Abstract
Staphylococcus aureus (S. aureus) is an independent risk factor for orthopaedic surgical site infection (SSI). To determine whether a preoperative decolonization protocol reduces S. aureus SSIs, we conducted a prospective observational study of patients undergoing elective total joint arthroplasty (TJA) at our institution, with two control groups. The concurrent control group comprised patients of surgeons who did not participate in the intervention study. The preintervention control group comprised patients of participating surgeons who had undergone elective TJA during the year before the study. Patients in the intervention group were screened preoperatively for S. aureus by nasal swab cultures. S. aureus carriers were decolonized with mupirocin ointment to the nares twice daily and chlorhexidine bath once daily for 5 days before surgery. All 164 of 636 participants (26%) who tested positive completed the decolonization protocol without adverse events and had no postoperative S. aureus SSIs at 1-year followup. In contrast, 1330 concurrent control patients had 12 S. aureus infections. If these infections had occurred in the 26% of patients expected to be nasal carriers of S. aureus at a given time, the infection rate would have been 3.5% (12 of 345) in the control group. In addition, the overall infection rate of the participating surgeons, including nonstaphylococcal infections, decreased from 2.6% during the preintervention period to 1.5% during the intervention period, translating to an adjusted economic gain of $231,741 for the hospital. The data suggest a preoperative decolonization protocol reduces S. aureus SSIs in patients undergoing TJA.
Level of Evidence: Level II, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Surgical site infection (SSI) is an infrequent but serious complication of total joint arthroplasty (TJA) [19], with rates ranging from 0.2% for primary total hip arthroplasty to 1.5% for total knee arthroplasty [6, 16]. Orthopaedic SSIs cause substantial morbidity, prolonging hospital stay by a median of 2 weeks, doubling rehospitalization rates, and more than tripling overall healthcare costs [32]. The economic burden is even greater in a capitated reimbursement structure, such as diagnostic-related groups that do not cover the costs of nosocomial infection, resulting in losses to the healthcare system [1].
Prevention of SSIs requires identification of risk factors and appropriate intervention [17]. There is a strong epidemiologic association between nasal carriage of S. aureus and development of S. aureus SSIs. Carriers are two to nine times more likely to acquire S. aureus SSIs than noncarriers [11, 22, 31]. In fact, nasal carriage was the only independent risk factor for S. aureus SSI in patients undergoing orthopaedic implant surgery [10]. Furthermore, in patients who acquire S. aureus SSIs, paired S. aureus isolates from the wound match those from the nares 85% of the time [21]. Intranasal mupirocin is an attractive prevention strategy because it is a safe and simple method that eradicates nasal colonization in a wide variety of patients [22, 31]. It also reduces S. aureus infections in patients undergoing hemodialysis [7, 12, 27] and SSIs in patients undergoing cardiovascular surgery [2, 13], orthopaedic surgery [5], and mixed surgery [21]. Recently, chlorhexidine baths have been added to intranasal mupirocin in an effort to eradicate carriage of methicillin-resistant S. aureus (MRSA) [25, 30] and to reduce nosocomial infections caused by MRSA in the intensive care unit [23]. The combination was simple and had no major side effects [25, 29, 30].
The rate of SSI following TJA at our institution was 1.1% between 2003 and 2005, with S. aureus accounting for more than 50% of infections (Table 1; unpublished data). We therefore hypothesized use of a decolonization protocol would lower the S. aureus infection rate in patients undergoing TJA and be cost-effective.
Table 1.
Pathogens isolated from patients with surgical site infections at our institution from 2003 to 2005
| Pathogen | Percent of infections | |
|---|---|---|
| Total hip arthroplasty | Total knee arthroplasty | |
| Methicillin-sensitive S. aureus | 34 | 32 |
| Methicillin-resistant S. aureus | 31 | 21 |
| Coagulase-negative staphylococci | 23 | 14 |
| Enterococci | 0 | 12 |
| Gram-negative bacilli | 12 | 21 |
Materials and Methods
To test these hypotheses, we performed a prospective observational study of patients scheduled for TJA at our institution and estimated the potential cost savings based on a previous report of the attributable cost of SSI [24]. The protocol consisted of preoperative screening for S. aureus nasal carriage and, in carriers, preoperative use of intranasal mupirocin with chlorhexidine body wash. We performed a prospective observational study of 1966 consecutive patients who underwent elective TJA at our institution between October 2005 and October 2006. Three orthopaedic surgeons (LSC, AJY, RM) agreed to participate; all 636 of their patients were eligible for enrollment in the preoperative screening intervention group and all 636 agreed to participate. All 1330 patients whose TJAs were performed by the remaining surgeons were eligible for enrollment in the concurrent control group. In addition, all 741 patients whose surgery was performed by participating surgeons between October 2004 and October 2005 served as a preintervention control group. The study was conducted under patient safety authority and therefore did not require approval by the Institutional Review Board.
Two to 4 weeks before surgery, patients in the intervention group were screened for S. aureus nasal carriage. Participants were educated about the rationale for nasal cultures, and informed consent was obtained. Samples were collected from both nares on a single swab (BBL™ CultureSwab™ Plus, BD Diagnostics, Sparks, Maryland). The inside circumference of each anterior nares was rubbed for 3 to 5 seconds to obtain adequate sampling. Specimens were inoculated onto CHROMagar MRSA and CHROMagar SA plates (BD Microbiology Systems, Sparks, MD), which were incubated for 20 to 28 hours at 35°C to 37°C. After 24 hours, we interpreted mauve colonies present on both plates as MRSA and on only the CHROMagar SA plate as methicillin-sensitive S. aureus (MSSA). Negative plates were incubated for an additional 24 hours. Mauve colonies present on either medium at 48 hours were verified as S. aureus by Gram’s stain and coagulase testing (Staphaurex, Remel, Lenexa, Kansas). Mauve colonies growing on both media were reported as MRSA while colonies growing only on CHROMagar SA were reported as MSSA [3, 4].
Approximately a week before surgery, patients with nasal cultures positive for MSSA or MRSA were educated about the rationale for the decolonization protocol, which was initiated in the outpatient setting. Patients were instructed to apply mupirocin nasal ointment twice daily to both nares and bathe with chlorhexidine daily for 5 days immediately prior to the scheduled surgery. During preoperative admission, we assessed compliance and safety by asking questions to determine whether patients had followed directions, completed the decolonization protocol, and experienced any adverse events.
During surgery, all patients received perioperative antibiotic prophylaxis. The standard regimen was cefazolin 2 g administered 30 to 60 minutes before surgery followed by 1 g every 8 hours for 24 hours. Alternatively, vancomycin 1 g 60 minutes before surgery followed by 1 g every 12 hours for 24 hours, was administered to patients with a history of MRSA infection or type I allergy to penicillin, and to MRSA carriers in the intervention group.
For 1 year after TJA, all patients were prospectively monitored for development of SSIs. We did not collect demographic or any other patient-specific data. No patients were lost to followup.
To estimate the potential cost savings, we compared hospital costs for infected patients in the preintervention and intervention groups based on data from a cohort designed to evaluate the attributable impact of methicillin resistance on clinical outcomes and hospital costs [24]. In that study of 55 patients with infected TJAs, the average direct hospital costs exceeded Medicare reimbursement by $27,000 and private insurance reimbursement by $18,000.
The primary outcome measure was the number of S. aureus SSIs over a 1-year followup period in the intervention group compared with that in the concurrent control group. To estimate the infection rate in the control group, we assumed all S. aureus SSIs occurred in nasal carriers and the carrier rate in the concurrent control group was identical to that in the intervention group. Rates were compared using the independent samples t test with equal variances assumed between the intervention and control groups. Analysis was performed using SPSS (Chicago, IL, United States).
Results
Screening yielded positive nasal cultures in 164 of 636 participants (26%), including 147 with MSSA (23%) and 17 with MRSA (3%). All 164 participants with positive nasal cultures received preoperative mupirocin and chlorhexidine body wash. Participants reported they had adhered to the decolonization protocol and had not experienced any adverse events. All participants with nasal cultures positive for MRSA subsequently received perioperative prophylaxis with vancomycin.
Postoperative followup for 1 year yielded no S. aureus SSIs in 164 patients in the intervention group, whereas 12 patients in the concurrent control group developed S. aureus SSIs (Table 2), including 7 due to MSSA and 5 due to MRSA. A total of 345 of 1330 control patients were expected to be S. aureus nasal carriers based on the carrier rate in the intervention group. If all 12 infections had occurred in the nasal carriers, the infection rate in the control group would have been 3.5% (12 of 345; p = 0.02; equal variances assumed with a 99% confidence interval [CI]).
Table 2.
Staphylococcus aureus (S. aureus) surgical site infections (SSIs) in patients with nasal cultures confirmed (intervention group) or assumed (concurrent control group) to be positive for S. aureus
| Patient group | Number of SSIs/Number of patients | Infection rate % |
|---|---|---|
| Intervention | 0/164 | 0 |
| Concurrent control | 12/345 | 3.5* |
*p = 0.016 (equal variances assumed; 99% confidence interval).
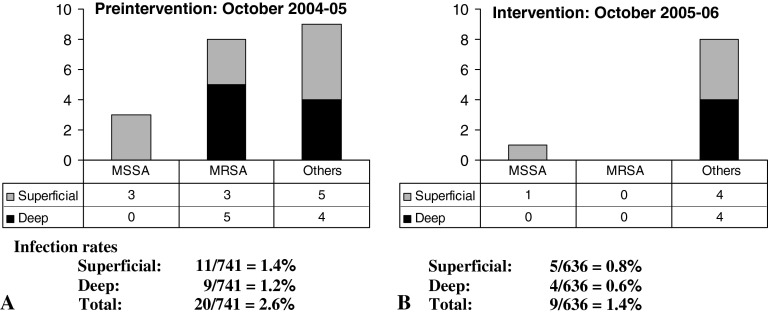
The overall infection rate, including nonstaphylococcal infections, decreased from 2.6% during the preintervention period to 1.5% during the intervention period (Fig. 1). Only one patient (0.15%) developed a superficial MSSA infection during the intervention period. This patient had a negative nasal screen for S. aureus and therefore did not undergo decolonization. In contrast, 11 patients had S. aureus SSIs during the preintervention period. The number of non-S. aureus SSIs was similar between groups, with 9 during the preintervention period and 8 during the intervention period.
Fig. 1.
The graph shows patients who underwent total joint arthroplasty by the same group of orthopaedic surgeons. (A) Infection rates during the preintervention period are shown. (B) The rate of surgical site infection was reduced during the intervention period. MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S. aureus.
The lower infection rate during the intervention period reduced the amount of lost revenue by $270,000, resulting in an economic gain compared with the preintervention period (Table 3). After adjusting for the lower number of TJAs performed during the intervention period, we estimated an economic gain of $231,741.
Table 3.
Cost analysis of patients with surgical site infections
| Payer | Intervention period | |
|---|---|---|
| Preintervention (n = 741) | Intervention (n = 636) | |
| Medicare | ||
| Number of infections | 15 | 7 |
| Lost revenue/infection | −$27,000 | −$27,000 |
| Subtotal | −$405,000 | −$189,000 |
| Private insurance | ||
| Number of infections | 5 | 2 |
| Lost revenue/infection | −$18,000 | −$18,000 |
| Subtotal | −$90,000 | −$36,000 |
| Total lost revenue | −$495,000 | −$225,000* |
* Lost revenue reduced by an absolute amount of $270,000 and, after adjusting for the number of surgeries, an adjusted amount of $231,741.
Discussion
Surgical site infection is a serious complication of total joint arthroplasty (TJA) [19], with rates ranging from 0.2% for primary total hip arthroplasty to 1.5% for total knee arthroplasty [6, 16]. Being a carrier is an independent risk factor for orthopaedic surgical site infection (SSI) [11, 22, 31]. We therefore hypothesized use of a decolonization protocol would lower the S. aureus infection rate in patients undergoing TJA and be cost-effective.
Our study had several limitations. First, the study was not randomized and we did not collect demographic or other patient-specific data, so selection bias is possible. On the other hand, our study had two control groups. The concurrent control group comprised patients whose surgeons did not participate in the decolonization protocol; the preintervention control group comprised patients whose TJAs were performed by the surgeons who subsequently participated in the intervention study. Trends were similar for both control groups, with infection rates higher than in the intervention group. Furthermore, the large numbers of patients should have helped to reduce the risk of selection bias. Second, we did not prospectively estimate the sample size required to detect between-group differences, but the main purpose for this calculation is to avoid type II or false negative error. Third, we did not repeat nasal screening to confirm S. aureus was eradicated after the decolonization protocol; however, it is reasonable to believe S. aureus was eradicated because of the success reported by others [22, 31].
Preoperative screening confirmed 26% of participants in the intervention group were nasal carriers, approximating the previously reported rate [20]. All patients with positive nasal cultures reported they had completed the decolonization protocol without adverse events. None of the carriers in the intervention group developed S. aureus SSIs during the 1-year followup period, whereas 12 patients in the concurrent control group developed S. aureus SSIs. We assumed these SSIs occurred in S. aureus carriers because nasal carriage is the most important risk factor for SSI [10] and paired S. aureus isolates from nares and wounds usually match [21]. Extrapolating from the estimated infection rate among assumed carriers, we calculated the decolonization protocol was associated with a reduction in S. aureus SSIs. In addition, the decolonization protocol appeared to be associated with a decrease in the overall infection rate compared with that during the preintervention period. Importantly, the decolonization protocol reduced S. aureus infections without increasing the rate of infections due to other pathogens.
The reduced incidence of infection during the intervention period translated to an adjusted economic gain of $231,741 compared with the preintervention period. This estimate was based on a report published in 1993 involving 55 patients with infected TJAs [24]. We did not adjust for inflation or consider indirect costs, such as impaired functional and mental capacity, and lost productivity, which can affect both patients and their caregivers. Consideration of these issues would have substantially increased savings and offset the nominal cost of the decolonization protocol, which was not included in our estimate.
One obvious concern about widespread mupirocin use is the potential for increased drug resistance and subsequent failure [8, 15, 18, 28]. However, selective use of short courses of mupirocin for nasal decolonization is thought not to cause widespread resistance [20].
Our findings add to those of previous studies designed to determine whether preoperative mupirocin reduces SSIs. Previous studies had different designs, sample sizes, and approaches to intervention, which probably affected the outcomes. Nonrandomized studies with concurrent or historic controls usually involved selective prophylaxis in nasal carriers [5, 13] and consistently demonstrated the beneficial effect of mupirocin in patients undergoing orthopaedic [6] or cardiac surgery [2, 13]. For example, Gernaat-van der Sluis et al. [5] reported prophylactic mupirocin reduced the overall SSI rate after orthopaedic surgery among S. aureus carriers (1.3% versus 2.7%), but not the rate among those with S. aureus SSIs (0.7% versus 1.1%). In contrast, randomized studies often failed to detect substantial differences favoring mupirocin in patients undergoing orthopaedic [9], cardiac [14], gastrointestinal [26], or mixed surgery [21]. Unexpected findings may have played a role, such as a lower-than-expected rate of S. aureus SSI in control groups, confounding the ability to detect differences within the sample size [9, 14]. Konvalinka et al. [14] reported nasal S. aureus colonization disappeared in almost half of control patients, further obscuring the anticipated benefit of mupirocin. The lack of benefit after gastrointestinal surgery is not surprising in view of the high percentage of Gram-negative infections; however, mupirocin was associated with less S. aureus pneumonia (none versus 2%) [26]. Perl et al. [21] reported mupirocin did not reduce S. aureus SSIs after mixed surgery (2.3% versus 2.4%) but reduced S. aureus nosocomial infections in the subset of S. aureus nasal carriers (4.0% versus 7.7%). Kalmeijer et al. [9] reported mupirocin did not notably reduce SSIs (3.8% versus 4.7%) or S. aureus SSIs (1.6% versus 2.7%) after orthopaedic surgery; however, these differences might have become relevant if the intervention group had had more than 315 patients or followup had been extended beyond 1 month.
The data from our prospective observational study suggest preoperative decolonization is a safe way to reduce S. aureus SSIs in patients undergoing TJA and may translate to economic savings for the hospital or healthcare institution. In this era of patient safety and cost containment, interventions that decrease morbidity and improve healthcare-related quality of life merit further evaluation.
Acknowledgments
We thank James A. Schnebel for his help with this study.We thank Cindy Hamilton and Anne Derbes for editing the manuscript. We thank James A. Schnebel for processing nasal swabs for recovery and confirmation of MSSA and MRSA.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution did not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Boyce JM, Potter-Bynoe G, Dziobek L. Hospital reimbursement patterns among patients with surgical wound infections following open heart surgery. Infect Control Hosp Epidemiol. 1990;11:89–93. doi: 10.1086/646127. [DOI] [PubMed] [Google Scholar]
- 2.Cimochowski GE, Harostock MD, Brown R, Bernardi M, Alonzo N, Coyle K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann Thorac Surg. 2001;71:1572–1578. doi: 10.1016/S0003-4975(01)02519-X. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza HA, Baron EJ. BBL CHROMagar Staph aureus is superior to mannitol salt for detection of Staphylococcus aureus in complex mixed infections. Am J Clin Pathol. 2005;123:806–808. doi: 10.1309/FVHR-F3GR-LEQX-GBAG. [DOI] [PubMed] [Google Scholar]
- 4.Flayhart D, Hindler JF, Bruckner DA, Hall G, Shrestha RK, Vogel SA, Richter SS, Howard W, Walther R, Carroll KC. Multicenter evaluation of BBL CHROMagar MRSA medium for direct detection of methicillin-resistant Staphylococcus aureus from surveillance cultures of the anterior nares. J Clin Microbiol. 2005;43:5536–5540. doi: 10.1128/JCM.43.11.5536-5540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.der Gernaat-van Sluis AJ, Hoogenboom-Verdegaal AM, Edixhoven PJ, Spies-van Rooijen NH. Prophylactic mupirocin could reduce orthopedic wound infections. 1,044 patients treated with mupirocin compared with 1,260 historical controls. Acta Orthop Scand. 1998;69:412–414. doi: 10.3109/17453679808999058. [DOI] [PubMed] [Google Scholar]
- 6.Hervey SL, Purves HR, Guller U, Toth AP, Vail TP, Pietrobon R. Provider volume of total knee arthroplasties and patient outcomes in the HCUP-Nationwide Inpatient Sample. J Bone Joint Surg Am. 2003;85:1775–1783. doi: 10.2106/00004623-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Herwaldt LA. Reduction of Staphylococcus aureus nasal carriage, infection in dialysis patients. J Hosp Infect. 1998;40 Suppl B:S13–23. doi: 10.1016/S0195-6701(98)90200-6. [DOI] [PubMed] [Google Scholar]
- 8.Irizarry L, Rupp J, Griffith J. Frequency of high-level mupirocin-resistant Staphylococcus aureus in a tertiary care facility. Antimicrob Agents Chemother. 1996;40:1967–1968. doi: 10.1128/aac.40.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GA, Stuurman A, van Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 10.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21:319–323. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 11.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluytmans JA, Manders MJ, van Bommel E, Verbrugh H. Elimination of nasal carriage of Staphylococcus aureus in hemodialysis patients. Infect Control Hosp Epidemiol. 1996;17:793–797. doi: 10.1086/647238. [DOI] [PubMed] [Google Scholar]
- 13.Kluytmans JA, Mouton JW, VandenBergh MF, Manders MJ, Maat AP, Wagenvoort JH, Michel MF, Verbrugh HA. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17:780–785. doi: 10.1086/647236. [DOI] [PubMed] [Google Scholar]
- 14.Konvalinka A, Errett L, Fong IW. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J Hosp Infect. 2006;64:162–168. doi: 10.1016/j.jhin.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leski TA, Gniadkowski M, Skoczynska A, Stefaniuk E, Trzcinski K, Hryniewicz W. Outbreak of mupirocin-resistant staphylococci in a hospital in Warsaw, Poland, due to plasmid transmission and clonal spread of several strains. J Clin Microbiol. 1999;37:2781–2788. doi: 10.1128/jcm.37.9.2781-2788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahomed NN, Barrett JA, Katz JN, Phillips CB, Losina E, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85:27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 18.Miller MA, Dascal A, Portnoy J, Mendelson J. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect Control Hosp Epidemiol. 1996;17:811–813. doi: 10.1086/647242. [DOI] [PubMed] [Google Scholar]
- 19.National Nosocomial Infections Surveillance (NNIS) System report, data summary from October 1986-April 1998, issued June 1998. Am J Infect Control. 1998;26:522–533. [DOI] [PubMed]
- 20.Perl TM. Prevention of Staphylococcus aureus infections among surgical patients: beyond traditional perioperative prophylaxis. Surgery. 2003;134:S10–17. doi: 10.1016/S0039-6060(03)00391-X. [DOI] [PubMed] [Google Scholar]
- 21.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 22.Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann Pharmacother. 1998;32:S7–16. doi: 10.1177/106002809803200104. [DOI] [PubMed] [Google Scholar]
- 23.Sandri AM, Dalarosa MG, de Ruschel Alcantara L, da Silva Elias L, Zavascki AP. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol. 2006;27:185–187. doi: 10.1086/500625. [DOI] [PubMed] [Google Scholar]
- 24.Sculco TP. The economic impact of infected total joint arthroplasty. Instr Course Lect. 1993;42:349–351. [PubMed] [Google Scholar]
- 25.Simor AE, Phillips E, McGeer A, Konvalinka A, Loeb M, Devlin HR, Kiss A. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178–185. doi: 10.1086/510392. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Kamigaki T, Fujino Y, Tominaga M, Ku Y, Kuroda Y. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br J Surg. 2003;90:1072–1075. doi: 10.1002/bjs.4269. [DOI] [PubMed] [Google Scholar]
- 27.Tacconelli E, Carmeli Y, Aizer A, Ferreira G, Foreman MG, D’Agata EM. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin Infect Dis. 2003;37:1629–1638. doi: 10.1086/379715. [DOI] [PubMed] [Google Scholar]
- 28.Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51:613–617. doi: 10.1093/jac/dkg127. [DOI] [PubMed] [Google Scholar]
- 29.Watanakunakorn C, Brandt J, Durkin P, Santore S, Bota B, Stahl CJ. The efficacy of mupirocin ointment and chlorhexidine body scrubs in the eradication of nasal carriage of Staphylococcus aureus among patients undergoing long-term hemodialysis. Am J Infect Control. 1992;20:138–141. doi: 10.1016/S0196-6553(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 30.Wendt C, Schinke S, Wurttemberger M, Oberdorfer K, Bock-Hensley O, von Baum H. Value of whole-body washing with chlorhexidine for the eradication of methicillin-resistant Staphylococcus aureus: a randomized, placebo-controlled, double-blind clinical trial. Infect Control Hosp Epidemiol. 2007;28:1036–1043. doi: 10.1086/519929. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31:13–24. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 32.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]