Abstract
Common peroneal nerve palsy has been reported to be the most frequent lower extremity palsy characterized by a supinated equinovarus foot deformity and foot drop. Dynamic tendon transposition represents the gold standard for surgical restoration of dorsiflexion of a permanently paralyzed foot. Between 1998 and 2005, we operated on a selected series of 16 patients with traumatic complete common peroneal nerve palsy. In all cases, we performed a double tendon transfer through the interosseous membrane. The posterior tibialis tendon was transferred to the tibialis anterior rerouted through a new insertion on the third cuneiform and the flexor digitorum longus was transferred to the extensor digitorum longus and extensor hallucis longus tendons. All 16 patients were reviewed at a minimum followup of 24 months (mean, 65 months; range, 24–114 months). The results were assessed using the Stanmore system questionnaire and were classified as excellent in eight, good in five, fair in two, and poor in one. Postoperative static and dynamic baropodometric evaluations also were performed. The proposed procedure, which provides an appropriate direction of pull with adequate length and fixation, is a reliable new method to restore balanced foot dorsiflexion correcting the foot and digit drop and producing a normal gait without the use of orthoses.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-008-0249-9) contains supplementary material, which is available to authorized users.
Introduction
Common peroneal nerve (CPN) palsy has been reported as the most frequent lower extremity palsy [16, 18]. It can be the result of several causative mechanisms such as ischemia, mechanical irritation, traction, crushing injuries, or laceration [5]. Although in most cases the lesion recovers spontaneously [2, 8, 11], in some cases, despite improvements in nerve repair and grafting, irreversible damage to the nerve can occur.
Paralysis of the CPN is characterized by a supinated equinovarus foot deformity resulting from the unopposed pull of the tibialis posterior muscle [27]. Foot drop and drop of the digits [3] also occurs causing a well known gait disability. An ankle-foot orthosis (AFO) or brace to prevent the foot drop sometimes may be poorly tolerated [29], especially in patients who have some degree of equinovarus contracture or in young patients who need to wear orthoses for the rest of their lives. In irreparable peroneal nerve paralysis, dynamic tendon transposition represents the gold standard for surgical restoration of functional dorsiflexion of a permanently paralyzed foot.
As reported by Watkins et al., Codivilla in 1899 and Putti in 1914 [26] are considered the pioneers of the anterior transposition of the posterior tibialis tendon (PTT) to the dorsum of the foot through the interosseous membrane. This technique has been widely used [6, 13, 17, 22], becoming the most accepted reconstructive method to correct drop-foot [6]. This technique, however, has several disadvantages relating to insufficient length of the PTT after harvest preventing easy tendon-to-bone (TtB) insertion on the dorsal aspect of the foot often requiring the ankle to be maximally dorsiflexed or altering the plan of location or the method of tendon insertion [6, 8, 12, 22, 24, 26, 29].
Tendon-to-tendon (TtT) suturing has been described [3, 8, 17, 22, 24] as an alternative to the TtB procedure eliminating the need for screws, staples, or pullout wires tied over a button [12, 26]. Direct TtT suture, however fails to achieve balanced dorsiflexion because the foot needs to be pulled medially (using anterior tibialis tendon [ATT] and/or extensor hallucis longus tendon [EHL]) and laterally (using the peroneus longus or tertius) using recipient tendons as reins [3, 8, 17, 18, 21, 22, 26, 27, 29]. Unfortunately, these methods are surgically demanding and the complexity of reconstruction dissipates the pull strength of the PTT reducing dorsiflexion power.
The technique recreates a new ATT origin on the third cuneiform with a transosseous tunnel through which the ATT is rerouted. A double tendon transfer [3] then is performed with a direct TtT suture at the distal third of the leg between the rerouted ATT and the PTT (transposed anteriorly through interosseous membrane) and between the flexor digitorum longus (FDL) tendon, similarly transposed and sutured side to side with the extensor digitorum longus (EDL) and extensor hallucis longus (EHL) tendons. This second transfer strengthens ankle dorsiflexion and reanimates toe extension [3].
We wished to document the advantages of this proposed new tendon transfer technique. We will show that this transfer allows the surgeon to achieve the most favorable biomechanical tendon insertion producing effective and balanced ankle dorsiflexion. Specifically, the analysis addresses whether the technique proposed in this study allows optimization of the position and length of the tendon transfer resulting in good to excellent restoration of active range of motion and reanimation of balanced toe extension. The biomechanical advantages of this technique will be reflected in elimination of the need for an AFO and improved Stanmore system scores and dynamic baropodometric evaluations.
Materials and Methods
In the current retrospective study, to create a homogenous cohort of patients, patients who had partial CPN palsy, moderate to severe middle to hindfoot deformity, and ankle or toe joint stiffness, which would have required complementary surgery, were excluded. Thus, data for 16 patients with complete traumatic CPN palsy operated on between 1998 and 2005 are presented; 10 were male with a mean age of 25.8 years (range, 11–44 years). Minimum followup was 24 months (mean, 65 months; range, 24–114 months).
Eight patients had previous microsurgery for CPN repair (Table 1) without any nerve recovery. To determine whether the CPN is recovering after microsurgical reconstruction, we waited a variable period between 18 and 24 months, during which progression of Tinel’s sign, muscular contractions, and nerve conduction studies were evaluated before considering tendon transfer [9, 28]. The remaining patients had been treated elsewhere first for their initial high-energy trauma at the knee or proximal tibia level. When referred to us for treatment of the nerve palsy, a mean of 36.4 months had elapsed from the time of the nerve lesion in each of the eight patients. This delay contraindicated any attempt at microsurgical reconstruction [7, 25]. In three cases (Patients 2, 3, 6) before tendon transfer, we explored the CPN at the fibular neck. In Patient 2, the nerve was avulsed from the muscle and in Patients 3 and 6, there was loss of nerve substance with a gap greater than 12 cm. In these three patients, as a result of the high risk of failure, we did not consider nerve reconstruction and proceeded directly to tendon transfer. Anterior tibialis tendon, EDL-EHL, and peroneal muscle function were assessed preoperatively by muscle testing during clinical evaluation and through electromyographic and nerve conduction studies. Patients 1, 8, 11, and 16 had residual peroneal muscle function graded between 1 and 2 [23], which was clinically ineffective in producing ankle eversion. Patients 1, 5, 7, 10, 13, 15, and 16 presented at clinical evaluation with a slight fixed hindfoot varus deformity. Patients underwent our surgical procedure after a mean of 33.3 months (average, 7 months–6 years) from initial trauma. At the time of surgery, all patients wore Codivilla’s orthoses (AFO). Three had a moderate passive ankle dorsiflexion deficit, which required several weeks of manual rehabilitation therapy of the Achilles tendons before surgery.
Table 1.
Patient data
| Patient number | Age (years) | Gender | Causative mechanism of CPN lesion | Duration from trauma to tendon transfer | Previous operations on CPN |
|---|---|---|---|---|---|
| 1 | 11 | M | Crash injuries | 6 years | Neurolysis (FA) |
| 2 | 44 | M | Knee dislocation | 9 months | * |
| 3 | 20 | M | Knee dislocation and femur fracture | 8 months | * |
| 4 | 30 | M | Traffic accident | 2 years | Neurolysis |
| 5 | 30 | F | Knee dislocation | 2 years 5 months | |
| 6 | 32 | M | Traffic accident | 7 months | * |
| 7 | 20 | M | Crush injury and exposed knee fracture | 1 year 6 months | |
| 8 | 24 | M | Traffic accident | 3 years | Nerve graft (FA) |
| 9 | 25 | F | Traffic accident | 2 years | |
| 10 | 19 | F | Traffic accident | 4 years 3 months | |
| 11 | 22 | M | Traffic accident | 2 years 8 months | Nerve graft and island flap (FA) |
| 12 | 26 | F | CPN cut lesion | 2 years 1 month | Direct nerve suture |
| 13 | 27 | M | CPN cut lesion | 4 years | Direct nerve suture |
| 14 | 34 | F | Traffic accident | 4 years | Neurolysis |
| 15 | 22 | M | Traffic accident | 5 years | |
| 16 | 28 | F | CPN cut lesion | 3 years 6 months | Direct nerve graft |
*After CPN exploration at the fibular neck, we proceeded directly to tendon transfer (see text); CPN = common peroneal nerve; M = male; F = female; FA = surgery performed by first author (AV); in the other cases, surgery was performed elsewhere; traffic accident = high-energy trauma involving the knee and/or the proximal aspect of the tibia.
Normal function and strength of foot intrinsic muscles, posterior tibialis and gastrocnemius muscles, FDL, and flexor hallucis longus muscles, together with full ankle and toe passive range of motion were primary inclusion requisites for patients to have surgery.
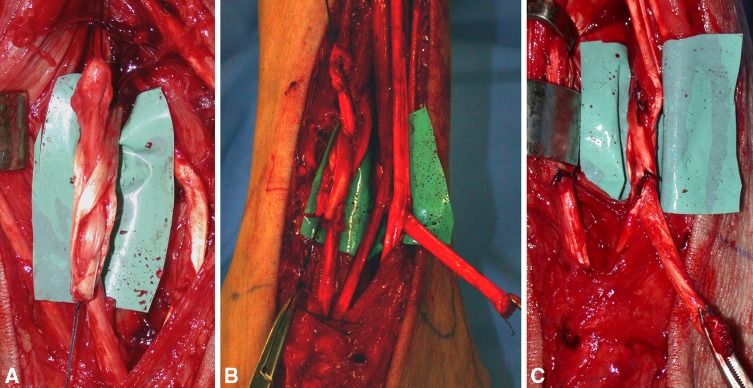
With the patient lying supine, a pneumatic tourniquet was applied to the thigh. An extended retromalleolar medial incision is made to the inferior medial third of the tibia to identify the posterior neurovascular bundle, and then expose PTT and FDL tendons to their muscular junction. The PTT and FDL tendons are divided at the retromalleolar level. A second incision is performed anteriorly and laterally to the tibial crest extended to the dorsum of the foot to identify and expose the anterior vascular bundle, ATT, EDL, and EHL tendons (Figs. 1, 2). The ATT is cut at its muscular junction and dissected to its insertion, and then extracted distally and medially preserving its bony insertion. When the muscular junction of the ATT is too distal or abundant muscular fibers shorten the tendon available for the transfer, we suggest releasing it from the excess of muscular tissue to get a longer portion of tendon to transfer. A 6-mm diameter hole (starting with a 2.5-mm drill) is drilled a few millimeters distally to tendon bony insertion through the cuneiform bones in the direction of the third cuneiform. Use of a preoperative radiograph-detectable marker on skin projection of the third cuneiform and intraoperative use of imaging (C-arm fluoroscope) and a Kirschner wire as a guide are advisable until the surgeon is confident with this technique. Tunnel direction can slightly shift medially or laterally from the third cuneiform to obtain balanced dorsiflexion. When the tunnel is created, care is taken to smooth its entrance and exit rounding off the sharp osseous edges, which could damage the tendon. The ATT then is passed through the osseous tunnel and extracted at that level without damaging the neurovascular anterior bundle (we use an arthroscopy grasper) and pulled proximally under the extensor retinaculum now located in the anterior compartment at the third distal level of the leg with its new origin on the third cuneiform (Figs. 3–5).
Fig. 4A–C.
(A) The tibialis anterior tendon (ATT) can be used entirely or it can be reduced in size by dividing it into two strips. (B) The ATT is folded over the skin (C) highlighting its new direction before the transosseous tunnel is performed.
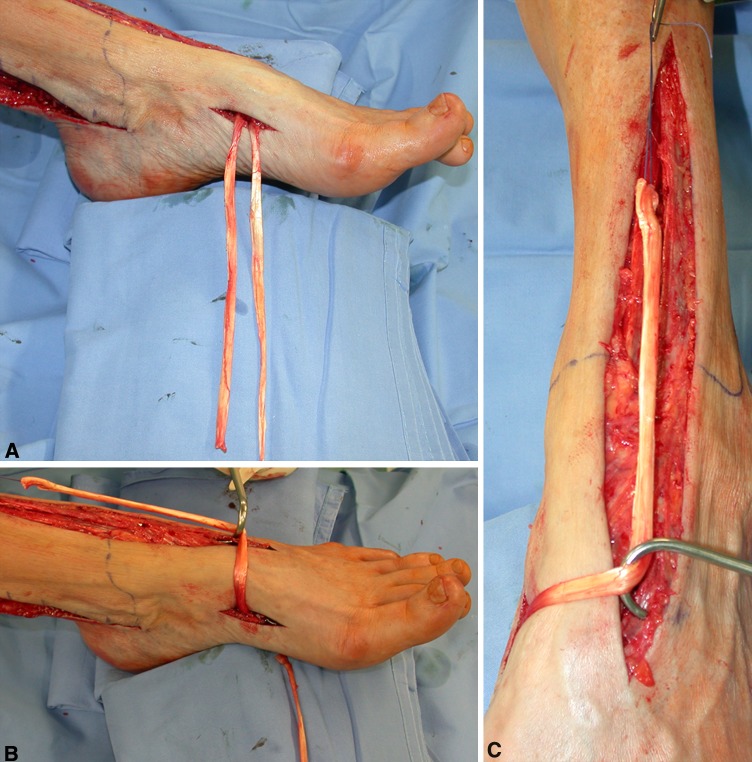
Fig. 1A–B.
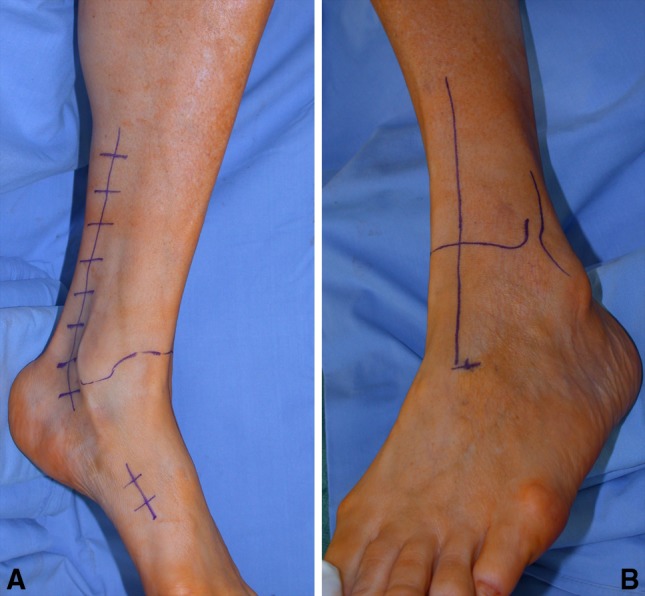
These intraoperative photographs show the skin incisions. (A) The proximal incision is extended from the retromalleolar groove to the inferior third of the leg, whereas the distal incision is limited at the anterior tibialis tendon (ATT) bony insertion. (B) A straight incision is started from the skin projection of the third cuneiform (preoperatively marked) up to the ATT muscular junction.
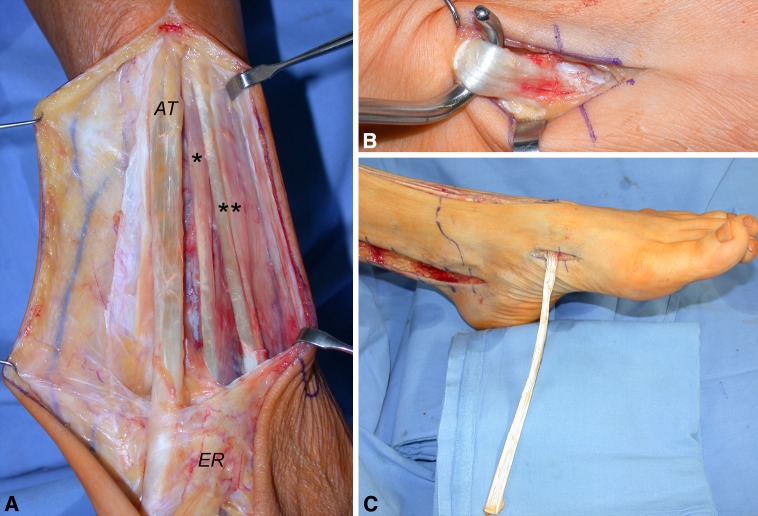
Fig. 2A–C.
These intraoperative photographs highlight the proposed procedure. (A) The tibialis anterior tendon (ATT; AT), extensor hallucis longus tendon (*), and extensor digitorum longus (**) tendons are exposed from muscular junction to the extensor retinaculum (ER). (B) The ATT bony insertion is identified. (C) The ATT is extracted distally.
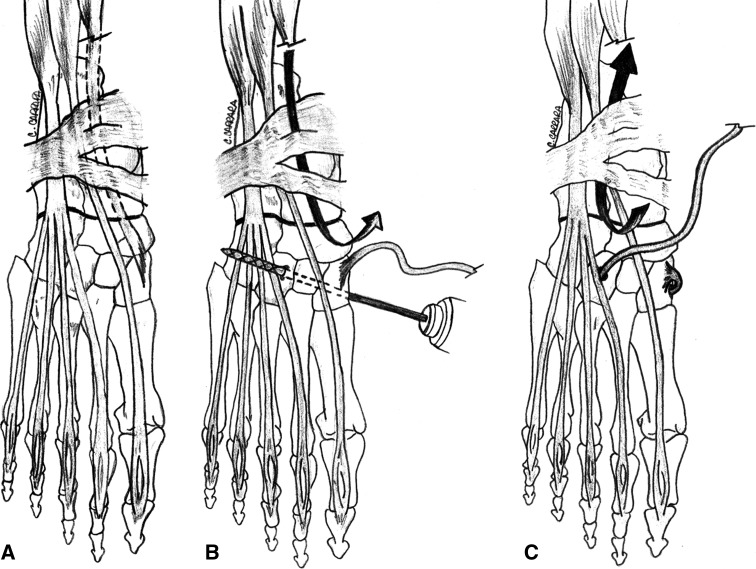
Fig. 3A–C.
A schematic drawing shows the proposed procedure. (A) The anterior tibialis tendon from its bony insertion to the muscular junction is identified. (B) The tendon is extracted distally and medially while a 6-mm drill hole is created from the first to the third cuneiform. (C) The tendon is passed transosseously to its new origin in the third cuneiform and pulled proximally under the retinaculum.
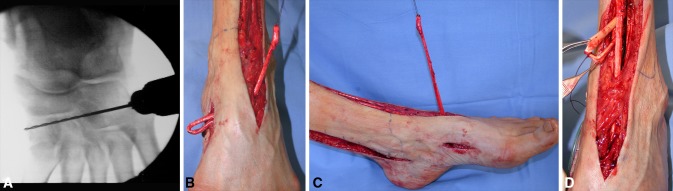
Fig. 5A–D.
(A) A C-arm fluoroscopic image of the drilling procedure from the third to the first cuneiform is shown. (B) The anterior tibialis tendon (ATT) is extracted anteriorly on the tarsus through the osseous tunnel. (C) The new origin of the ATT now is located at the third cuneiform level. (D) The ATT is passed under the extensor retinaculum, whereas the posterior tibialis and flexor digitorum longus tendons are transposed anteriorly through the interosseous membrane.
From the posterior incision, through blunt dissection protecting the neurovascular bundle, we create an interosseous window with an appropriate length, accurately removing remnants of the interosseous membrane and all the soft tissues that could create impingement between and with tendons (the windows preferably are made at the distal portion of the interosseous membrane [13]). Posterior tibialis and FDL tendons are transposed anteriorly avoiding the two tendons twisting on each other. If the muscular junction of the PTT and the FDL tendon are too bulky, to prevent any adherence at the interosseous window and to make tendon gliding easier, we suggest debulking the excess muscular fibers from the tendons taking care not to weaken them.
The ankle is maintained at 90° with digits in slight extension (approximately 20°) while tendon sutures are performed as follows: the PTT is sutured end to end to the ATT and the FDL tendon is sutured side-to-side to the EDL and EHL tendons using the Pulvertaft technique [19] (Fig. 6). Before suturing, it is crucial in this phase to provide the correct tension for the EDL and EHL by evaluating the posture of the toes, avoiding excessive or insufficient traction. After this procedure, the suture falls a few centimeters proximal to the extensor retinaculum, allowing the bulk of the suture to be partially deepened in the surrounding soft tissues and the transferred tendons to glide without impingement on the retinaculum.
Fig. 6A–C.
(A) Pulvertaft’s tendon-to-tendon suturing is performed weaving the anterior tibialis tendon into the posterior tibialis tendon. (B) The flexor digitorum longus tendon is woven into the extensor hallucis longus and EDL tendons. (C) 3–0 absorbable suture is used in this phase.
A nonweightbearing below-knee plaster cast extending to support the toes is applied with the ankle at 90° dorsiflexion and is maintained for 30 days. After removal of the plaster, the patient wears an AFO and remains nonweightbearing for another 20 days. In the meantime, daily and prolonged physiotherapy is started. The AFO is maintained for 2 months until the new motor scheme is corticalized. Full weightbearing walking is permitted after 45 to 50 days.
Followup was performed by the same observer (IM). Patients were asked about their condition before surgery and retrospective chart analysis was done (Table 2). Preoperative and postoperative data were collected according to the Stanmore system questionnaire [30], which was preferred over the American Orthopaedic Foot and Ankle Society scale because it is specifically conceived for outcome evaluation after a tendon transfer technique [10]. The results for the Stanmore grading system were classified as excellent for scores between 85 and 100, good between 70 and 84, fair between 55 and 69, and poor if the score was less than 55. Patients also were asked to rate the results of their surgery using a nonnumeric grading system, in which the categories were excellent, good, fair, and poor (Table 3).
Table 2.
Preoperative evaluation using the Stanmore system (SS) questionnaire and toe dorsiflexion scale
| Modified Stanmore system questionnaire | Points | Patient number | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Pain (15 points) | |||||||||||||||||
| No pain at any time | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |||||||||
| Mild pain | 10 | 10 | 10 | 10 | 10 | 10 | |||||||||||
| Moderate pain | 5 | 5 | 5 | 5 | |||||||||||||
| Severe pain | 0 | 0 | |||||||||||||||
| Need for orthoses (15 points) | |||||||||||||||||
| No | 15 | ||||||||||||||||
| Occasionally (once a week) | 10 | ||||||||||||||||
| Frequently (twice a week) | 5 | ||||||||||||||||
| Regularly (greater than twice a week) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Normal shoes (5 points) | |||||||||||||||||
| Yes | 5 | ||||||||||||||||
| Yes, but prefers certain types | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||
| No | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| Functional outcome (10 points) | |||||||||||||||||
| Normal daily activity and normal recreation | 10 | ||||||||||||||||
| Normal daily activity and limited recreation | 6 | ||||||||||||||||
| Limited daily activity and recreation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||
| Severe limitation on daily activity and recreation | 0 | 0 | 0 | ||||||||||||||
| Muscle power (25 points) (modified Medical Research Council grading) | |||||||||||||||||
| Grade 4+ or 5 | 25 | ||||||||||||||||
| Grade 4 | 20 | ||||||||||||||||
| Grade 3 | 10 | ||||||||||||||||
| Grade 2 or less | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Degree of active dorsiflexion (degrees) | |||||||||||||||||
| Greater than 6° | 25 | ||||||||||||||||
| 0°–5° | 20 | ||||||||||||||||
| −5° to −1° | 10 | ||||||||||||||||
| −10° to −6° | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Less than −11° | |||||||||||||||||
| Foot posture (5 points) | |||||||||||||||||
| Plantigrade, balanced, no deformity | 5 | ||||||||||||||||
| Plantigrade, mild deformity | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Obvious deformity or malalignment | 0 | 0 | |||||||||||||||
| Total score of SS | 16 | 14 | 11 | 24 | 8 | 24 | 0 | 24 | 19 | 19 | 24 | 24 | 24 | 19 | 24 | 19 | |
| Toe dorsiflexion | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | |
Table 3.
Followup and postoperative results
| Parameter | Patients | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Followup (months) | 113 | 27 | 62 | 29 | 83 | 24 | 75 | 35 | 42 | 97 | 37 | 51 | 110 | 56 | 87 | 114 | |
| Stanmore system questionnaire | Points | ||||||||||||||||
| Pain (15 points) | |||||||||||||||||
| No pain at any time or not worse | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | ||
| Mild pain or slightly worse | 10 | 10 | |||||||||||||||
| Moderate pain or markedly worse | 5 | 5 | |||||||||||||||
| Severe pain or markedly worse | 0 | ||||||||||||||||
| Need of orthoses | |||||||||||||||||
| No | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | ||
| Occasional (once a week) | 10 | 10 | |||||||||||||||
| Frequently (twice a week) | 5 | ||||||||||||||||
| Regularly (greater than twice a week) | 0 | 0 | |||||||||||||||
| Normal shoes (5 points) | |||||||||||||||||
| Yes | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||||
| Yes, but prefers certain types | 3 | 3 | 3 | 3 | 3 | ||||||||||||
| No | 0 | ||||||||||||||||
| Functional outcome | |||||||||||||||||
| Normal daily activity and normal recreation | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |||||||||
| Normal daily activity and limited recreation | 6 | 6 | 6 | 6 | 6 | 6 | 6 | ||||||||||
| Limited daily activity and recreation | 3 | 3 | 3 | ||||||||||||||
| Severe limitation on daily activity and recreation | 0 | 0 | |||||||||||||||
| Muscle power (25 points) (modified Medical Research Council grading) | |||||||||||||||||
| Grade 4+ or 5 | 25 | 25 | 25 | 25 | 25 | ||||||||||||
| Grade 4 | 20 | 20 | 20 | 20 | 20 | ||||||||||||
| Grade 3 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |||||||||
| Grade 2 or less | 0 | 0 | |||||||||||||||
| Degree of active dorsiflexion (degrees) | |||||||||||||||||
| Greater than 6° | 25 | 25 | 25 | 25 | |||||||||||||
| 0–5° | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | ||||||
| −5 to −1° | 10 | 10 | 10 | ||||||||||||||
| −10 to −6° | 0 | 0 | |||||||||||||||
| Less than −11° | |||||||||||||||||
| Foot posture | |||||||||||||||||
| Plantigrade, balanced, no deformity | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||
| Plantigrade, mild deformfity | 3 | 3 | 3 | ||||||||||||||
| Obvious deformity or malalignement | 0 | 0 | |||||||||||||||
| Patient Ratings | F | E | G | E | P | E | P | G | G | G | E | E | E | E | E | E | |
| Total Score of SS | 62 | 90 | 74 | 100 | 59 | 100 | 8 | 90 | 70 | 86 | 100 | 86 | 76 | 73 | 80 | 91 | |
| Toes dorsiflexion | 0 | 1 | 0 | 2 | −1 | 1 | −1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 2 | |
E = excellent; G = good; F = fair; P = poor.
Muscle power of dorsiflexion was assessed using the Medical Research Council grading system modified using Seddon’s method [23]. Degree of active dorsiflexion (measured with a goniometer) and muscle function testing also were recorded (Table 3).
Because the Stanmore system was conceived only for evaluation of the PTT and because our surgical reconstruction aimed also to correct the digit drop, we added another evaluation method to the Stanmore system. It consists of visual evaluation of the digits’ posture and active movements at the metatarsophalangeal and proximointerphalangeal joints, in which neutral position is defined as a 180° angle between the metatarsal bone and the first phalanx independently from the posture of the more distal phalanxes. From the neutral position, corresponding to number 0, results were divided into −1, 1, and 2 according to the following patterns: −1 (poor) in which there is drop of the toes with no active extension; 0 (fair) neutral position with no toe drop but no active extension; 1 (good) if from neutral position some toe active extension at the metatarsophalangeal joint is achieved; and 2 (excellent) the same as 1 with some extension of the distal phalanxes.
Baropodometric study completed the postoperative patient evaluation. For this purpose, we used a registering device that was 60 cm in length and 40 cm wide supplied with a 9600 pressure sensor (4 cm2) situated approximately at the distal three-fourths of a platform measuring 5 m in length. Static and dynamic analyses were performed by an independent observer (FZ) who was blinded to the patients’ disorder and any surgery performed.
During static analysis, plantar pressure images were registered and calculated with a bipodalic underload scanner. Dynamic analysis allowed us to study step phases through the acquisition of single-footprint frames, which gave us the progression of the barycenters along the single foot during gait.
Results
In all cases, transosseous rerouting of the ATT provided a sufficient tendon length, which permitted TtT suturing between the ATT and PTT to be performed proximal to the extensor retinaculum eliminating tendon length-related problems of the transfer. The new origin of the ATT at the third cuneiform was confirmed to be the optimal traction line [6] to achieve maximum dorsiflexion with minimal imbalance in accompanying pronation and supination. Thirteen patients (81%) exceeded 0° active dorsiflexion and had, at clinical examination, a balanced foot posture without deformity and although none of the patients had dorsiflexion power graded as 5, eight patients (50%) had grades of 4 or 4+ (Table 3). Fourteen patients (87.5%) showed no toe drop and among these, 11 patients (69%) could actively dorsiflex their toes (Table 3). Eight patients (50%) were able to stand on their heels with no support and two (Patients 4, 11) were able to walk heel-to-heel for a short distance even if a difference could be observed when compared with the contralateral side.
At the time of followup, 14 patients (87.5%) abandoned use of the AFO (Table 3; Figs. 7–9). None of the patients reported pain referred to the osseous tunnel at the dorsum of the foot or at the midfoot during gait and while wearing shoes. Patient 5 had a slight flat foot on the footprint. Patient 3 had digit hyperextension as a consequence of excessive tendon pull during the suture phase and Patient 7’s treatment failed as a result of tendon adherence. At followup, he still needed Codivilla’s orthoses (AFO) and when proposed, he refused triple arthrodesis. Tendon transfer was probably the wrong indication (it was a crush injury with subsequent skin necrosis that required a free flap).
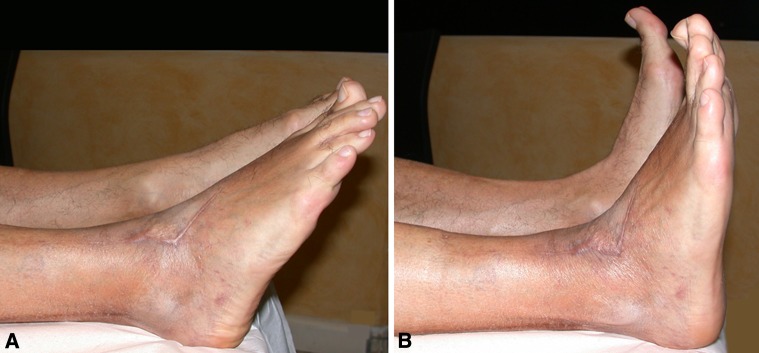
Fig. 8A–B.
The surgical procedure was performed on the right foot (Patient 4). (A) Ankle plantar and (B) dorsal flexion show normal range of motion compared with the contralateral side. The bulk of the suture is hidden in the soft tissues at the inferior third of the leg.
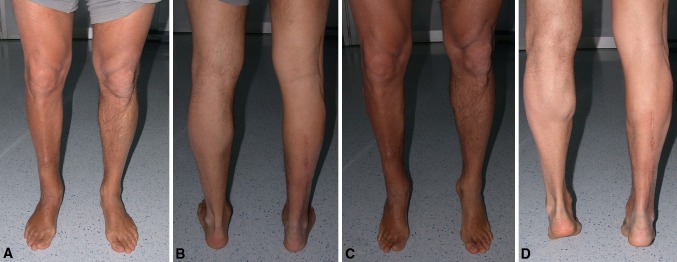
Fig. 7A–D.
The surgical procedure was performed on the right foot (Patient 4). (A) Front and (B) back photographs show no forefoot or hind-foot deformities. The tendon transfer does not compromise ankle plantar flexion against gravity as seen in these (C) front and (D) back views.
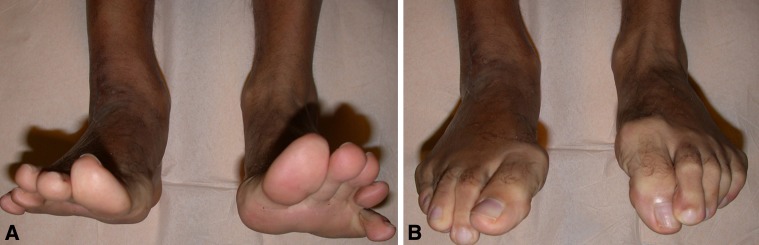
Fig. 9A–B.
For Patient 4, (A) toe plantar flexion is normal compared with the contralateral side, having preserved the interosseous and intrinsic muscles of the foot. (B) Toe dorsiflexion is possible at the metacarpal and proximal phalangeal joints.
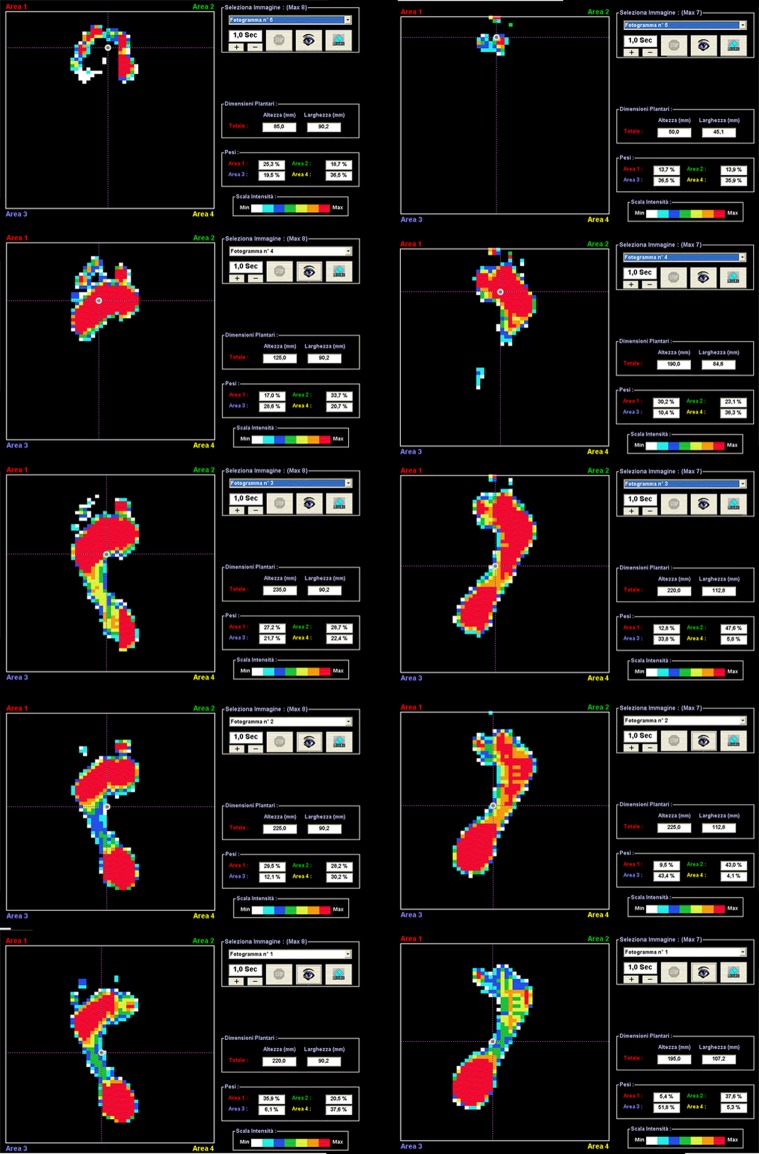
Static footprint analysis showed, in all but one patient (Patient 5), the absence of flat foot, metatarsal external overload, and/or forefoot adduction. The patient’s postural balance gained global improvement as a result of the reduction of compensation in maintaining bipodalic station (Fig. 10). Dynamic analysis showed an overall satisfying progression of gait characterized by the absence of external overload in toe plantar flexion and by reduction of foot contact time with the ground (stance phase) with improvement of heel contact and pushoff phase with evidence of a longer step. This positively affects dynamicity of the gait cycle, because the increase of ankle plantar and dorsiflexion range of motion produces elongation of the sural triceps muscle improving pushoff phase power and venous return for compression of deep veins (Fig. 11) (Videos 1 and 2, Supplemental Website Materials; supplemental materials are available with the online version of CORR).
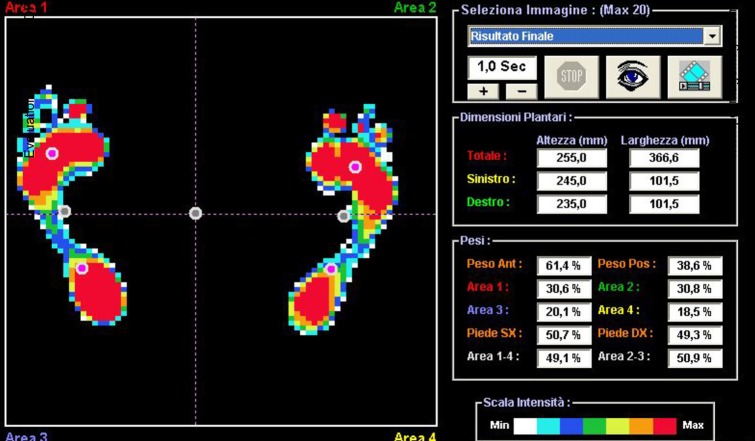
Fig. 10.
A plantar pressure image for the right foot of Patient 4 shows the static footprint is excellent without any incongruences in weight distribution between the right (50.3%) and left (49.7%) feet. No flatfoot, no overload at the external metatarsal bones, and no adduction and/or supination of the forefoot are present in the right foot. Round spots represent the seven barycenters, six of the feet and one corporeal (central spot).
Fig. 11.
A dynamic evaluation of the right foot of Patient 4 is shown. Single footprint frames of both feet during gait do not show any substantial alteration in any phase of the gait.
According to the Stanmore system rating, the final results were excellent in eight (50%), good in five (31.2%), fair in two (12.5%), and poor in one (6.2%), with an average score of 77.8 representing a good overall result (the preoperative average score was 18.31). Results of patient ratings were excellent in nine (56.2%), good in four (25%), fair in one (6.2%), and poor in two (12.5%) (Figs. 7–9).
Discussion
The objectives of tendon transfer for treatment of posttraumatic paralysis are (1) to improve functional deficit by restoring or reinforcing lost functions; (2) to neutralize deforming forces; and (3) to gain stability eliminating the need for bracing during gait [17, 28]. These objectives can be achieved by static and dynamic surgical procedures. The former (arthrodesis, osteotomy, tenodesis) changes uncorrected fixed and nonfunctional postures into more functional postures and results in better overall function but does not restore lost movements. Generally, static procedures are used as a support or after failure of dynamic procedures and/or when dynamic procedures are not indicated (severe articular incongruence, wide traumatic palsy, cerebropathy, neuropathy, etc). Dynamic surgical procedures act by transferring functional muscles or changing their osseous insertions, improving function, and, most of all, restoring lost movement. In the last 30 years, indications for arthrodesis have narrowed, limited mainly to cases of cerebral and neuromuscular disease. Tendon transfers are preferred particularly for treatment of posttraumatic nerve palsy [12, 22]. For common peroneal nerve palsy the functions that must be restored are foot dorsiflexion, supination, hallux and toe dorsiflexion, avoiding a steppage gait, correcting equinus and varus deformities, and digit drop. The tendon transfer is more effective if dynamic, voluntary movement is achieved rather than just a static tenodesis effect [4, 29]. To make a transfer functional, some authors [3, 8, 20] claim the tension between the transposed muscle and sutured tendon, or tendon attachment into bone, must be under slight to moderate tension, or even under high tension [1], with the foot held at 90°. When the tension is greater than necessary, as could happen when the PTT harvest is not long enough to reach the desired insertion, the surgeon is forced to maximally dorsiflex the ankle. This limits tendon excursion which causes the transfer to act more like a tenodesis than a dynamic transfer [21]. Tenodesis effect is more likely in patients who have difficulties cooperating in a rehabilitation program (ie, older patients) or in cases in which the surgical technique results in tendon adherence. Ideally, the length of the transferred tendon should be sufficient to allow full, passive, plantar flexion permitting the potential for normal range of motion [21]. Before suturing the tendons, free gliding of the PTT and FDL tendons through the interosseous membrane must be ensured. If necessary, corrections should be made such as widening the interosseous membrane, removal of excess muscle bulk that impinges on the interosseous window, or checking to be sure that the two tendons are not twisted on each other.
The novelty of our proposed technique is that of moving the insertion of the recipient tendon (ATT) toward the donor transferred tendon (PTT) and not the contrary. By creating a new ATT origin on the third cuneiform, we bring the recipient tendon directly up to the PTT solving tendon harvest length problems and preserving a straight line of pull. This has the potential to better preserve the strength of the transfer which other techniques may sacrifice when the PPT is split into two tails and sutured to other tendons [22, 24, 27, 29].
In our series, eight patients (50%) had dorsiflexion strength graded as 4 or 4+, which is uncommon in tendon transfer procedures. We believe these good results could be related to the direct TtT suture performed between the ATT and PTT that produces straight traction at the third cuneiform strengthened by the associated transfer [3] of the FDL on the EDL-EHL tendons. Tendon to tendon suture, introduced in 1968 [24], simultaneously addresses the difficulties related to TtB procedures and donor tendon length. It allows the surgeon to adjust and modulate tendon tension, appropriately verifying foot and digit posture before suturing is completed [24] for the FDL on EDL-EHL tendons and the PTT on ATT. The technique also can be used for terminolateral and terminoterminal tendon suturing allowing multiple tendon reconstruction.
The overall advantages of the new technique might be summarized as follows: (1) the rerouted ATT has a sufficient length to allow TtT suturing with the PTT. The suturing is performed not as usually happens at the dorsal aspect of the foot, but at the inferior third of the leg where the bulk of the suture can be easily deepened into soft tissues; (2) extraction of the rerouted ATT at the tarsus (ATT new origin) can be shifted medially or laterally according to the correction called for. Medial extraction can be indicated when peroneus tendons are functioning in contrast to their lateral pull. In total CPN palsy, we suggest extracting the tendon at the third cuneiform level; (3) using this technique, suturing the FDL tendon can be performed in a terminolateral fashion creating, according to the reconstructive need, a solid and variable tension construct. This also permits avoiding not only drop of the toes, but also allows some extension of the hallucis; (4) transfer of the FDL tendon improves the power of foot dorsiflexion compared with transfer of the PTT alone and allows active dorsiflexion of the toes. During the dissection, it is mandatory to preserve the flexor hallucis longus, which plays an important role during the propulsive phase of gait.
Sacrifice of both tendons (PTT, FDL) in our experience did not produce any deficits in terms of power and flexion of the ankle and toes.
Contraindications to tendon transfer through the interosseous route are crush injuries, bone fractures, and soft tissue lesions at the distal half of the leg creating an increased risk of tendon adherence. Development of a flat foot, as in Patient 5, seems to not be related specifically to the technique used [29]. We are not able to comment on this phenomenon as sacrifice of the sole PTT for treatment of CPN palsy usually does not result in an acquired flat foot [15] which happens in a normal foot. The reason for this may be that in the normal foot, the primary evertor of the foot, the peroneus brevis muscle [14], is unopposed in contrast to the palsied foot where loss of peroneus brevis function (as a result of nerve palsy) and loss of the PTT (as a result of tendon transfer) result in a new dynamic balance [15].
For treatment of complete CPN palsy, transosseous rerouting to the third cuneiform of the ATT and dual transfer of the PTT and FDL tendons is a reliable method to restore balanced foot and toe dorsiflexion producing a normal gait without the need for orthoses. In future studies we hope to show that this technique improves the patients’ endurance during gait, helping to avoid fatigue-related stride asymmetry. In addition we hope to show that alterations in the insertion site of the ATT on the dorsum of the foot allows the surgeon to address a wide variety of muscle imbalances unhindered by the length of the tendon available.
Electronic supplementary material
For Patient 4, the surgical procedure was performed on the right foot . The patient walks over a baropodometric platform. Before walking, the patient is asked to stand on tiptoe. During gait, ankle dorsiflexion is well balanced and toe movements are functionally and aesthetically satisfying. At the end, the patient is asked to dorsiflex the ankle greater than 90°. (MPG 4738 kb)
For Patient 11, the surgical procedure was performed on the left foot. The video is registered during one of the clinical assessments in our division laboratory. During gait, ankle dorsiflexion is well balanced and toe movements are functionally and aesthetically satisfying. (MPG 1526 kb)
Acknowledgments
We thank “Il Podologo srl” in the person of Zucchini Franco for baropodometric evaluation and Carrara Claudio for the drawings.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Andersen JG. Indications and contra-indications in reconstructive surgery in leprosy. Lepr Rev. 1963;34:127–131. doi: 10.5935/0305-7518.19630021. [DOI] [PubMed] [Google Scholar]
- 2.Birch R, Bonney G, Wynn Parry CB. Surgical Disorders of the Peripheral Nerves. London, England: Churchill-Livingstone; 1988. pp. 235–243. [Google Scholar]
- 3.Carayon A, Bourrel P, Bourges M, Touze M. Dual transfer of the posterior tibial and flexor digitorum longus tendons for drop foot: report of thirty-one cases. J Bone Joint Surg Am. 1967;49:144–148. [PubMed] [Google Scholar]
- 4.De Marchi F, Malerba F, Montrasio Alfieri U, Ferrarin M, Rabufetti M. Tibialis posterior tendon transfer through the interosseal membrane in paralysis of the common peroneal nerve. Foot Ankle Surg. 2000;6:19–25. doi: 10.1046/j.1460-9584.2000.00184.x. [DOI] [Google Scholar]
- 5.Garland DE, Hughston JC. Peroneal nerve paralysis: a complication of extensor reconstruction of the knee. Clin Orthop Relat Res. 1979;140:169–171. [PubMed] [Google Scholar]
- 6.Goh JC, Lee PY, Lee EH, Bose K. Biomechanical study on tibialis posterior tendon transfers. Clin Orthop Relat Res. 1995;319:297–302. [PubMed] [Google Scholar]
- 7.Green DP. Radial nerve palsy. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, editors. Green’s Operative Hand Surgery. Philadelphia, PA: Elsevier Inc Churchill-Livingstone; 2005. pp. 1113–1129. [Google Scholar]
- 8.Hove LM, Nilsen PT. Posterior tibial tendon transfer for drop-foot: 20 cases followed for 1–5 years. Acta Orthop Scand. 1998;69:608–610. doi: 10.3109/17453679808999265. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Kline DG. Management and results of peroneal nerve lesions. Neurosurgery. 1996;39:312–310. doi: 10.1097/00006123-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 11.Kline DG, Hudson AR. Lower extremity nerves. In: Kline DG, Hudson AR, editors. Nerve Injuries. Philadelphia, PA: WB Saunders; 1995. pp. 316–323. [Google Scholar]
- 12.Lipscomb PR, Sanchez JJ. Anterior transplantation of the posterior tibial tendon for persistent palsy of the common peroneal nerve. J Bone Joint Surg Am. 1961;43:60–66. [Google Scholar]
- 13.Mallet J. Transplantation du jambier postérieur dans le paralysie du SPE. Chirurgie. 1975;101:909–912. [PubMed] [Google Scholar]
- 14.Mann RA, Thompson FM. Rupture of the posterior tibial tendon causing flat foot: surgical treatment. J Bone Joint Surg Am. 1985;67:556–561. [PubMed] [Google Scholar]
- 15.Mizel MS, Temple HT, Scranton PE, Jr, Gellman RE, Hecht PJ, Horton GA, McCluskey LC, McHale KA. Role of the peroneal tendons in the production of the deformed foot with posterior tibial tendon deficiency. Foot Ankle Int. 1999;20:285–289. doi: 10.1177/107110079902000502. [DOI] [PubMed] [Google Scholar]
- 16.Mont M, Dellon AL, Chen F, Hungerford MW, Krackow KA, Hungerford DS. The operative treatment of peroneal nerve palsy. J Bone Joint Surg Am. 1996;78:863–869. doi: 10.1302/0301-620X78B6.6714. [DOI] [PubMed] [Google Scholar]
- 17.Mulier T, Moens P, Molonaers G, Spaepen D, Dereymaeker G, Fabry G. Split posterior tibial tendon transfer through the interosseous membrane in spastic equinovarus deformity. Foot Ankle Int. 1995;16:754–759. doi: 10.1177/107110079501601203. [DOI] [PubMed] [Google Scholar]
- 18.Ninkovic M, Sucur D, Starovic B, Markovic S. A new approach to persistent traumatic peroneal nerve palsy. Br J Plast Surg. 1994;47:185–189. doi: 10.1016/0007-1226(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 19.Pulvertaft RG. Suture materials and tendon junctures. Am J Surg. 1965;109:346–352. doi: 10.1016/S0002-9610(65)80083-6. [DOI] [PubMed] [Google Scholar]
- 20.Reidy JA, Broderick TF, Barr JS. Tendon transplantations in the lower extremity: a review of end results in poliomyelitis. I. Tendon transplantations about the foot and ankle. J Bone Joint Surg Am. 1952;34:900–908. [PubMed] [Google Scholar]
- 21.Richard BM. Interosseous transfer of tibialis posterior for common peroneal nerve palsy. J Bone Joint Surg Br. 1989;71:834–837. doi: 10.1302/0301-620X.71B5.2555372. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez RP. The Bridle procedure in the treatment of paralysis of the foot. Foot Ankle. 1992;13:63–69. doi: 10.1177/107110079201300203. [DOI] [PubMed] [Google Scholar]
- 23.Seddon HJ. Surgical Disorders of the Peripheral Nerves. 2. Edinburgh: Churchill Livingstone; 1975. Results of repairs of nerves; pp. 303–307. [Google Scholar]
- 24.Srinivasan H, Mukherjee SM, Subramaniam RA. Two-tailed transfer of tibialis posterior for correction of drop foot in leprosy. J Bone Joint Surg Am. 1968;50:623–628. [PubMed] [Google Scholar]
- 25.Tubiana R. Transferts tendineux. Considération pratiques. In: Tubiana R, ed. Traite de Chirurgie de la Main. Vol 4. Paris, France: Masson; 1991:81–95.
- 26.Watkins MB, Jones JB, Ryder CT, Jr, Brown TH., Jr Transplantation of the posterior tibial tendon. J Bone Joint Surg Am. 1954;36:1181–1189. [PubMed] [Google Scholar]
- 27.Wiesseman GJ. Tendon transfers for peripheral nerve injuries of the lower extremity. Orthop Clin North Am. 1981;12:459–467. [PubMed] [Google Scholar]
- 28.Wood MB. Peripheral nerve injuries to the lower extremity. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction. Philadelphia, PA: JB Lippincott Co; 1991. pp. 489–504. [Google Scholar]
- 29.Yeap JS, Birch R, Singh D. Long-term results of tibialis posterior tendon transfer for drop-foot. Int Orthop. 2001;25:114–118. doi: 10.1007/s002640100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeap JS, Singh D, Birch R. A method for evaluating the results of tendon transfers for foot drop. Clin Orthop Relat Res. 2001;383:208–213. doi: 10.1097/00003086-200102000-00024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For Patient 4, the surgical procedure was performed on the right foot . The patient walks over a baropodometric platform. Before walking, the patient is asked to stand on tiptoe. During gait, ankle dorsiflexion is well balanced and toe movements are functionally and aesthetically satisfying. At the end, the patient is asked to dorsiflex the ankle greater than 90°. (MPG 4738 kb)
For Patient 11, the surgical procedure was performed on the left foot. The video is registered during one of the clinical assessments in our division laboratory. During gait, ankle dorsiflexion is well balanced and toe movements are functionally and aesthetically satisfying. (MPG 1526 kb)