of the antibiotic to inhibit bacterial growth. The data also indicated pellets manufactured from a commercially available calcium sulfate kit, when prepared within a clinically relevant time frame, eluted daptomycin similarly to the methods previously described by Richelsoph et al. [14].
Eluates drawn from each time point showed some inhibitory effects on the growth of the two strains of bacteria tested. One measurement from each of days 10 and 14 was higher than the other measurements for the same day in the group, resulting in a large standard deviation. The source of this variation is unknown, although an open elution environment may have introduced potential contaminates. Eluates collected through day 28 inhibited growth for both strains of bacteria.
Our preliminary study demonstrated daptomycin eluted from calcium sulfate retained its inhibitory effects on the growth of the selected strains of bacteria. Daptomycin retained bacterial inhibitory activity after several days of near-body temperatures, remained active when mixed and eluted from calcium sulfate, and was active after exposure to gamma irradiation. The study also indicated daptomycin can be clinically produced with a commercially available CaSO4 kit with a modified mixing solution. The calcium sulfate/daptomycin local delivery combination should be further investigated in a preclinical in vivo study to determine potential applications for the treatment of osteomyelitis.
Acknowledgments
We thank Judith Steenbergen, PhD, of Cubist Pharmaceuticals (Lexington, MA) for technical support and advice. We thank Amber Jennings, PhD, of the University of Memphis (Memphis, TN) for technical support.
Footnotes
One or more of the authors (NW, WH) receive funding from Cubist Pharmaceuticals, Lexington, MA. Materials were donated from Wright Medical Technology, Ltd. (Arlington, TN).
References
- 1.Akins R, Haase K. Gram-positive resistance: pathogens, implications, and treatment options: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2005;25:1001–1010. doi: 10.1592/phco.2005.25.7.1001. [DOI] [PubMed] [Google Scholar]
- 2.Earnshaw R, Smith D. The tensile and compressive strength of plaster and stone. Austr Dent J. 1966;11:415–422. doi: 10.1111/j.1834-7819.1966.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 3.Heijink A, Yaszemski M, Patel R, Rouse M, Lewallen D, Hanssen A. Local antibiotic delivery with OsteoSet, DBX, and Collagraft. Clin Orthop Relat Res. 2006;451:29–33. doi: 10.1097/01.blo.0000229319.45416.81. [DOI] [PubMed] [Google Scholar]
- 4.Intini G, Andreana S, Entini F, Buhite R, Bobek L. Calcium sulfate and platelet-rich plasma make a novel osteoinductive biomaterial for bone regeneration. J Transl Med. 2007;5:13. doi: 10.1186/1479-5876-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai J, Brodeur S. Physical and chemical compatibility of daptomycin with nine medications. Ann Pharmacother. 2004;38:1612–1616. doi: 10.1345/aph.1E124. [DOI] [PubMed] [Google Scholar]
- 6.Lee R, Volz R, Sheridan D. The role of fixation and bone quality on the mechanical stability of tibial knee components. Clin Orthop Relat Res. 1991;273:177–183. [PubMed] [Google Scholar]
- 7.Linde F, Hvid I, Pongsoipeetch B. Energy absorptive properties of human trabecular bone specimens during axial compression. J Orthop Res. 1989;7:432–439. doi: 10.1002/jor.1100070316. [DOI] [PubMed] [Google Scholar]
- 8.Mader J, Adams K. Comparative evaluation of daptomycin (LY146032) and vancomycin in the treatment of experimental methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob Agents Chemother. 1989;33:689–692. doi: 10.1128/aac.33.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaren A. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res. 2004;427:101–106. doi: 10.1097/01.blo.0000143554.56897.26. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2002. Performance Standards for Antimicrobial Susceptibility Testing–12th Informational Supplement: Approved Standard M100-S12. Wayne, PA: NCCLS; 2002.
- 11.Neut D, van de Belt H, van Horn J, van der Mei H, Busscher H. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 2003;24:1829–1831. doi: 10.1016/S0142-9612(02)00614-2. [DOI] [PubMed] [Google Scholar]
- 12.Peltier L. The use of plaster of Paris to fill large defects in bone. Am J Surg. 1959;97:11–15. doi: 10.1016/0002-9610(59)90305-8. [DOI] [PubMed] [Google Scholar]
- 13.Peltier L, Bickel E, Lillo R, Thein M. The use of plaster of Paris to fill defects in bone. Ann Surg. 1957;146:61–69. doi: 10.1097/00000658-195707000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richelsoph K, Webb N, Haggard W. Elution behavior of daptomycin-loaded calcium sulfate pellets: a preliminary study. Clin Orthop Relat Res. 2007;461:68–73. doi: 10.1097/BLO.0b013e3181123889. [DOI] [PubMed] [Google Scholar]
- 15.Rouse M, Piper K, Jacobson M. Daptomycin treatment of Staphylococcus aureus experimental chronic osteomyelitis. J Antimicrob Chemother. 2006;57:301–305. doi: 10.1093/jac/dki435. [DOI] [PubMed] [Google Scholar]
- 16.Salzer W. Antimicrobial-resistant gram-positive bacteria in PD peritonitis and the newer antibiotics used to treat them. Perit Dial Int. 2005;25:313–319. [PubMed] [Google Scholar]
- 17.Thomas M, Puleo D, Al-Sabbagh M. Calcium sulfate: a review. J Long Term Eff Med Implants. 2005;15:599–607. doi: 10.1615/jlongtermeffmedimplants.v15.i6.30. [DOI] [PubMed] [Google Scholar]
- 18.Turner T, Urban R, Gitelis S, Kuo KN, Andersson GB. Radiographic and histologic assessment of calcium sulfate in experimental animal models and clinical use as a resorbable bone-graft substitute, a bone-graft expander, and a method for local antibiotic delivery: one institution’s experience. J Bone Joint Surg Am. 2001;83(Suppl 2 Pt 1):8–18. doi: 10.2106/00004623-200100021-00003. [DOI] [PubMed] [Google Scholar]