Abstract
Since publication of the results of a first-generation intercalary humeral spacer, a newer design has been available that addressed the weaknesses of the first. This study evaluated the hypothesis that the second-generation lap joint junction intercalary humeral spacer reduced complications compared with the original male-female taper design. We retrospectively reviewed the charts of 32 consecutive patients who had undergone placement of an intercalary humeral spacer. Twenty-one with the male-female taper (minimum followup 0 months, mean 19.2 months) were compared with 11 with the lap joint configuration (minimum followup 0 months, mean 20.3 months). Demographic, tumor, treatment, and radiographic variables were similar between groups. We observed a lower complication rate in the lap joint group (three of 11 versus 11 of 21). The most common complications in the male-female group, neuropraxia, periprosthetic fracture, and disengagement, were not seen in the lap joint group. Aseptic loosening was more frequent in the lap joint group. There were no differences in blood loss, operative time, or Musculoskeletal Tumor Society scores between groups. We noted improvement in Musculoskeletal Tumor Society scores from preoperatively to postoperatively in both groups. Use of these implants should be reserved for patients with limited life expectancy.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The humerus is second only to the femur among long bones involved by metastatic disease [5, 6, 13–15]. As with other sites of metastatic disease, the goals of treatment for humeral lesions are pain relief and functional restoration to improve quality of life [7]. When there is adequate remaining intact bone proximal and distal to the area of involvement that allows placement of a standard fixation device, many authors recommend either a locked intramedullary nail or plate fixation [2, 4–6, 9–11, 13–15]. Sometimes supplemental cement enhances fixation [7]. However, in exceptional situations with extensive cortical destruction or segmental diaphyseal bone loss, conventional treatment may not yield stable fixation even with supplemental cement. In these situations, standard means of fixation may have a higher risk of device failure or at least a lower likelihood of providing immediate stability to most completely restore function [1, 3, 8, 12].
One option in this situation is the use of a cemented intramedullary spacer device [1, 3, 8, 12]. This device has previously been reported in a smaller series of patients in whom an early version of the device was implanted [3]. The earlier version used a male-female taper that required distraction of the cemented components in situ to reduce and secure the two components together. In the original series using this device [3], there were three nerve palsies in 17 patients, predominantly transient but occurring after implantation, perhaps related to the distraction required to assemble the device. Furthermore, in the initial series, there were limited stem lengths available. Perhaps related to the limited stem selection was the occurrence of two periprosthetic fractures postoperatively among the 17 earlier reported patients: failure at the junction was encountered as well with disengagement of the male-female taper (2/17) [3].
Subsequent to the initial report, a newer generation of the prosthesis was introduced that addressed the concerns with the initial device. The second-generation device used a lap joint rather than the male-female taper and also provided a wider array of stem sizes and lengths. In addition to the newer taper, the second-generation device also offered increased sizes of stem diameter, stem length, and body size combinations. The goal of these design modifications was to lower the incidence of nerve palsies and to reduce periprosthetic fractures by allowing a greater portion of the humeral canals to be covered by longer and larger diameter stems.
We asked whether the use of this second-generation device in a selected group of patients would: (1) provide substantial pain relief; (2) have functional results comparable to the original prosthesis; and (3) have implant survivorship comparable to the original prosthesis at similar followup duration without increasing blood loss or operative time, while also reducing the complications of nerve palsy, disengagement, and periprosthetic fracture compared with the original version of the prosthesis.
Materials and Methods
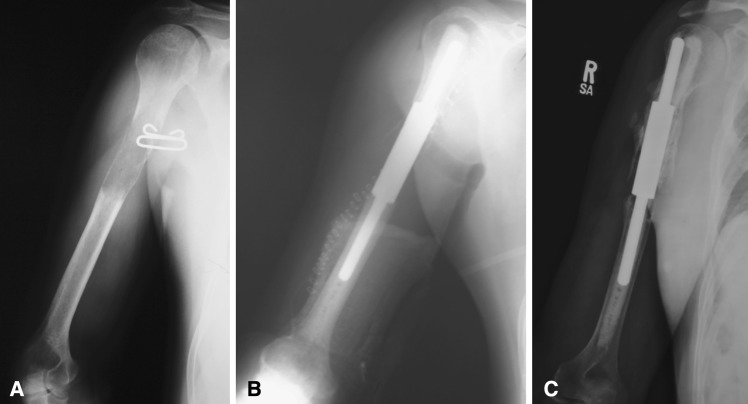
We retrospectively reviewed the medical records of 39 patients who had intercalary humeral spacers placed at the Mayo Clinic and the State University of New York Upstate Medical University from January 1989 to December 2004. The period of collection spanned the evolution of this device from a male-female taper junction secured by a single set screw in 1989 to a lap joint construct secured by two set screws first introduced in January 1999 (Stryker, Mahwah, NJ). Intercalary humeral spacers had been implanted in a total of 39 patients at the two institutions. However, there was essentially no postoperative information available for seven patients. Hence, the study population consisted of 32 patients treated with intercalary spacers for humeral lesions. Before 1999, there were 21 of the first-generation devices implanted at the senior author’s (FHS) institution (Fig. 1A–B). Subsequent to that date, 11 of the second-generation devices were implanted at the two study institutions (TAD, FHS) (Fig. 2). Power analysis showed that for the effect size and standard deviation of one of the primary independent variables, MSTS pain score, at the samples sizes available (11 for the lap joint group and 21 for the male-female taper group) and alpha 0.050, two-tailed, power was only 0.117. In other words, only 12% of studies would be expected to yield a significant effect, rejecting the null hypothesis that the two population means are equal.
Fig. 1A–B.
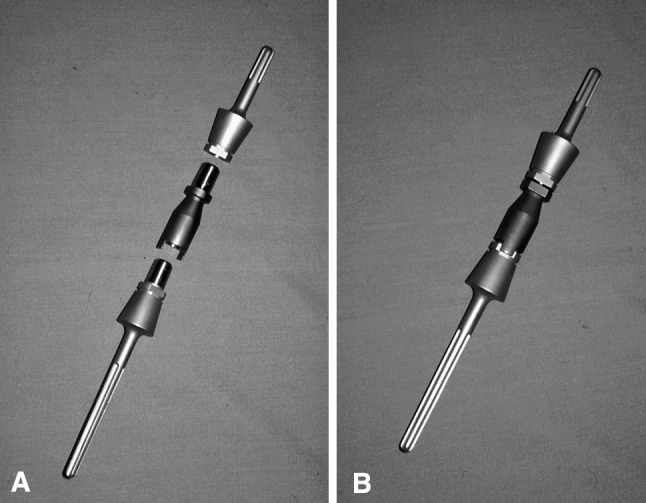
The male-female taper junction of the original intercalary humeral spacer prosthesis is shown. Note that although rotation is not fixed with implantation of the individual components, distraction of 2.04 cm is needed to reduce the junction. (A) The components of this spacer show a proximal and distal stem along with a separate body segment. (B) This spacer has been assembled.
Fig. 2.
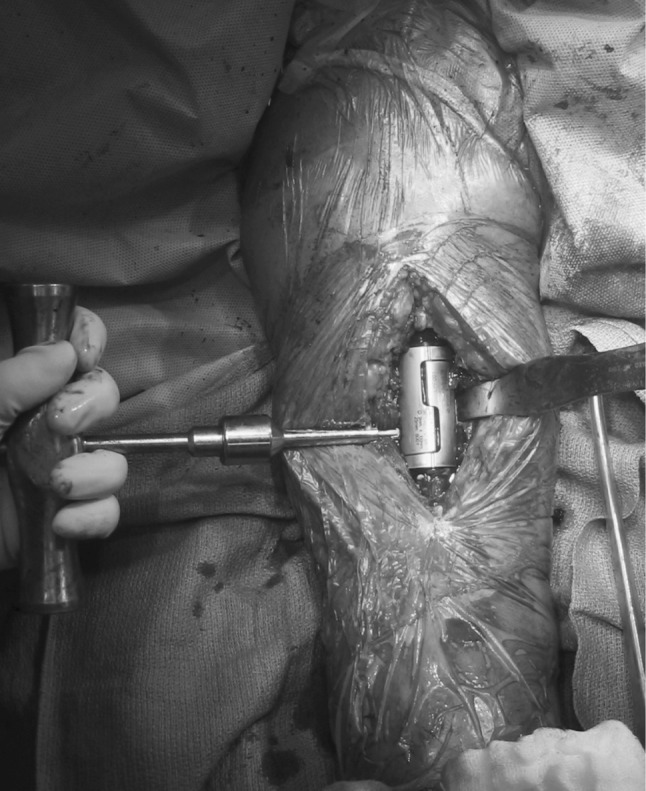
The lap joint junction of the second-generation intercalary humeral spacer is shown. There are holes for the set screws positioned 180° apart, one over each portion of the lap joint, which allow for compression at the dual taper. Note that although no distraction is necessary, rotation is fixed on cementation of the individual stems into the humerus, so appropriate rotation must be ensured before cementing.
To define the patient population, the data reviewed included patient demographics, underlying disease characteristics, preoperative and postoperative adjuvant treatments, surgical indications, anatomic location within the humerus, and operative findings including size of the implant. We reviewed preoperative radiographs to assess the site of involvement. None of the 32 patients were lost to followup but some patients died before clinical or radiographic followup was obtained. Clinical followup was a minimum of zero months (mean, 19.9 months; range, 0–110 months). Radiographic followup was a minimum of zero months (mean, 8.7 months; range, 0–72 months). We had prior Institutional Review Board approval.
Twenty-one patients received 21 male-female first-generation intercalary spacers, and 11 patients received 11 lap joint second-generation intercalary spacers. The demographic features of these two groups of patients did not differ except more (p = 0.05) of the male-female group had undergone humeral operative intervention that subsequently failed before placement of the spacer (six of 21 versus zero of 11) and more (p = 0.03) of the male-female group had undergone humeral operative intervention before the index procedure overall (seven of 21 versus zero of 11) than for the lap joint group (Table 1).
Table 1.
Comparative features between spacer groups
| Comparative features | Male-female (first generation) | Lap joint (second generation) | p Value |
|---|---|---|---|
| Patient and tumor demographics | |||
| Age (years; mean ± SD) | 64.8 ± 13.1 | 62.8 ± 11.4 | 0.67 |
| Primary cancer | 0.64 | ||
| Histology | 0.24 | ||
| Metastatic/myeloma (%) versus primary | 90.5% metastases | 90.9% metastases | 0.97 |
| Preoperative treatment | |||
| Preoperative radiation treatment (%) | 23.8 | 9.1 | 0.31 |
| Preoperative chemotherapy (%) | 28.6 | 45.5 | 0.19 |
| Previous operations for cancer (%) | 76.2 | 36.4 | 0.06 |
| Previous humeral operations (%) | 33.3 | 0 | 0.03 |
| Index surgery indications | |||
| Impending fracture (%) | 28.6 | 9.1 | 0.21 |
| Actual fracture (%) | 61.9 | 81.8 | 0.25 |
| Failed open reduction with internal fixation (%) | 28.6 | 0 | 0.05 |
| Resection solitary metastasis (%) | 19.0 | 9.1 | 0.46 |
| Segmental defect (%) | 90.5 | 100 | 0.29 |
| Lesion characteristics | |||
| Segmental defect size (cm; mean ± SD) | 6.5 ± 1.9 | 6.5 ± 2.5 | 0.93 |
| Defect site (%) (proximal-middle/middle/middle-distal/distal) | 42.9/38.1/14.3/4.8 | 36.4/54.5/9.1/0 | 0.84 |
| Radiodensity (lytic/mixed/blastic; %) | 81.0/4.8/0* | 90.9/0/0* | 0.60 |
| Implant sizes (mean ± SD) | |||
| Proximal stem length (cm) | 7.5 ± 1.2 | 8.2 ± 2.0 | 0.35 |
| Body size (cm) | 7.4 ± 2.7 | 6.3 ± 1.6 | 0.26 |
| Distal stem length (cm) | 6.4 ± 1.7 | 7.5 ± 1.1 | 0.09 |
| Operation details (mean ± SD) | |||
| Operative time (minutes) | 165 ± 55 | 165 ± 105 | 0.98 |
| Estimated blood loss (mL) | 665 ± 417 | 520 ± 399 | 0.37 |
| Postoperative treatment | |||
| Radiotherapy (%) | 28.6 | 63.6 | 0.19 |
| Radiotherapy dose (Gy; mean ± SD) | 28 ± 2.7 | 40.1 ± 15.7 | 0.12 |
| Hormonal/immunotherapy (%) | 28.6 | 11.1† | 0.26 |
| Chemotherapy (%) | 30.0‡ | 22.2‡ | 0.30 |
*Three cases had missing data on radiodensity from the male-female group, one missing from the lap joint group; †two cases had missing data on hormonal/immunotherapy postoperative treatment from the lap joint group; ‡one case had missing data on chemotherapy postoperative treatment from the male-female group and two from the lap joint group; SD = standard deviation.
The median age of the 32 patients at implantation was 64.3 years (range, 37–84 years). The underlying disease was most commonly either multiple myeloma (eight patients) or metastatic clear cell renal carcinoma (seven patients). Four patients had adenocarcinoma of the breast, four metastatic adenocarcinoma of unknown primary, and two non-small cell lung cancer. We observed the following diagnoses in one patient each: dedifferentiated chondrosarcoma, postradiation malignant fibrous histiocytoma, pancreatic carcinoma, malignant peripheral nerve sheath tumor, other high-grade soft tissue sarcoma, Leydig cell testicular tumor, and prostate cancer. Overall, 29 of the humeral lesions were the result of disseminated malignancy (metastatic carcinoma or myeloma) and three were the result of a primary bone or soft tissue tumor.
There were two patients with sarcoma in the male-female group and two in the lap joint group. However, only three of the four patients with sarcoma were primary at the arm. The fourth was a solitary (at the time) humeral metastasis of a fibrosarcoma from a previous femoral primary. Of the three primary sarcomas, two were in the lap joint group. One of these underwent placement of an intercalary humeral spacer for a pathologic fracture through irradiated bone after sarcoma resection. Sarcomas in the male-female group consisted of the solitary fibrosarcoma metastasis and another patient associated with primary soft tissue sarcoma resection.
Radiotherapy was administered preoperatively in six patients (range, 3000–4500 rads in 10–18 fractions). Two other patients with soft tissue sarcomas received preoperative chemotherapy. Preoperative chemotherapy was given to 11 patients (regimens including VBMCP/Cytoxan® [Mead Johnson Oncology Products, Princeton, NJ]/interferon, Taxotere®[Sanofi-Aventis, Bridgewater, NJ]/carboplatin, MAID/Taxotere®/gemcitabine, Cytoxan®/adriamycin, vincristine/adriamycin/Decadron® [Merck, Whitehouse Station, NJ], melphalan/prednisone, and Cytoxan®/5-fluorouracil/prednisone).
Because the indications were not mutually exclusive, some patients had more than one indication for use of the spacer. Surgical indications were impending pathologic fracture in seven cases, actual fracture in 22 cases, segmental defect in 30 cases, and failure of internal fixation in six cases. No prior cancer-related surgery had been performed for 12 patients, but another 12 had nonhumeral cancer-related surgery before the surgical procedure for placement of an intercalary humeral spacer. Seven patients had previously undergone ipsilateral humeral surgery. One patient had undergone resection of another primary lesion at a separate site.
We classified anatomic location of the epicenter of the defect and/or fracture as being within the proximal/middle junction in 13 patients, the central third in eight patients, within the distal/middle junction in 10 patients, and within the distal third in one. Maximal dimension of the defect ranged from 3.5 cm to 12.5 cm (mean, 7.1 cm). Distance from the proximal articular surface to the proximal end of the defect ranged from 8 cm to 17 cm (mean, 12.6 cm). Distance from the distal articular surface to the distal end of the defect ranged from 7.5 cm to 20 cm (mean, 13.2 cm). Bone lesions were nearly always lytic (27 cases) and rarely mixed lytic/blastic (one case). There were no purely blastic lesions in this series. At latest followup, 21 patients were dead of disease, three patients were alive with disease, and two patients were free of disease. We did not know the oncologic outcome in six patients.
Radiotherapy was administered postoperatively to 14 patients who had not had preoperative radiotherapy (2500–6800 rads in 5–34 fractions). The highest postoperative dose and longest fractionation scheme was given to a patient with a high-grade malignant fibrous histiocytoma. Postoperative chemotherapy was given to eight patients (regimens including vincristine/adriamycin/Decadron®, VBMCP, VBMCP and prednisone, VP16/Cytoxan®/adriamycin/cisplatin, and Velcade® [Millennium Pharmaceuticals, Inc., Cambridge, MA]/Cytoxan®). Preoperative hormonal therapy or immunotherapy was given to five patients (including tamoxifen, Megace® [Par Pharmaceutical Companies, Inc, Woodcliff Lake, NJ], and Arimidex® [AstraZeneca, Wilmington, DE]). Postoperative hormonal therapy or immunotherapy was used for seven patients (including diethylstilbestrol, interferon, Lupron [TAP Pharmaceutical Products Inc., Lake Forest, IL], suramin, and tamoxifen). One patient with myeloma received postoperative bone marrow transplantation.
The technique of surgical implantation was very similar between the two devices. Typically, we used a brachialis-splitting approach to expose the anterior aspect of the humerus. At this point, for patients with a solitary lesion to be resected, the segment of involved humerus was removed. For patients with failed internal fixation, we removed the previous device at this point. For those with failed internal fixation, pathologic fracture, and/or segmental destruction, the bone edges were freshened with a saw blade back to a circumferential or nearly circumferential rim of intact bone. We prepared the intramedullary spaces proximally and distally using rigid or flexible intramedullary reamers beginning at a small size and reaming to at least 1-mm diameter greater than the diameter of the stem to be implanted. Trial implants were used to determine the appropriate combination of stem lengths and diameters as well as body sizes. We then selected the final implants and individually cemented them into the intramedullary canals of the humerus after canal preparation. Cement was most often introduced using a syringe rather than a standard intramedullary cement gun as a result of the small size of the humeral canal. For the early-generation implants, we made sure the body portions were positioned to allow access for set screw placement after reduction. For those early implants, 2.04 cm of distraction was needed to reduce the male-female taper (Fig. 1). For the second-generation implants, we positioned the implants so their faces were aimed directly medial on one end and lateral on the adjoining end of the bone with the arm in neutral position not only to allow access to both set screws after reduction, but also to ensure correct rotational alignment (Fig. 2). A torque-limiting screwdriver was then used to seat the screws fully (Fig. 2).
Functional outcome was determined retrospectively using the rating system of the Musculoskeletal Tumor Society (MSTS). Postoperatively we (TD, TL, RH) radiographically determined distance of the implants from the bone ends, loosening, and implant survival. Loosening of each stem was evaluated based upon radiolucencies and change in position. Circumferential radiolucencies at either the bone-cement or the prosthetic-cement interface were considered conclusive evidence of loosening. Implant survival was based upon the implant remaining in place without removal for any reason, including amputation, tumor recurrence, and implant failure. We classified complications as intraoperative or postoperative. Disease progression alone was not considered a complication unless it led to periprosthetic fracture or implant loosening.
We compared demographic (gender, age at discovery, age at procedure) and other descriptive variables (primary cancer, preoperative or postoperative radiotherapy treatment, chemotherapy treatment, hormonal/immunotherapy treatment, other surgeries, prior humeral surgeries, surgical indication, segmental defect size, distal distance from defect to end of bone, proximal distance from defect to end of bone, lytic/blastic/mixed nature of defect, proximal and distal stem lengths, body size, preoperative Musculoskeletal Tumor Society functional ratings overall and for each component, duration clinical followup, duration radiological followup, recurrences or metastases) as well as outcome variables (operative time, estimated blood loss as determined from operative notes, intraoperative complications, postoperative complications, subsequent operations) between the male-female and lap joint groups using chi square and unpaired student’s t-tests with a p value of 0.05 considered significant. Analysis was accomplished using StatView Version 5.0.1 (SAS Institute, Inc, Cary, NC).
Results
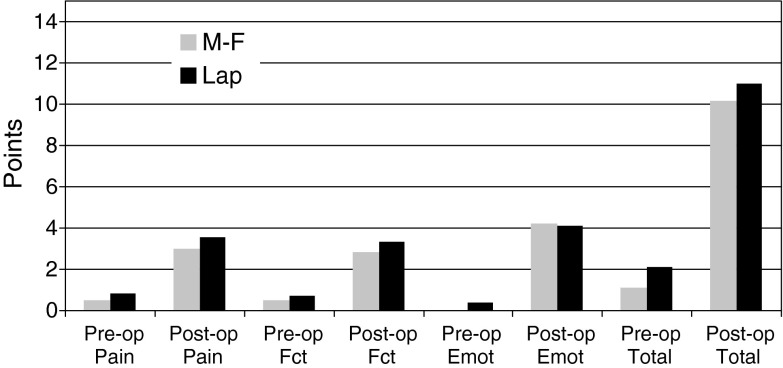
Although substantial pain relief was achieved using each type of prosthesis, pain relief was similar between the two groups (Table 2) (Fig. 3). On average, the MSTS pain score improved preoperatively (mean, 0.75; range, 0–5) to postoperatively (mean, 3.2; range, 0–5). Typically, patients were able to use the operative extremity to assist with activities of daily living on the first postoperative day. All but two patients, both in the second-generation group, achieved improvement in their MSTS pain scores from preoperatively to postoperatively. The first was the only patient whose overall MSTS score decreased from preoperatively (5) to postoperatively (1). This patient had no discomfort before resection of a soft tissue sarcoma involving the adjacent humerus but required occasional narcotic pain medications at latest followup. The second was another patient who was originally treated for a soft tissue sarcoma. In that patient, a postradiation fracture occurred 2 years after the resection necessitating open reduction and internal fixation with a plate and screws supplemented with a vascularized fibular graft. The intercalary humeral spacer in that patient was placed after early failure of the internal fixation procedure. The patient required constant narcotic pain medications, so the preoperative and postoperative scores were identical (0).
Table 2.
Comparative outcome variables between spacer groups
| Outcome variables | Male-female (n = 20; first generation) | Lap joint (n = 12; second generation) | p Value |
|---|---|---|---|
| Complications | |||
| Total (intraoperative, postoperative, disease; %) | 52.4 | 27.3 | 0.17 |
| Intraoperative* (%) | 9.5 | 0.0 | 0.29 |
| Postoperative* (%) (nondisease progression) | 38.1 | 27.3 | 0.54 |
| Postoperative neuropraxia (%) | 14.3 | 0.0 | 0.19 |
| Postoperative (disease progression; %) | 9.5 | 0.0 | 0.29 |
| Aseptic loosening (%) | 9.5 | 27.3 | 0.19 |
| Time to postoperative complications (months; mean ± SD) | 12.9 ± 11.5 | 12.0 | 0.93 |
| Need for subsequent operation (%) | 14.3 | 0.0 | 0.19 |
| Preoperative MSTS function (mean ± SD) | |||
| Pain score | 0.52 ± 0.81 | 0.82 ± 1.47 | 0.47 |
| Function score | 0.52 ± 1.0 | 0.73 ± 1.56 | 0.65 |
| Emotional acceptance score | 0.0 ± 0 | 0.36 ± 1.21 | 0.17 |
| Total score | 1.10 ± 1.61 | 2.10 ± 4.31 | 0.35 |
| Postoperative MSTS function (mean ± SD) | |||
| Pain score | 3.00 ± 1.59 | 3.6 ± 1.59 | 0.41 |
| Function score | 2.82 ± 1.19 | 3.3 ± 1.32 | 0.33 |
| Emotional acceptance score | 4.24 ± 1.35 | 4.11 ± 1.36 | 0.83 |
| Total score | 10.19 ± 3.39 | 11.00 ± 3.64 | 0.68 |
| Followup (mean ± SD) | |||
| Clinical (months) | 20.3 ± 25.9 | 19.2 ± 23.7 | 0.91 |
| Radiographic (months) | 6.9 ± 11.4 | 12.1 ± 23.3 | 0.40 |
*Not mutually exclusive (eg, one intraoperative complication also incurred postoperative complications); MSTS = Musculoskeletal Tumor Society; SD = standard deviation.
Fig. 3.
Preoperative and postoperative Musculoskeletal Tumor Society (MSTS) pain, function, emotional acceptance, and total scores are shown according to type of intercalary humeral spacer. Within each prosthesis group (eg, male-female [MF] group and lap joint [LJ] group), comparison showed improvement (p ≤ 0.05) from preoperative to postoperative scores. No differences were observed comparing the MF with LJ groups for any of the individual or total scores.
Functional results with the newer type of prosthesis were comparable to those of the original prosthesis. Neither total MSTS scores nor the functional or emotional components differed between the two implant groups (Fig. 3). Total MSTS scores improved from preoperatively to postoperatively in all but one patient (previously described for a decrease in both pain and function scores).
Implant survivorship for the newer humeral spacer was comparable to the original prosthesis. At a median 20-month followup, the implant survival rate was 94% (30 of 32). Implant survival was 100% among the 11 patients with second-generation spacers compared with 90.5% (19 of 21) in those with first-generation spacers.
There was no increase in blood loss or operative time required for implantation of the lap joint prosthesis when compared to the male-female-type prosthesis. Operative time ranged from 49 to 407 minutes (mean, 164 minutes), but often included other portions of the procedure such as resection of soft tissue sarcomas. Estimated blood loss ranged from 200 to 1800 mL (mean, 617 mL). We found no difference in either operative time or estimated blood loss between the two types of spacers (Table 1).
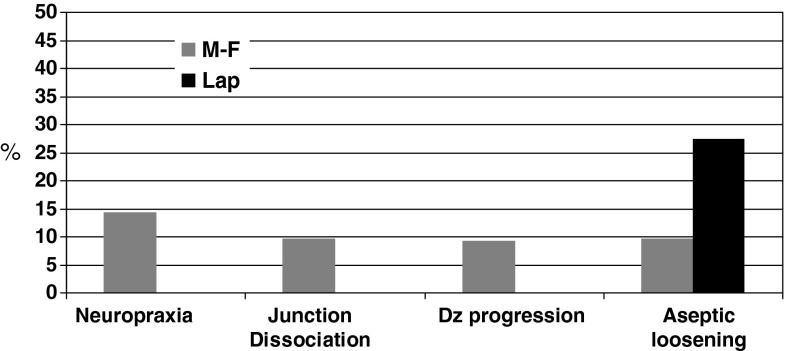
The newer lap joint prostheses reduced the complications of nerve palsy, disengagement, and periprosthetic fracture compared with the original version of the prosthesis. Postoperative nerve palsies were encountered in three of 21 patients who received first-generation spacers and none of the 11 patients who received second-generation implants (Fig. 4). One of the latter groups of patients had preoperative radial nerve palsy, which gradually improved during followup. Disengagement of the prosthesis affected two of the 21 patients who received first-generation spacers but none of the 11 patients who received second-generation implants (Fig. 4). A periprosthetic fracture was diagnosed as having occurred intraoperatively in one patient who received a first-generation spacer and in none of the patients who received a second-generation implant. Two other patients with male-female prostheses incurred pathologic fractures, both postoperatively.
Fig. 4.
Postoperative complications according to prosthesis type (male-female [MF] versus lap joint [LJ]) show that although there were fewer postoperative complications of most types in the LJ prosthesis group compared with the MF prosthesis group, the LJ prostheses showed a concerning rate of aseptic loosening considering the relatively short followup for these prostheses.
There were some unexpected findings. Symptomatic aseptic loosening independent of disease progression was observed in two of the 21 patients who received a first-generation implant and three of 11 patients who received a second-generation implant (Fig. 5A–C). Overall, we observed complications, including those related to disease progression, intraoperative complications, and postoperative complications, in 11 of the 21 patients in the former group and in three of the 11 patients in the latter group. Complications other than disease progression affected nine of 21 patients who underwent implantation of the first-generation spacer and three of 11 patients who underwent implantation of the second-generation spacer.
Fig. 5A–C.
A case example of aseptic loosening of a patient with a lap joint intercalary spacer prosthesis is shown. (A) A solitary focus of clear cell carcinoma from renal cell cancer is shown in the proximal portion of the humeral diaphysis. (B) Immediately after resection and reconstruction with an intercalary humeral spacer, note the absence of any radiolucent lines at the prosthesis-cement interface. (C) Six years later, the patient has developed aseptic loosening of the proximal stem at the prosthesis-cement interface (arrow). Note that heterotopic bone has formed around the body of the spacer. The patient did not have to undergo revision surgery.
Discussion
The goal of treatment for metastatic bone disease in the humerus, as for other sites, is pain relief, lasting rigid implant fixation, and improved quality of life [4–6, 9–11, 13–15]. In the humerus, this is typically achieved with a locked intramedullary rod or a plate-screw construct often supplemented by bone cement [2, 4–6, 9–11, 13–15]. When a large region of segmental destruction is created by a metastatic lesion, myeloma, failure of internal fixation in the setting of pathologic fracture, or after resection of a solitary metastatic lesion, rigid and stable fixation is difficult to obtain by standard means. It is for these rare instances that a metallic intercalary cemented humeral spacer device was developed initially at the Mayo Clinic [1]. Early reports on this device suggested efficacy in terms of pain relief and functional restoration, but complications related to the need for overdistraction to reduce the male-female junction, disengagement of the male-female junction, and periprosthetic fractures attributed to a relatively limited array of intramedullary stems, potentially limiting the surgeons’ ability to prophylactically protect the proximal and distal intramedullary canals [4]. In this study, we posed five questions: Are (1) pain relief, (2) functional results, (3) implant survivorship, (4) blood loss and (5) operative time with the second generation intercalary humeral prosthesis comparable to that of the first generation prosthesis?
This study had some of the limitations of a retrospective chart review: our pain data and functional followup were collected from chart review rather than from patient- or physician-completed forms. However, in these patients who are typically at the end of life, access is limited by their need to attend to multiple other issues with various physicians. Radiographic data were also incomplete, a result of the fact that patients had sometimes been referred with radiographs from outside institutions that were no longer available at the time of latest followup. Followup for the implant is also short but obviously limited by the patients’ short life expectancy. The power to detect differences between these two small groups is limited, but these are rare procedures, and the indications are infrequent, so there simply are not large groups of these patients to analyze.
We compared the results of the newer second-generation lap joint intercalary spacer devices with a wider array of intramedullary stems with those using the earlier first-generation implants. We found comparable pain relief and functional restoration between the two devices. We also observed a lower incidence of neural dysfunction, disengagement of the junction, and periprosthetic fracture that were attributed to the original implant design features. Further, there is a concerning (3/11) incidence of aseptic loosening evident with the newer prosthesis.
In the initial series of 17 patients who underwent implantation of the intercalary humeral spacer device, five incurred complications independent of disease progression [3]. Those patients are included among the currently reported 21 patients who underwent implantation of the first-generation implant. Three of those 17 patients incurred at least transient postoperative neuropraxia, which was attributed, at least in part, to the need for distraction to reduce the male-female body junction. Two of those 17 patients encountered early postoperative instability at the male-female junction, which was attributed to the difficulty in achieving solid impaction of the taper because the device had to be assembled and secured after implantation of the intramedullary stems. Hence, there was no exposed portion of the device that allowed seating with a mallet as is typically the case with other male-female taper junctions such as those on femoral and humeral prostheses. Despite these failings, the intercalary humeral spacer was believed a valuable option for the immediately stable reconstruction of a segmental deficit in patients with shortened lifespans resulting from metastatic disease or myeloma [4].
The second-generation lap joint intercalary spacer devices have also been studied in the biomechanics laboratory using a 5-cm middle third segmental defect model in allograft bone [8]. Single-cycle torque to failure showed the spacer devices to have a greater peak torque (mean, 41.4 N-m) and stiffness (mean, 2.1 N-m/degrees) than intramedullary nail specimens with cemented stems and cement in the defect (mean peak torque, 23.1 N-m; mean stiffness, 1.6 N-m/degrees). Both the intercalary spacers and the cement/intramedullary nail constructs were stronger and stiffer than an intercalary allograft nail composite specimen without supplemental cement (mean peak torque, 12.4 N-m; mean stiffness, 0.6 N-m/degrees) [8].
Although our study suggests the newer lap joint body junction design may reduce distraction-associated neuropraxia and failure of the junction to remain secure, the occurrence of symptomatic aseptic loosening in three of 11 patients who underwent implantation of the lap joint device is of concern. Longer followup and larger numbers of patients may show an even higher incidence of failure by this mechanism. Hence, this device should not be used in a more liberal fashion than that for which it was designed.
We believe there is a role for the continued use of this device with the appropriate limited indications. Our current indications are limited to segmental destruction in the setting of a disseminated malignancy (metastatic disease or myeloma) or solitary renal-thyroid metastatic carcinoma requiring resection involving the middle third of the humerus and extending to no more proximal or distal than to allow a remaining 5 cm of intramedullary canal. We believe the radiographic findings here should underscore the potential for problems with symptomatic aseptic loosening at longer followup should this implant be used in patients with primary tumors that carry a greater potential for cure and, hence, longer-term survival.
Acknowledgments
We thank Babak Khamsi for his assistance with local chart review in Syracuse, NY.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Chin HC, Frassica FJ, Hein TJ, Shives TC, Pritchard DJ, Sim FH, Chao EY. Metastatic diaphyseal fractures of the shaft of the humerus. The structural strength evaluation of a new method of treatment with a segmental defect prosthesis. Clin Orthop Relat Res. 1989;248:231–239. [PubMed] [Google Scholar]
- 2.Damron TA, Rock MG, Choudhury SN, Grabowski JJ, An KN. Biomechanical analysis of prophylactic fixation for middle third humeral impending pathologic fractures. Clin Orthop Relat Res. 1999;363:240–248. doi: 10.1097/00003086-199906000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res. 1996;324:233–243. doi: 10.1097/00003086-199603000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra S, Stapert J, Boxma H, Wiggers T. Treatment of pathological fractures of the humeral shaft due to bone metastases: a comparison of intramedullary locking nail and plate osteosynthesis with adjunctive bone cement. Eur J Surg Oncol. 1996;22:621–626. doi: 10.1016/S0748-7983(96)92450-6. [DOI] [PubMed] [Google Scholar]
- 5.Frassica FJ, Frassica DA. Evaluation and treatment of metastases to the humerus. Clin Orthop Relat Res. 2003;415(Suppl):S212–218. doi: 10.1097/01.blo.0000093052.96273.a7. [DOI] [PubMed] [Google Scholar]
- 6.Frassica FJ, Frassica DA. Metastatic bone disease of the humerus. J Am Acad Orthop Surg. 2003;11:282–288. doi: 10.5435/00124635-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Harrington KD, Sim FH, Enis JE, Johnston JO, Diok HM, Gristina AG. Methylmethacrylate as an adjunct in internal fixation of pathological fractures. Experience with three hundred and seventy-five cases. J Bone Joint Surg Am. 1976;58:1047–1055. [PubMed] [Google Scholar]
- 8.Henry JC, Damron TA, Weiner MM, Higgins ME, Werner FW, Sim FH. Biomechanical analysis of humeral diaphyseal segmental defect fixation. Clin Orthop Relat Res. 2002;396:231–239. doi: 10.1097/00003086-200203000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Lewallen RP, Pritchard DJ, Sim FH. Treatment of pathologic fractures or impending fractures of the humerus with Rush rods and methylmethacrylate. Experience with 55 cases in 54 patients, 1968–1977. Clin Orthop Relat Res. 1982;166:193–198. [PubMed] [Google Scholar]
- 10.Lin J, Hou SM, Hang YS, Chao EY. Treatment of humeral shaft fractures by retrograde locked nailing. Clin Orthop Relat Res. 1997;342:147–155. doi: 10.1097/00003086-199709000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Redmond BJ, Biermann JS, Blasier RB. Interlocking intramedullary nailing of pathological fractures of the shaft of the humerus. J Bone Joint Surg Am. 1996;78:891–896. doi: 10.2106/00004623-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Schurmann M, Gradl G, Andress HJ, Kauschke T, Hertlein H, Lob G. Metastatic lesions of the humerus treated with the isoelastic diaphysis prosthesis. Clin Orthop Relat Res. 2000;380:204–214. doi: 10.1097/00003086-200011000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Sim FH, Pritchard DJ. Metastatic disease in the upper extremity. Clin Orthop Relat Res. 1982;169:83–94. [PubMed] [Google Scholar]
- 14.Vail TP, Harrelson JM. Treatment of pathologic fracture of the humerus. Clin Orthop Relat Res. 1991;268:197–202. [PubMed] [Google Scholar]
- 15.Vandeweyer E, Gebhart M. Treatment of humeral pathological fractures by internal fixation and methylmethacrylate injection. Eur J Surg Oncol. 1997;23:238–242. doi: 10.1016/S0748-7983(97)92460-4. [DOI] [PubMed] [Google Scholar]