Abstract
The neoadjuvant treatment of osteosarcoma using intravenous agents has resulted in survival rates of 55% to 77% [3, 5, 6, 20, 22, 35]. We designed a neoadjuvant chemotherapy protocol using combined intraarterial and intravenous agents to treat high-grade osteosarcoma and malignant fibrous histiocytoma of bone in an attempt to improve survival. We report the results of treating 53 adults (age 18–77 years) diagnosed with nonmetastatic extremity osteosarcoma or malignant fibrous histiocytoma. Preoperative chemotherapy consisted of intravenous doxorubicin followed by intraarterial cisplatinum administered repetitively every 3 weeks for three to five cycles, depending on tumor response assessed by serial arteriography. Dose and duration of cisplatin were adjusted for tumor size. After resection, good responders (90% or greater necrosis) underwent treatment with the same agents and poor responders were treated with alternative agents for longer duration. Minimum followup was 24 months (mean, 111 months; range, 24–235 months). Estimated Kaplan-Meier survival at 10 years was 82% and event-free survival was 79%. Forty-one patients (77%) had a good histologic response and 92% (49 of 53) underwent limb-sparing procedures. Local recurrence occurred in two patients (4%). These results compared favorably with those reported in the current literature.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteosarcoma or osteogenic sarcoma (OGS) most frequently occurs between the ages of 15 and 25 years. Although it is the most common bone sarcoma in pediatric patients and in young adults, it can occur at any age. Malignant fibrous histiocytoma (MFH) of bone is more common in adults than children and responds to the same treatment as OGS [39]. The treatment regimens and survival rates for these bone sarcomas have improved over the last decade; however, some reports suggest a worse prognosis in patients younger than 10 years and older than 30 [8, 12, 36, 39].
In 1986, we formed a multidisciplinary team to treat musculoskeletal pathology of the extremities, including malignant soft tissue and bone neoplasms, such as OGS and MFH. We reviewed the standard chemotherapeutic protocols for OGS at the time and made the following observations: (1) most treatment plans used multiagent drugs with cumulative side effects that effectively limited the dose and duration of any one drug in the regimen; (2) no adjustments were made in either dose or duration of therapy for tumor size; (3) there was no accurate way to measure tumor response before surgery, leading to a “one size fits all” approach; and (4) there was no concentration of chemotherapy at the site of the primary tumor and normal healthy tissues received chemotherapeutic doses equal to that of the diseased tissues. Based on these observations, and in an attempt to improve the prognosis of patients with OGS and MFH, an individualized neoadjuvant approach was developed by the Extremities at Risk team using two active agents: dose-intensified intraarterial (IA) cisplatin (CDDP) in combination with intravenous (IV) doxorubicin (DOX) [7, 12, 28, 29]. We repeatedly administered these two agents only. In addition, this response-based regimen used arteriography to serially assess tumor neovascularity and treatment response [11, 13].
Our primary objective was to evaluate the efficacy of this intraarterial protocol in adult patients. We hypothesized: (1) the use of a dose-intensive, repetitive chemotherapy regimen of IV DOX and IA CDDP would improve adult survival rates; and (2) a good histologic response could be achieved by individualizing the duration of neoadjuvant therapy through the use of serial arteriography as a feedback mechanism. We addressed three secondary research questions: (1) Does the dose adjustment of CDDP for tumor size negate the poor prognostic factor of large tumors?; (2) Does this therapy influence the ability to perform limb preservation surgery?; (3) Does this dose-intensive regimen increase the rate of chemotoxicities?
Materials and Methods
The protocol was designed as a single-arm prospective study in 1987. Patients were eligible for this study if they had: (1) a histologically proven, previously untreated, primary high-grade extremity lesion; (2) no evidence of metastatic disease; (3) no history of previous cancers; (4) normal cardiac function; and (5) age of 18 years or greater. To be considered evaluable, patients had visible tumor neovascularity (TNV) on baseline arteriogram, had completed a minimum of three neoadjuvant chemotherapy intraarterial cycles, and had undergone definitive tumor resection. Between July 1987 and April 2005, 64 patients at least 18 years of age were diagnosed with high-grade nonmetastatic OGS or MFH of an extremity at our institution. One patient was not eligible for the study due to abnormal cardiac function, and six declined to participate. Fifty-seven patients were enrolled in the study. Four patients were unevaluable: one declined definitive surgery despite having good arteriographic response and three failed to complete the minimum required neoadjuvant chemotherapy. Fifty-three patients (35 men and 18 women) with an average age of 32 (range, 18–77 years) at diagnosis fulfilled the criteria for analysis. Sites of involvement included the femur (25), tibia/fibula (23), humerus (four), and ulna (one). Eight of 53 patients were diagnosed with MFH of bone. Six patients presented with pathologic fractures. The majority of tumors (36) were 10 cm or smaller; 17 patients had tumors greater than 10 cm and underwent 24-hour infusions of CDDP. All patients had clinical and arteriographic evidence of response from administration of neoadjuvant chemotherapy as demonstrated by initial symptomatic relief of pain at the primary disease site and decreased TNV on serial arteriograms. Patients required an average of 3.6 neoadjuvant courses (range, 3–5) before achieving 90% or greater reduction in TNV. We had a minimum followup of 24 months (mean, 111 months; range, 24–235 months). The protocol was approved by our Institutional Review Board.
Diagnosis was made by needle or open biopsy. Patients were then staged to confirm Stage II-B [17] disease with MRI of the involved extremity, total body bone scan, and chest CT. All patients were required to have baseline CBC, normal cardiac function by echocardiogram or MUGA, and normal renal and liver tests.
Preoperative chemotherapy consisted of a 72-hour continuous IV infusion of 90 mg/m2 DOX followed the next day by IA CDDP. In 1992 the protocol was amended due to a high incidence of grades 3 and 4 mucositis in young patients to state that the duration of DOX infusion would be decreased to 48 hours in young patients only. The dose of DOX was not adjusted. Dose and duration of CDDP were 120 mg/m2 for 6 hours for tumors 10 cm or smaller in maximum dimension at diagnosis and 160 mg/m2 for 24 hours for tumor dimensions greater than 10 cm. Chemotherapy cycles consisting of DOX and CDDP were repeated every 3 to 4 weeks as determined by hematologic recovery (absolute neutrophil count > 750, platelets ≥ 100,000). All arteriograms and IA chemotherapy were administered at a single institution.
The regimen and arteriographic procedure have been described in detail in previous publications [11, 13, 50, 51] and will be briefly reviewed here. Intraarterial catheterization for administration of CDDP was performed under IV conscious sedation by one of the trained interventional radiologists at our institution. The tip of the catheter was positioned in the affected extremity to infuse the vessels feeding the neoplasm. We took care to place the catheter tip proximal to the tumor and as far away from skin perforators as possible to avoid regional skin necrosis. The CDDP was infused in a heparinized solution using a volumetric and pulsatile jet infusion pump (Gianturco-Wallace Chemo-Pulser Pump; Cook, Inc, Bloomington, IN). The patient was restricted to bed rest with hourly monitoring of the distal limb color, temperature, and pulses during the CDDP administration. These CDDP infusions were accompanied by vigorous IV hydration, hypertonic saline diuresis, and close monitoring of intake, output, and electrolytes.
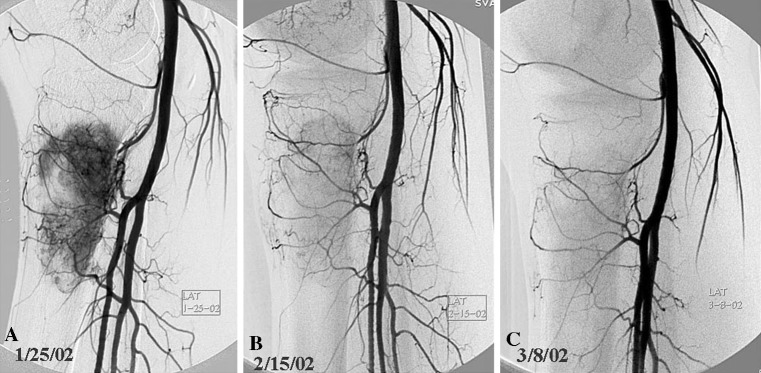
We obtained arteriograms before the administration of each dose of IA CDDP. The initial arteriogram provided volume and intensity of TNV, which was graded by the interventional radiologist as mild (Grade 1), moderate (Grade 2), or marked (Grade 3). Subsequent arteriograms were compared with the baseline to assess percent change in TNV as an indicator of tumor response as described in a previous publication [13]. The number of preoperative chemotherapy cycles administered was based on arteriographic response. Neoadjuvant therapy was discontinued when one of three criteria was achieved: (1) 90% or greater disappearance of tumor neovascularity (“good response” radiographically) (Fig. 1A–C); (2) initial response was followed by a plateau of effect (partial response); and (3) no response or progression of disease (Fig. 2A–C). After a minimum of three IA cycles, the serial arteriograms were comparatively assessed by the three specialists involved: the medical oncologist (IH), orthopaedic oncologist (RRH, RMW or CMK), and interventional radiologist. If there was a lack of consensus as to the percent reduction in neovascularity after the third arteriogram, the patient would undergo a fourth cycle. Arteriographic response was quantified as to percent reduction in TNV to predict histologic response. We gave consideration to details such as: (1) visible decrease in tumor blush; (2) diminished size and number of feeder vessels; and/or (3) overall diminished arteriographic “footprint” as seen on serial images. If there were differences of opinion regarding tumor response, the team erred on the side of administering an additional cycle of IA/IV therapy. Once one of the criteria was met for cessation of chemotherapy and after hematologic recovery, a window for definitive surgery was planned.
Fig. 1A–C.
Serial arteriograms of a patient with osteosarcoma of the proximal tibia are shown. (A) Baseline arteriogram assessed at 3+ (marked) neovascularity, which shows a considerable decrease on (B) the second lateral arteriogram, and (C) at least 90% reduction of neovascularity on the third arteriogram. This was concordant with a good response.
Fig. 2A–C.
A poor response to chemotherapy is depicted on these serial arteriograms. (A) A lateral view (2+) arteriogram of a distal femur with osteosarcoma is shown. (B) A second arteriogram demonstrates a mild decrease in neovascularity and considerable decrease in mass effect on the popliteal artery. (C) A third arteriogram demonstrates an increase in blush greater than seen at baseline, judged to represent progression of disease. The decision was made to discontinue neoadjuvant therapy and proceed to definitive surgery. Histopathologic necrosis was confirmed at 65%. (This figure was published in Cullen JW, Jamroz BA, Stevens SL, Madsen W, Hinshaw I, Wilkins RM, Cullen P, Camozzi AB, Fink K, Peck SD, Kelly CM. The value of serial arteriography in osteosarcoma: delivery of chemotherapy, determination of therapy duration, and prediction of necrosis. J Vasc Interv Radiol, 16:1107–1119, ©Elsevier 2005, with permission.)
We restaged patients before surgery with chest CT. MRI of the entire involved bone was used to plan the extent of the definitive resection. If the neurovascular structures appeared free of tumor and the patient was expected to retain adequate limb function, a limb-preservation procedure was recommended. In addition, we used feedback from the arteriographic response to aid in surgical planning. Amputation was reserved for patients who were believed at high risk for local recurrence due to involvement of the neurovascular bundle or lack of arteriographic response.
We (WM) determined the percentage of tumor necrosis after definitive surgery according to the method reported by Huvos et al. [26]. Patients with less than 10% viable tumor cells (90% or greater tumor necrosis) were deemed good responders. All others were considered poor responders (less than 90% necrosis).
Postoperative chemotherapy was initiated 2 to 3 weeks after surgery. The agents used and the duration were dependent on the patient’s histologic response. Good responders continued with the same agents (CDDP and DOX), both administered by IV every 4 weeks. The duration of postoperative chemotherapy depended on the number of preoperative chemotherapy cycles administered to equal a total of six cycles of therapy. For example, if a patient underwent four preoperative chemotherapy cycles before reaching maximum arteriographic response and was a good histologic responder, only two cycles would be required after surgery. When the dose of DOX had been maximized (540 mg/m2), etoposide was substituted for DOX. Ifosfamide was substituted for CDDP-induced grade 3 or 4 ototoxicity. Alternative chemotherapy for poor responders incorporated high-dose methotrexate, etoposide, and ifosfamide administered over 32 weeks for a total of six postoperative chemotherapy cycles.
At completion of therapy, plain radiographs of the primary disease site, chest CT scan, and a total body bone scan were obtained. Chest radiographs every 3 months and chest CT scans at 6-month intervals were performed unless indicated sooner by the chest radiograph.
Postoperative patient satisfaction and limb function were measured using the Musculoskeletal Tumor Society (MSTS) functional evaluation system for the upper or lower extremity [16]. In order to avoid physician bias, this clinician-based evaluation was modified to a patient-based questionnaire using lay language and was completed at clinic visits by the patient in the waiting room prior to seeing the surgeon. A corresponding clinical evaluation was completed by the surgeon to record complications, range of motion, and evidence or absence of recurrent disease. These data were then entered into the Extremities at Risk electronic patient registry maintained by the clinical research coordinator and scored using a maximum 30-point or 100% scale.
Efficacy of the treatment regimen was judged by rates of good histologic response. The Kaplan-Meier method [30] was used to calculate survival and event-free survival at 5 and 10 years. We defined an adverse event as tumor recurrence or death as a result of the disease or its treatment. The log-rank test was used to compare patients with large (greater than 10 cm) versus small (10 cm or less) primary tumors for survival and event-free survival.
Results
At 10 years, the Kaplan-Meier estimate of overall survival was 82% (range, 68% to 90.2%; 95% confidence interval) and the event-free survival was 78.5% (range, 64.5% to 87.6%; 95% confidence interval). At a minimum followup of 24 months 45 patients (85%) were alive and disease-free. We observed no tumor recurrences or deaths from disease 5 years from the date of diagnosis. Age predicted (p = 0.048) overall survival. The 10-year survival for patients 18 to 30 years old was 92.5% compared to 71% for patients aged 31–77 years (Table 1).
Table 1.
Survival and confidence intervals (CI)
| Group | Survival | Disease-free survival | |||||
|---|---|---|---|---|---|---|---|
| N | 10-Year | 95% CI | p Value | 10-Year | 95% CI | p Value | |
| All patients | 53 | 81.9% | 68.0, 90.2 | — | 78.5% | 64.5, 87.5 | — |
| Tumor size | 0.37 | 0.28 | |||||
| ≤ 10 | 36 | 85.7% | 68.9, 93.8 | 83.1% | 66.2, 92.1 | ||
| > 10 | 17 | 74.7% | 45.5, 89.7 | 69.1% | 40.7, 85.9 | ||
| Age | 0.048 | 0.16 | |||||
| 18–30 years | 29 | 92.5% | 85.2% | ||||
| 31–77 years | 24 | 70.6% | 70.6% | ||||
| Histologic response | 0.08 | 0.035 | |||||
| Poor (≤ 90) | 12 | 64.8% | 31.0, 85.2 | 56.3% | 24.4, 79.1 | ||
| Good (> 90) | 41 | 87.3% | 72.2, 94.5 | 85.1% | 69.9, 93.0 | ||
Serial arteriography assisted in determining the duration of neoadjuvant therapy and was highly predictive of tumor necrosis (histologic response). We were accurate in predicting 92.5% of the cases, yielding a sensitivity of 100%. The use of serial arteriography predicted a poor histologic response correctly in eight of 12 patients. The four cases in which arteriographic prediction did not correlate with the final pathology were poor responders who had been assessed on arteriogram to have 90% or greater tumor necrosis. A good histologic response was achieved by 77% of patients, which correlated with event-free survival. Greater than 90% tumor necrosis was achieved in 41 of 53 patients. Ten patients relapsed, eight of whom died of disease and two who have been disease-free over 8 years after surgery and further therapy. Twelve percent (five of 41) of good responders relapsed, one locally and four to the lung. Five of 12 poor responders relapsed, one with local recurrence and four with distant metastases. Patients with a poor histologic response had a 56% event-free survival versus 85% at 10 years for good responders.
Patients with tumors greater than 10 cm (n = 17) had similar overall survival of 75% and event-free survival of 69% compared to patients with small tumors (n = 36) with survival of 86% and event-free survival of 83%. As might be expected, patients with smaller tumors had improved projected survival. However, our numbers were small and we found no differences in response with varying tumor size (Table 1).
We performed limb-preservation surgery in 92% (49 of 53) of patients. Four primary amputations were required because of tumor proximity to the neurovascular bundle or poor response to neoadjuvant chemotherapy. Because most lesions occurred around the knee, the most common limb-preservation surgery by far consisted of wide local resection and endoprosthetic replacement. Other limb-preservation reconstructions included structural allograft (intercalary or osteochondral) or allograft-prosthetic composite, depending on the location of the primary tumor and the extent of resection required. Secondary amputations were necessary as a result of persistent infection (two) or local tumor progression (two) that occurred after cessation of postoperative chemotherapy. With over 9 years of average length of followup, MSTS scores in the limb salvage group averaged 65% (range, 17% to 100%).
The majority of toxicities were manageable and tolerable with no toxic deaths. Two patients developed clinical evidence of cardiomyopathy secondary to DOX. Both were alive and stable on cardiac medications. Painful mucositis after DOX infusions was infrequently reported. Myelosuppression was common and cumulative. Occurrences of febrile neutropenia and bacteremia were also infrequent and manageable. One patient with OGS of the distal femur developed osteonecrosis of her contralateral femoral head. This may have been therapy-related because patients are prescribed a small dose of decadron if they experienced severe nausea during therapy. At any rate, this was an isolated case and there was no way to establish the etiology. Three patients developed a solitary episode of myocutaneous inflammation in the area of the tumor bed after 24-hour IA CDDP infusion. The area of inflammation was excised at the time of definitive tumor resection and the ability to perform a limb-preservation procedure was never compromised. There were no other arterial catheter-related untoward effects.
Discussion
There was no effective chemotherapy for OGS and survival rates were consistently reported below 20% despite amputation prior to 1970 [14]. Rates of survival and limb preservation were improved with advances in adjuvant chemotherapy [25, 38, 49]. In 1974, Rosen and colleagues [46] initiated the concept of “neoadjuvant” chemotherapy to allow time for construction of custom-made endoprostheses for limb salvage. This approach also made it possible to evaluate the histologic effect of chemotherapy and greater than 90% tumor necrosis correlated with long-term survival. In a sequel study by Rosen et al. [45], postoperative chemotherapy was intensified in patients having a poor histologic response that resulted in survival comparable to that of good responders. Ettinger et al. [18] reported excellent results using postoperative CDDP and DOX in patients with nonmetastatic OGS. After the initiation of neoadjuvant chemotherapy, the most notable advances in survival rates involved intraarterial chemotherapy [28, 29, 41, 48]. We hypothesized by using dose-intensive, repetitive cycles of intraarterial cisplatin and intravenous doxorubicin to treat primary high-grade extremity OGS and MFH of bone, we would improve survival rates in adults compared to those in previously published studies. We also hypothesized serial arteriography would accurately predict tumor necrosis by assessing change in tumor neovascularity, an ability that would enhance our rate of good histologic response and, in turn, improve long-term survival.
There are limitations to this method of treatment and this study. First, the regimen adds a level of complexity with the required teamwork and arteriography. A labor-intensive approach, it requires assemblage of an experienced team and good communication. Physicians involved with the Extremities at Risk program hold a weekly hour-long conference in which complex cases are presented. Patients with newly diagnosed OGS and MFH of bone are presented, arteriograms are reviewed, and surgical options discussed. Second, IA therapy requires a more invasive approach for the administration of chemotherapy with the additional risk of complications. In our hands, however, the rate of complications related to the intraarterial procedure and arteriogram were quite low. Third, the extra cost involved with intraarterial administration, including the expertise of an interventional radiologist, must be factored in. However, we believe the benefit in survival outweighs the economic disadvantage. Fourth, a degree of subjectivity is involved in the radiographic assessment of tumor response. For accurate prediction of tumor response we depend on multispecialty consensus reached by an experienced team of oncologic, orthopaedic, and radiologic specialists. By erring on the side of administering an additional neoadjuvant cycle in the few cases of disagreement, we have been able to sustain our rates of good response and survival. Ideally, a more objective form of measuring tumor response would be desirable, and our interventional radiology department is currently exploring the application of new technologies such as digital subtraction analysis. Lastly, this series is limited by relatively small numbers of patients treated at a single institution and was a single-arm study without concurrent controls.
Intraarterial use of CDDP was first reported by Mavligit et al. [37] in 1981 in a small series of patients, the majority of whom had advanced and/or metastatic disease. Pharmacologic studies demonstrated equivalent drug levels in peripheral blood with IV or IA administration; however, levels were two to five times higher in the draining vein of an arterially infused area [48]. Jaffe et al. [28] utilized IA CDDP (150 mg/m2) as a single agent every 2 to 3 weeks and reported 16 of 42 patients had at least 90% tumor necrosis. In another study led by Jaffe et al. [29], IA CDDP was compared with IV high-dose methotrexate (HDM) as primary treatment for OGS. The response rate of patients treated with IA CDDP was higher (p = 0.065) to those treated with HDM (60% versus 27%). In addition, two patients who failed to respond to HDM achieved a complete response with IA CDDP.
We hypothesized by using dose-intensive, repetitive cycles of intraarterial cisplatin and intravenous doxorubicin to treat primary high-grade extremity OGS and MFH of bone, we would improve survival rates in adults compared to those in previously published studies. Our data suggest this intraarterial dose-intensive chemotherapy regimen was effective for treating adults with nonmetastatic extremity OGS and MFH of bone and compared favorably to historical controls (Table 2). The neoadjuvant response-based approach implemented by the Extremities at Risk team used two of the best agents known to successfully treat OGS and MFH of bone, DOX and CDDP [7, 12, 22, 28]. These two agents were used in a repetitive, dose-intensified fashion. Our survival rates in treating adult patients were 82% and 79% (event-free survival). When broken down into groups by age, 18–30 years (n = 29) versus 31–77 years (n = 24), 10-year survival rates were 92.5% and 71%, respectively (Table 1). This suggests that although age may be of prognostic importance, long-term survival using this regimen is sustained in older adults, a population that may be more difficult to treat due to inability to tolerate chemotherapy and comorbidities. These results compare favorably to those reported in the literature (Table 2) [3, 5, 6, 20, 22, 35].
Table 2.
Treatment results of nonmetastatic extremity OGS: historical controls
| Reference | Type of study | Age | # Pts | Overall survival | EFS | Site | >90% Response | Rate of local recurrence |
|---|---|---|---|---|---|---|---|---|
| Lewis et al. [35] | Randomized 2 arm | <40 | 497 | 55% (C) | NA | Extremity | 36% (C) | 18% (C) |
| 58% (D) | 50% (D) | 9% (D) | ||||||
| 5 yr | ||||||||
| Ferrari et al. [22] | Single arm | <40 | 182 | 77% | 64% | Extremity | 42% | 4% |
| 8 yr | 8 yr | |||||||
| Bacci et al. [6] | Retrospective | All | 789 | 68% | 60% | All | 63% | 5% |
| 5 yr | 5 yr | |||||||
| Ferrari et al. [20] | Randomized 2 arm | <40 | 303 | 72% (1) | 63% (1) | Extremity | NA (1) | Not included |
| 61% (2) | 54% (2) | 64% (2) | ||||||
| 8 yr | 8 yr | |||||||
| Bacci et al. [5] | Retrospective | All | 1126 | 66% | 55% | Extremity | NA | 5% |
| 5 yr | 5 yr | |||||||
| Bacci et al. [3] | Single arm | All | 43 | 68% | 53% | Extremity | 56% | 5% |
| 7 yr | 7 yr | |||||||
| Present Study | Retrospective | 18–77 | 53 | 82% | 79% | Extremity | 77% | 4% |
| 10 yr | 10 yr |
Other intraarterial regimens have been tried. In 1996, the Rizzoli Institute in Bologna, Italy, treated 40 patients with preoperative IA CDDP chemotherapy [2]. Patients were randomized in their study to receive CDDP either IA or IV as part of a three- (CDDP, methotrexate, DOX) or four-drug regimen (CDDP, methotrexate, DOX, ifosfamide). Although the rate of favorable histologic response was better in the IA group (78% versus 46%), their study showed no advantage in survival. It was concluded the increased procedural risk of delivering IA CDDP was not warranted and the IA protocol was discontinued. They used, however, a relatively low dose of the IA CDDP that was not adjusted for tumor size. They did not monitor tumor response preoperatively. In addition, they used multiple agents for a predetermined number of cycles with no adjustment for tumor size [2].
Our second hypothesis was that serial arteriography could be used to accurately predict tumor necrosis by assessing change in tumor neovascularity, an ability that would enhance our rate of good histologic response and, in turn, improve long-term survival. The theory that good histologic response correlates with improved prognosis has been supported in the literature [9, 42]. The use of serial arteriography in our study had a 92.5% rate of accuracy and 100% sensitivity in predicting good tumor response (greater than 90%). Other modalities have been evaluated and compared for their ability to preoperatively predict tumor necrosis, including radiography, CT, scintigraphy, dynamic MR imaging, MR arteriography, and positron emission tomography. None of these imaging modalities predicted duration of neoadjuvant chemotherapy [23, 31–34, 40, 47]. Only serial arteriography as used in this study accurately predicted tumor necrosis [13]. Tumor necrosis is reportedly one of the most important prognostic factors impacting long-term survival in nonmetastatic osteosarcoma [9, 15, 42]. By using serial arteriography to monitor tumor response and individualize the duration of neoadjuvant therapy, we achieved a 77% rate of good histologic response. We deduce that our high rate of good response likely translated into an exceptional survival rate as opposed to more traditional protocols which prescribe a set number of preoperative cycles and result in survival rates in the 70% range [1, 3, 4, 7, 19, 21, 24, 42–44, 52].
A secondary research question addressed in our manuscript is whether our agent dose and duration adjustments for tumor size would be effective in negating the poor prognostic factor of large tumor size (> 10 cm). Patients with large tumors were administered 160 mg/m2 versus 120 mg/m2 for smaller tumors, an adjustment of 33%. Duration of IA CDDP for large tumors was increased from 6 to 24 hours. Our treatment plan resulted in 75% survival for patients with tumors greater than 10 cm as compared to 86% for those with tumors ≤ 10 cm (p = 0.37). Although the difference was minimal, the overall trend persisted towards worse survival in those with larger tumors despite the dose/duration adjustments.
Another secondary research question was whether this regimen affected our ability to perform limb-sparing surgery. Arteriography afforded a useful serial assessment of reduction in tumor neovascularity and greatly assisted in surgical planning for either limb preservation or amputation. Our rate of limb preservation (92%) is comparable if not better than others reported in the literature [1, 3]. Two patients had local recurrence at a rate of 3.7%. This compares favorably to historical controls [3, 5, 6, 10, 20, 22, 35] (Table 2).
Our final secondary research question addressed the toxicity of our treatment strategy. We had no deaths related to chemotoxicity. Serial arteriography allowed us to individualize the duration of therapy, allowing us to limit the administration of chemotherapy to what was necessary to gain response. Two patients developed cardiomyopathy related to DOX infusion. Myelosuppression was common as it is in most modern treatment strategies. Mucositis, febrile neutropenia, and bacteremia were also infrequent and manageable. There were three patients who developed a myocutaneous inflammation in the area of the tumor bed after 24-hour IA CDDP infusion. This was the only side effect noted that would be considered specific to this treatment strategy. These events did not adversely affect patient outcomes. There were no other arterial catheter-related untoward effects.
The success with this regimen is attributable to a number of factors. We selected and have remained committed to using the most efficacious agents in treating OGS. These agents were alternated and repeated every 3 weeks to achieve maximum response. Thus the antineoplastic effect of the agents is dose-intensified and their impact is not diluted by the use of other less superior couplets or agents. The concentration of cisplatin at the tumor site has been reported to be four to six times higher when delivered intraarterially rather than by traditional IV administration, and avoids bathing normal healthy tissue with the same chemotherapeutic doses as the diseased tissues [27, 28, 48]. Even though IA CDDP has previously been employed, it has not been administered in a repetitive fashion, and no protocol has used serial arteriography to monitor tumor necrosis in newly diagnosed OGS.
Response-based therapies have been routinely used to treat childhood cancers such as acute lymphocytic leukemia (ALL) and Hodgkin’s disease. The standard treatment of ALL dictates that patients who are not in remission by day 29 are treated with 2 additional weeks of inductive chemotherapy. This maximizes the number of patients who achieve complete remission. In Hodgkin’s disease, following an initial response to therapy, treatment may be discontinued once a complete response is documented. For partial or inadequate responders, the regimen was continued with the same agents or adjusted to include radiation therapy or different agents. These types of regimens result in avoiding long-term toxicity in patients who are early rapid responders while increasing the cure rate for patients who are slow early responders.
Our patients underwent between three and five neoadjuvant cycles and the assessment of serial arteriograms enabled us to identify early rapid responders in much the same way. Not only was the arteriogram valuable as an endpoint to proceed with surgery, but poor arteriographic responders (< 90% decrease in TNV) underwent early definitive surgery and were switched to receive an alternative therapy if indeed tumor necrosis was less than 90%. Additionally, arteriography was successful in individualizing the duration of therapy based on response. If, like most standard protocols, our regimen had empirically mandated three neoadjuvant cycles, 51% (27 of 53) of patients may have been undertreated and had a poor response. Conversely, empirical administration of four neoadjuvant cycles would have resulted in overtreatment of 49% (26 of 53) of patients and undertreatment of 5% (three of 53). Finally, administration of five neoadjuvant cycles regardless of response may have overtreated at least 94% (50 of 53) of patients. It appears that duration of neoadjuvant therapy can be based on response. This improves the chances of achieving a good histologic response and avoids unnecessary toxicity for rapid responders.
It has been difficult to compare survival results across the board in different treatment plans for osteosarcoma. The presence of metastasis at presentation is a poor prognostic factor. Other factors such as axial sites of the primary tumor, size of the series, and various age group limitations often confound easy comparisons of survival. Inclusion of such a variety of patients hinders the ability to statistically analyze the success of treatment and should be analyzed separately.
In general, our treatment strategy has continued to demonstrate considerably improved survival when compared with other published results for treating primary nonmetastatic extremity OGS. Our rates of tumor response and survival demonstrate the importance of repetitive administration of the best available agents, serial arteriography to evaluate rapidity of response, and dose adjustment for large tumors. We believe our treatment strategy deserves further investigation in a multiinstitutional randomized study comparing it to the best intravenous regimen available.
Acknowledgments
We thank Dr. Lorrie Odom, Rocky Mountain Pediatric Hematology Oncology; Dr. Brandt Jamroz, interventional radiologist; Dr. Sanford Peck, pathologist; and the departments of audiology, rehabilitation, and cardiology at Presbyterian/St Luke’s Medical Center. We are especially grateful to Dr. John Cullen for his help in designing the protocol and his continued commitment toward research in pediatric oncology.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bacci G, Ferrari S, Bertoni F, Ruggieri P, Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M, Campanacci M. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18:4016–4027. doi: 10.1200/JCO.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Forni C, Mercuri M, Picci P, Bertoni F, Capanna R, Manfrini M, Donati D, Del Brach Prever A, Baldini N. The effect of intra-arterial versus intravenous cisplatinum in the neoadjuvant treatment of osteosarcoma of the limbs: the experience at the Rizzoli Institute. Chir Organi Mov. 1996;81:369–382. [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Longhi A, Forni C, Bertoni F, Fabbri N, Zavatta M, Versari M. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13:93–99. doi: 10.1179/joc.2001.13.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Ferrari S, Mercuri M, Longhi A, Capanna R, Tienghi A, del Brach Prever A, Comandone A, Cesari M, Bernini G, Picci P. Neoadjuvant chemotherapy for extremity osteosarcoma: preliminary results of the Rizzoli’s 4th study. Acta Oncol. 1998;37:41–48. doi: 10.1080/028418698423168. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities. A 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Fifteen year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin RS, Chawla SP, Carrasco CH, Raymond AK, Murray JA, Armen T, Patel S, Wallace S, Ayala A, Papadopoulos NE, et al. Preoperative chemotherapy for osteosarcoma with intravenous adriamycin and intra-arterial cisplatinum. Ann Oncol. 1992;3(Suppl 2):S3–S6. doi: 10.1093/annonc/3.suppl_2.s3. [DOI] [PubMed] [Google Scholar]
- 8.Bentzen SM, Poulsen HS, Kaae S, Jensen OM, Johansen H, Mouridsen HT, Daugaard S, Arnoldi C. Prognostic factors in osteosarcomas: a progression analysis. Cancer. 1988;62:194–202. doi: 10.1002/1097-0142(19880701)62:1<194::AID-CNCR2820620129>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 10.Brosjö O. Surgical procedure and local recurrence in 223 patients treated 1982–1997 according to two osteosarcoma chemotherapy protocols. The Scandinavian Sarcoma Group experience. Acta Orthop Scand. 1999;285(Suppl):58–61. [PubMed] [Google Scholar]
- 11.Carrasco CH, Charnsangavej C, Raymond AK, Richli WR, Wallace S, Chawla SP, Ayala AG, Murray JA, Benjamin RS. Osteosarcoma: angiographic assessment of response to preoperative chemotherapy. Radiology. 1989;170:839–842. doi: 10.1148/radiology.170.3.2916040. [DOI] [PubMed] [Google Scholar]
- 12.Cores EP, Holland JF, Wang JJ, Sinks LF. Doxorubicin in disseminated osteosarcoma. JAMA. 1972;221:1132–1138. doi: 10.1001/jama.221.10.1132. [DOI] [PubMed] [Google Scholar]
- 13.Cullen JW, Jamroz BA, Stevens SL, Madsen W, Hinshaw I, Wilkins RM, Cullen P, Camozzi AB, Fink K, Peck SD, Kelly CM. The value of serial arteriography in osteosarcoma: delivery of chemotherapy, determination of therapy duration and prediction of necrosis. J Vasc Interv Radiol. 2005;16:1107–1119. doi: 10.1097/01.RVI.0000167856.31329.F8. [DOI] [PubMed] [Google Scholar]
- 14.Dahlin DC, Coventry MB. Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am. 1967;49:101–110. [PubMed] [Google Scholar]
- 15.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12:423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
- 16.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard D. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 17.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 18.Ettinger LJ, Douglass HO, Higby DJ. Adjuvant Adriamycin and Cis-diamminedichloroplatinum (cis-platinum) in primary osteosarcoma. Cancer. 1981;47:248–254. doi: 10.1002/1097-0142(19810115)47:2<248::AID-CNCR2820470208>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari S, Bacci G, Picci P, Mercuri M, Briccoli A, Pinto D, Gasbarrini A, Tienghi A, del Brach Prever A. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann Oncol. 1997;8:765–771. doi: 10.1023/A:1008221713505. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari S, Bertoni F, Mercuri M, Picci P, Giacomini S, Longhi A, Bacci G. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity. An analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol. 2001;12:1145–1150. doi: 10.1023/A:1011636912674. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari S, Mercuri M, Picci P, Bertoni F, del Brach Prever A, Tienghi A, Mancini A, Longhi A, Rimondini S, Donati D, Manfrini M, Ruggieri P, Biagini R, Bacci G. Nonmetastatic osteosarcoma of the extremity: results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with high-dose methotrexate, intra-arterial or intravenous cisplatin, doxorubicin, and salvage chemotherapy based on histologic tumor response. Tumori. 1999;85:458–464. doi: 10.1177/030089169908500607. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Müller C, Tienghi A, Wiebe T, Comandone A, Böhling T, Del Prever AB, Brosjö O, Bacci G, Saeter G. Italian and Scandinavian Sarcoma Groups. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, Cisplatin, and doxorubicin for patients with localized ostesarcoma of the extremity. A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 23.Franzius C, Scluk J, Brinkschmidt C, Jurgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med. 2000;25:874–881. doi: 10.1097/00003072-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Körholz D, Graf N, Heise U, Jürgens H, Kotz R, Salzer-Kuntschik M, Weinel P, Werner M, Winkler K. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–899. doi: 10.1023/A:1008391103132. [DOI] [PubMed] [Google Scholar]
- 25.Honegger HP, Cserhati MD, Exner GU, von Hochstetter A, Groscurth P. Zürich experience with preoperative, high dose methotrexate-containing chemotherapy in patients with extremity osteosarcomas (OSA) Ann Oncol. 1991;2:489–494. doi: 10.1093/oxfordjournals.annonc.a057998. [DOI] [PubMed] [Google Scholar]
- 26.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 10 patients after treatment with chemotherapy, en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14–18. [PubMed] [Google Scholar]
- 27.Jaffe N, Knapp J, Chuang VP, Wallace S, Ayala A, Murray J, Cangir A, Wang A, Benjamin RS. Osteosarcoma. Intra-arterial treatment of the primary tumor with cis-diamminedichloroplatinum II (CDP); angiographic, pathologic, and pharmacologic studies. Cancer. 1983;51:402–407. doi: 10.1002/1097-0142(19830201)51:3<402::AID-CNCR2820510308>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe N, Raymond AK, Ayala A, Carrasco CH, Wallace S, Robertson R, Griffiths M, Wang YM. Effect of cumulative courses of intra-arterial cis-diamminedichloroplatinum II on the primary tumor in osteosarcoma. Cancer. 1989;63:63–67. doi: 10.1002/1097-0142(19890101)63:1<63::AID-CNCR2820630110>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Jaffe N, Robertson R, Ayala A, Wallace S, Chuang V, Anzai T, Cangir A, Wang YM, Chen T. Comparison of cis-diamminedichloroplatinum II with high-dose methotrexate and citrovorum factor rescue in the treatment of primary osteosarcoma. J Clin Oncol. 1985;3:1101–1104. doi: 10.1200/JCO.1985.3.8.1101. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 31.Kawai A, Sugihara S, Kunisada T, Uchida Y, Inoue H. Imaging assessment of the response of bone tumors to preoperative chemotherapy. Clin Orthop Relat Res. 1997;337:216–225. doi: 10.1097/00003086-199704000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Knop J, Delling G, Heise U, Wingler K. Scintigraphic evaluation of tumor regression during preoperative chemotherapy of osteosarcoma. Correlation of 99 mTc-methylene diphosphonate parametric imaging with surgical histopathology. Skeletal Radiol. 1990;19:165–172. doi: 10.1007/BF00204090. [DOI] [PubMed] [Google Scholar]
- 33.Kunisada T, Ozaki T, Kawai A. Imaging assessment of the responses of osteosarcoma patients to preoperative chemotherapy: angiography compared with thallium-201 scintigraphy. Cancer. 1999;86:949–956. doi: 10.1002/(SICI)1097-0142(19990915)86:6<949::AID-CNCR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Lang P, Grampp S, Vahlensieck M. Primary bone tumors: value of MR angiography for preoperative planning and monitoring response to chemotherapy. AJR Am J Roentgenol. 1995;165:135–142. doi: 10.2214/ajr.165.1.7785572. [DOI] [PubMed] [Google Scholar]
- 35.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AH. MRC BO06 and EORTC 80931 collaborators; European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy. A randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 36.Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286–291. doi: 10.1097/01.blo.0000145991.65770.e6. [DOI] [PubMed] [Google Scholar]
- 37.Mavligit GM, Benjamin RS, Patt YZ. Intra-arterial cis-platinum for patients with inoperable skeletal tumors. Cancer. 1981;48:1–4. doi: 10.1002/1097-0142(19810701)48:1<1::AID-CNCR2820480102>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Mercuri M, Capanna R, Manfrini M, Bacci G, Picci P, Ruggieri P, Ferruzzi A, Ferraro A, Donati D, Biagini R, de Maio M, Cazzola A, Campanacci M. The management of malignant bone tumors in children and adolescents. Clin Orthop Relat Res. 1991;264:156–168. [PubMed] [Google Scholar]
- 39.Mirra JJ, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989:255–344, 766–794.
- 40.Nair N, Ali G, Green AA. Response of osteosarcoma to chemotherapy. Evaluation with F-18 FDG-PET scans. Clin Positron Imaging. 2000;3:79–83. doi: 10.1016/S1095-0397(00)00037-6. [DOI] [PubMed] [Google Scholar]
- 41.Nitschke R, Starling KA, Vats T, Bryan H. Cis-diamminedichloroplatinum (NSC 119875) in childhood malignancies: A Southwest Oncology Group Study. Med Pediatr Oncol. 1978;4:127–132. doi: 10.1002/mpo.2950040208. [DOI] [PubMed] [Google Scholar]
- 42.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy. A report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 43.Quintana J, Beresi V, DelPozo H, Latorre JJ, Henriquez A, Chamas N, Diaz V, Geldres V, Sepulveda L, Macho L, Dolz G. Intra-arterial cisplatin given prior to surgery in osteosarcoma: grade of necrosis and size of tumor as major prognostic factors. Am J Pediatr Hematol Oncol. 1991;13:269–273. doi: 10.1097/00043426-199123000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Rha SY, Chung HC, Gong SJ, Shim KY, Ahn JB, Yang WI, Shin KH, Yoo NC, Kim JH, Roh JK, Lee CI, Kim BS. Combined preoperative chemotherapy with intra-arterial Cisplatin and continuous intravenous Adriamycin for high grade osteosarcoma. Oncol Rep. 1999;6:631–637. doi: 10.3892/or.6.3.631. [DOI] [PubMed] [Google Scholar]
- 45.Rosen G, Caparros B, Nirenberg A. The successful management of metastatic osteogenic sarcoma: A model for the treatment of primary osteogenic sarcoma. In: van Oosterom AT, Muggia FM, Cleton FJ, eds. Therapeutic Progress in Ovarian Cancer, Testicular Cancer and the Sarcomas. Boston, MA: Leiden University Press; 1979:349–366.
- 46.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma - the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2192. doi: 10.1002/1097-0142(197906)43:6<2163::AID-CNCR2820430602>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 47.Schulte M, Brecht-Krauss D, Werner M. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG-PET. J Nucl Med. 1999;40:1637–1643. [PubMed] [Google Scholar]
- 48.Stewart DJ, Benjamin RS, Zimmerman S, Caprioli RM, Wallace S, Chuang V, Calvo D, 3rd, Samuels M, Bonura J, Loo TL. Clinical pharmacology of intra-arterial cis-diamminedichloroplatinum (II) Cancer Res. 1983;43:917–920. [PubMed] [Google Scholar]
- 49.Uchida A, Myoui A, Araki N, Yoshikawa H, Shinto Y, Ueda T. Neoadjuvant chemotherapy for pediatric osteosarcoma patients. Cancer. 1997;79:411–415. doi: 10.1002/(SICI)1097-0142(19970115)79:2<411::AID-CNCR26>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 50.Wilkins RM, Cullen JW, Camozzi AB, Jamroz BA, Odom L. Improved survival in primary nonmetastatic pediatric osteosarcoma of the extremity. Clin Orthop Relat Res. 2005;438:128–136. doi: 10.1097/01.blo.0000179736.10871.76. [DOI] [PubMed] [Google Scholar]
- 51.Wilkins RM, Cullen JW, Odom L, Jamroz BA, Cullen PM, Fink K, Peck SD, Stevens SL, Kelly CM, Camozzi AB. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10:498–507. doi: 10.1245/ASO.2003.03.061. [DOI] [PubMed] [Google Scholar]
- 52.Winkler K, Bielack S, Delling G, Salzer-Kuntschik M, Kotz R, Greenshaw C, Jürgens H, Ritter J, Kusnierz-Glaz C, Erttmann R, Gadicke G, Graf N, Ladenstein R, Leyvraz S, Mertens R, Weinel P. Effect of intra-arterial versus intravenous cisplatin in addition to systemic doxorubicin, high-dose methotrexate, and ifosfamide on histologic tumor response in osteosarcoma (Study COSS-86) Cancer. 1990;66:1703–1710. doi: 10.1002/1097-0142(19901015)66:8<1703::AID-CNCR2820660809>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]