Abstract
Bone cements loaded with combinations of antibiotics are assumed more effective in preventing infection than bone cements with gentamicin as a single drug. Moreover, loading with an additional antibiotic may increase interconnectivity between antibiotic particles to enhance release. We hypothesize addition of clindamycin to a gentamicin-loaded cement yields higher antibiotic release and causes larger inhibition zones against clinical isolates grown on agar and stronger biofilm inhibition. Antibiotic release after 672 hours from Copal bone cement was more extensive (65% of the clindamycin and 41% of the gentamicin incorporated) than from Palacos R-G (4% of the gentamicin incorporated). The higher antibiotic release from Copal resulted in a stronger and more prolonged inhibition of bacterial growth on agar. Bacterial colony counting and confocal laser scanning microscopy of biofilms grown on the bone cements suggest antibiotic release reduced bacterial viability, most notably close to the cement surface. The gentamicin-sensitive Staphylococcus aureus formed gentamicin-resistant small colony variants on Palacos R-G and therefore Copal more effectively decreased biofilm formation than Palacos R-G.
Introduction
Several studies report the effectiveness of antibiotic-loaded bone cement in primary and revision arthroplasties [3, 7, 9, 10, 11]. Gentamicin is the most frequently used antibiotic for loading bone cement because it has a broad antimicrobial spectrum and it can withstand the high temperatures reached during polymerization of the bone cement. Although gentamicin-loaded bone cement has been used for decades, its antibiotic release is not very effective because maximally 15% of the total amount of antibiotic incorporated is released [12, 33], and release is poorly controlled and often confined to a high initial release burst followed by an extremely low release [6], lingering for several weeks. Low levels of antibiotic release from bone cements have been reported years after implantation [31], but this long-lasting low antibiotic release is not considered therapeutically effective. More important, release of subinhibitory concentrations with time theoretically can contribute to the development of gentamicin resistance among infecting bacteria [21, 28].
Musher reported that nearly 50% of the staphylococci in prosthesis-related infections are gentamicin-resistant [19]. Occurrence of gentamicin-resistant bacterial strains in prosthesis-related infections has led to the development of antibiotic-loaded bone cements in which gentamicin is combined with a second antibiotic [15, 25, 34]. A combination of antibiotics is not only expected to broaden the antimicrobial spectrum of the cement, but also may reduce the occurrence of antibiotic resistance [18]. Copal (Biomet Merck, Darmstadt, Germany) is a new commercially available bone cement in Europe designed for revision cases and possesses a similar polymer matrix as Palacos R-G bone cement (Schering-Plough, Maarssen, The Netherlands), which was FDA-approved in 2003. Copal contains twice the amount of gentamicin as Palacos R-G and has clindamycin added. One report suggests a combination of gentamicin with clindamycin would act synergistically and kill greater than 90% of the bacterial strains involved in common prosthesis-related infections [13].
We hypothesized addition of clindamycin to gentamicin-loaded cement yields higher antibiotic release and results in larger inhibition zones against clinical isolates grown on agar, and causes stronger biofilm inhibition.
Materials and Methods
We made bone cement discs of two commercially available gentamicin-loaded bone cements: Palacos R-G (0.5 g gentamicin base per batch of cement) and Copal (1 g gentamicin base and 1 g clindamycin base per batch of cement). To investigate whether Copal increased antibiotic release when compared with Palacos R-G, samples of both bone cements were immersed in buffer and the antibiotic concentration was measured at different times. Possible larger inhibition zones by Copal were qualitatively inferred from growth inhibition on agar plates created by both bone cements at different intervals. Possible increased biofilm inhibition of Copal was investigated with bacterial plate counting and with a confocal laser scanning microscope after 1 and 7 days of growth on samples of both cements. Twelve discs from each gentamicin-loaded cement were grouped into four groups of three discs: one group of three discs was used for the release studies, one group of discs was used to study the antibacterial efficacy, one group of three discs was used for bacterial plate counting to evaluate the number of living bacteria in biofilms on samples of both cements, and the last group was used to observe the biofilms on samples of both cements.
We used commercially available, antibiotic-loaded Copal bone cement containing 1.62 w/w% gentamicin base and 1.62 w/w% clindamycin base and Palacos R-G containing 0.84 w/w% gentamicin base. Palacos R (Schering-Plough), containing no antibiotics, was included as a control (Table 1). We performed mixing and preparation of the bone cements under sterile conditions according to the manufacturers’ instructions. Cements were prepared by mixing the powdered methylmethacrylate with the liquid monomer in a bowl with a spatula under atmospheric pressure. We spread the doughy bone cement in a polytetrafluoroethylene mold after which the mold was compressed between two glass plates and covered with copier overhead film (MC 110, Océ, The Netherlands) to facilitate removal after hardening. We manually compressed the glass plates up to the time specified for final hardening and left them in place for at least 24 hours. This resulted in cylindrical bone cement samples of 1.2 cm2 with a diameter of 6 mm and a height of 3 mm. The average weight of a Copal disc was 129 ± 7 mg and 122 ± 2 mg for a Palacos R-G disc.
Table 1.
Composition of Palacos R-G and Copal bone cement provided by manufacturers
| Components | Palacos R-G | Copal |
|---|---|---|
| Powder | 40.8 g | 42.85 g |
| Gentamicin sulphate | 0.84 g (0.5 g gentamicin) | 1.67 g (1 g gentamicin) |
| Clindamycin hydrochloride | – | 1.18 g (1 g clindamycin) |
| Methylmethacrylate | 33.8 g | 35.41 g |
| Benzoyl peroxide | 0.2 g | 0.32 g |
| Zirconium dioxide | 6.0 g | 4.27 g |
| Chlorophyll | 0.008 g | * |
| Liquid | ||
| Methylmethacrylate | 18.4 g | 18.4 g |
| N,N-dimethyl-p-toluidine | 0.4 g | 0.38 g |
| Chlorophyll | 0.005 g | * |
| Hydroquinone | † | † |
*Chlorophyll copper complex E 141; †amount not quantified.
We first studied antibiotic release of Palacos R-G and Copal bone cement. To be able to detect a true difference of 600 μg/cm2 with a power of 90%, assuming a standard deviation of 5 μg/cm2 in the Palacos group and 150 μg/cm2 in the Copal group, we needed three samples in each group. We immersed three samples of each Palacos R-G and Copal bone cement in 20 mL phosphate-buffered saline (NaCl 8.76 g/L, K2HPO4 0.87 g/L, KH2PO4 0.68 g/L; pH 7.0) at 37° C. Each of the three samples was put in separate 20-mL volumes of buffer and at designated times (6, 24, 72, 168, 336, 504, 672 hours), 0.5-mL aliquots were taken and the gentamicin concentration in these aliquots was measured by fluorescence polarization immunoassay (AxSYM; Abbott Laboratories, Abbott Park, IL). We determined the clindamycin concentration as released from Copal bone cement by high-performance liquid chromatography.
Second, we investigated the direct inhibitory effect on bacterial growth of Palacos R-G and Copal bone cement with time. We immersed bone cement samples in separate 20-mL volumes of phosphate-buffered saline and two samples of each bone cement were removed after 6, 24, 72, 168, 336, 504, and 672 hours. After removal, the samples were air-dried and pressed firmly in the center of a Tryptone Soya Broth (TSB; Oxoid, Basingstoke, UK) agar plate inoculated with a gentamicin-sensitive Staphylococcus aureus 7323 (minimum inhibitory concentration [MIC] gentamicin, 0.75 μg/mL; MIC clindamycin, 0.25 μg/mL) or a gentamicin-resistant coagulase-negative staphylococcus (CNS) 5277 (MIC gentamicin greater than 256 μg/mL; MIC clindamycin, 0.032 μg/mL). Both strains were clinical isolates retrieved from infected joint prostheses and cultured from cryopreservative beads (Protect Technical Service Consultants Ltd, Heywood, UK) onto blood agar plates at 37° C in ambient air for 24 hours, after which the cultures were suspended in NaCl 0.9% to a concentration of 108 bacteria/mL. This suspension was used to inoculate TSB agar plates. We pressed bone cement samples in the agar 10 minutes after inoculation, after which the plates were incubated aerobically at 37° C. We qualitatively inferred inhibitory effects from the zones of inhibition around the bone cement samples after 24 hours incubation.
Bacteria in a biofilm have a profoundly reduced susceptibility to antibiotic treatment and, therefore, an ideal antibiotic-loaded carrier should not only have effective bacterial growth inhibition, but also should inhibit biofilm formation [27]. Therefore, as a final experiment, we explored the inhibitory effects of Palacos R-G and Copal bone cement on biofilm formation. We took one colony of each strain from a blood agar plate to inoculate a preculture in 10 mL TSB, which was grown aerobically at 37° C for 24 hours. Then, 100 μL of this preculture was used to inoculate 10 mL TSB. We placed a bone cement sample of Palacos R, Palacos R-G, or Copal bone cement into the TSB to allow biofilm formation on the cement samples. Biofilms were evaluated after 1 or 7 days. In the formation of 7-day biofilms, TSB was refreshed every 2 days.
To determine the number of viable bacteria in the biofilms, we placed bone cement samples in separate test tubes with 2 mL of NaCl 0.9% solution, vortexed them for 10 seconds, and placed them in an ultrasonic bath for 60 seconds to remove the biofilm. The NaCl solution with bacteria subsequently was diluted serially and 100 μL of these dilutions was plated on TSB agar plates and incubated for 24 hours, after which the number of bacteria was counted. We performed this experiment three times with different precultures.
We used confocal laser scanning microscopy (CLSM) to observe the biofilm architecture because it allows nondestructive examination. By moving the sample in the z-direction, stacks of optical sections can be generated, which can be used to localize labeled structures in three dimensions and consequently provide a better understanding of the biofilm structure. To analyze the biofilms with CLSM, we took one sample of each group out of the TSB and stained them with 5 μL of LIVE/DEAD Baclight viability stain (Molecular Probes Europe BV, Leiden, The Netherlands) containing SYTO 9 dye (fluorescent green) and propidium iodide (fluorescent red) to differentiate between living and dead bacteria, respectively [26]. In addition, we stained biofilms with 5 μL calcofluor white (0.1 mmol/L; fluorescent blue), a polysaccharide-binding dye used to visualize the extracellular polymeric substance (EPS). Confocal images were collected using a Leica TCS-SP2 CSLM (Leica Microsystems Heidelberg GmbH, Heidelberg, Germany) with beam path settings for fluorescein isothiocyanate, tetramethylrhodamine isothiocyanate, and 4′-6-diamidino-2-phenylindole labels. We collected a cross section of the biofilm with a x40 objective, and images were obtained at approximately 1- to 2-μm intervals down through the biofilm. The number of images, therefore, corresponded with the thickness of the biofilm.
To compare antibiotic release and the number of living bacteria in biofilms on samples of Copal and Palacos R-G bone cement, we performed pairwise comparisons using the Student’s t-test for independent samples. The number of study units was three for all experiments and a 95% (p < 0.05, two-tailed) confidence interval was applied for significance.
Results
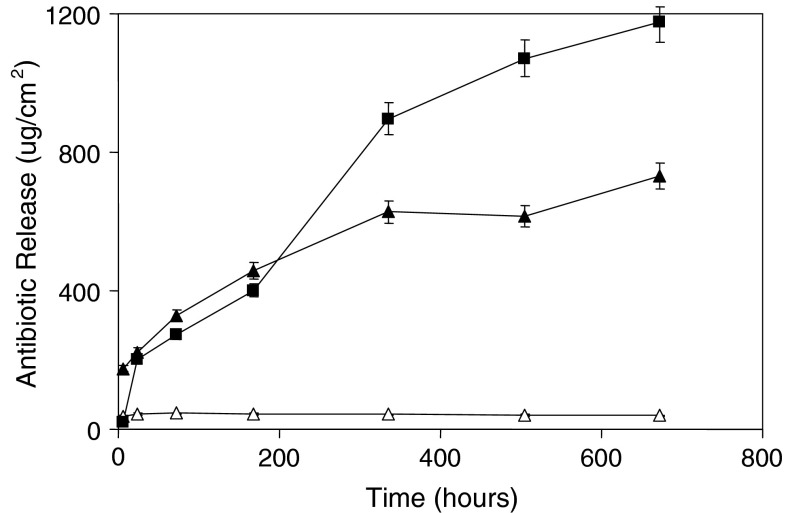
Addition of clindamycin to gentamicin-loaded cement enhances antibiotic release. At all times (Fig. 1), gentamicin release from Copal was higher (p = 0.002) than from Palacos R-G. Sixty-five percent of the clindamycin and 41% of the gentamicin incorporated in Copal were released after 672 hours, whereas only 4% of the gentamicin incorporated in Palacos R-G was released. Gentamicin release from Palacos R-G stopped almost completely after the first 24 hours, but antibiotic release from Copal continued for at least 672 hours.
Fig. 1.
Cumulative gentamicin and clindamycin concentrations in 20 mL phosphate-buffered saline as a function of time after release from Palacos R-G (open symbols) and Copal (closed symbols) bone cement are shown. The triangles denote gentamicin release, whereas the squares indicate clindamycin release. The error bars denote the average standard deviation over three experiments performed per group. Copal increased antibiotic release compared with Palacos R-G.
Qualitative examination showed Copal more strongly inhibits growth on agar plates at all times when compared with Palacos R-G (Table 2). Palacos R-G initially was effective in inhibiting growth of a gentamicin-sensitive S. aureus, but after 72 hours of elution, we no longer observed inhibition. In comparison, Copal yielded a stronger and more prolonged bacterial inhibition for at least the duration of the experiment (672 hours). Palacos R-G did not inhibit bacterial growth of the gentamicin-resistant CNS. In contrast, Copal inhibited growth of the gentamicin-resistant CNS at all times after elution.
Table 2.
Antibacterial efficacy as established using the modified Kirby-Bauer test*
| Time after elution (hours) | 0 | 6 | 24 | 72 | 168 | 336 | 504 | 672 |
|---|---|---|---|---|---|---|---|---|
| Palacos R-G | ||||||||
| Staphylococcus aureus 7323 | + | ± | ± | ± | − | − | NT | NT |
| Coagulase-negative staphylococcus 5277 | − | − | − | − | − | − | NT | NT |
| Copal | ||||||||
| Staphylococcus aureus 7323 | ++ | + | + | + | + | + | + | + |
| Coagulase-negative staphylococcus 5277 | ++ | ++ | ++ | ++ | + | + | + | + |
* Inhibition zones against a gentamicin-sensitive Staphylococcus aureus 7323 and a gentamicin-resistant coagulase-negative staphylococcus 5277 strain for Palacos R-G and Copal bone cement at different times after elution; sensitivity is recorded with modified Kirby-Bauer test if an inhibition zone of at least 3 mm is present around the cement sample; therefore, zones with a width 3 mm or less were scored with (−), zones between 4 and 10 mm were scored with (±), zones between 11 and 20 mm were scored with (+), and zones larger than 21 mm were scored with (++); modified Kirby-Bauer test was performed in duplicate; NT = not tested.
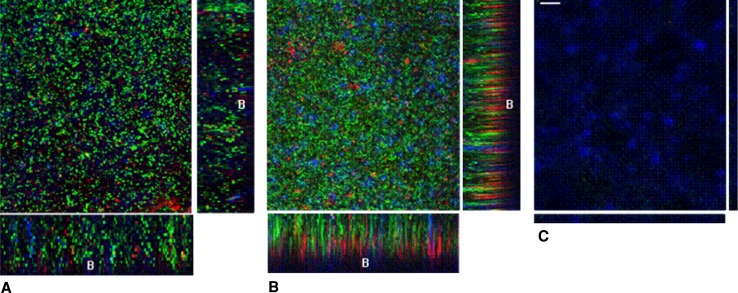
Addition of clindamycin to gentamicin-loaded cement had an additional effect on biofilm inhibition as observed with CLSM and after determination of the number of viable bacteria in the biofilms. Copal increased inhibition of 1- (p = 0.031) and 7-day-old (p = 0.044) S. aureus biofilms as compared with Palacos R and Palacos R-G (Table 3). Analysis by CLSM of the cross-sectional buildup of S. aureus biofilms (Fig. 2) indicated thicker biofilms after 7 days on Palacos R (±100 μm) than on Palacos R-G (± 90 μm) and Copal (± 20 μm). The biofilm on Palacos R-G bone cement had a high density of live bacteria compared with the low density or total absence of live bacteria on Copal bone cement. Moreover, the dead bacteria present in the biofilm on Palacos R-G bone cement were located on the bone cement surface, where the concentration of released antibiotic was the highest. For gentamicin-resistant CNS, the average number of viable bacteria after Day 1 was lower (p = 0.006) on Palacos R-G (23 × 103 CFU/cm2) and Copal (35 × 103 CFU/cm2) than on Palacos R (11 × 106 CFU/cm2) with no difference between both gentamicin-loaded bone cements. On Day 7, viable counts on Palacos R (10 × 107 CFU/cm2) and Palacos R-G (16 × 107 CFU/cm2) had increased (p = 0.008), whereas the number of bacteria on Copal had decreased (p = 0.001) to below detection. Analysis by CLSM of the cross-sectional buildup of CNS biofilms (data not shown) indicated the biofilms were approximately three- to fourfold thinner than S. aureus biofilms.
Table 3.
Number of bacteria (10log CFU/cm2) in 1- and 7-day-old Staphylococcus aureus 7323 and Coagulase-negative Staphylococci 5277 Biofilms*
| 10Log CFU/cm2 | Staphylococcus aureus 7323 | Coagulase-negative staphylococcus 5277 | ||
|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | |
| Palacos R | 8.1 ± 0.2 | 8.0 ± 0.2 | 7.0 ± 0.3 | 8.0 ± 0.1 |
| Palacos R-G | 7.0† ± 0.4 | 8.3† ± 0.1 | 4.4 ± 0.3 | 8.2 ± 0.2 |
| Copal | 3.1 ± 0.6 | 0 ± 0 | 4.6 ± 0.2 | 0 ± 0 |
* Bacterial growth results are averages ± standard deviations from three experiments with separately cultured bacteria and different cement blocks; †±80% small colony variants; CFU = colony-forming units.
Fig. 2A–C.
Confocal laser scanning microscopy images of biofilms on bone cement discs after LIVE/DEAD Baclight and Calcofluor staining are shown. The live bacteria are green, dead bacteria are red, and extracellular polysaccharides are blue. Overlay projections show the 7-day Staphylococcus aureus biofilms on (A) Palacos R, (B), Palacos R-G, and (C) Copal bone cement. The projection image (square image, x–y plane, 375 μm × 375 μm) includes all the slices in an image stack. The rectangular micrographs on the sides represent the x–z plane and y–z optical cross sections through the thickness of the biofilms (the bottom of each biofilm is indicated B [bone cement] in each cross section). Bar = 10 μm. Copal reduced biofilm to a level that no or only a few bacteria were visible on confocal laser scanning microscopy.
Discussion
Bone cements loaded with combinations of antibiotics are assumed more effective in preventing infection than bone cements with gentamicin as a single drug. Moreover, loading with an additional antibiotic may increase interconnectivity between antibiotic particles to enhance release. We hypothesized addition of clindamycin to a gentamicin-loaded cement yields higher antibiotic release, and causes larger inhibition zones against clinical isolates grown on agar and stronger biofilm inhibition.
As a potential limitation of this study, we compared Copal and Palacos R-G bone cement in 20 mL volumes of fluid, whereas the surface area of a cement disc exposed relative to the fluid volume is 0.06 cm−1. In vivo, the ratio between cement area and surrounding fluid volume is approximately 100 times larger [32]. A recent report suggested surface to volume concentration has a strong impact on the gentamicin concentration that can be achieved and, consequently, on the antibacterial effect of an antibiotic-loaded bone cement [8]. Moreover, we established the antibacterial efficacy of both cements by measuring the inhibition zones against different bacterial strains. Studies of antibiotic diffusion through agar media are helpful [1], but zones of inhibition cannot be directly correlated with the local and systemic antibiotic concentrations. Finally, to confirm whether these findings had any clinical importance, a prospective, randomized-controlled (multicenter) study would be required to compare the rates of eradication of infection between the two bone cements.
The manufacturer of Copal bone cement claims their bone cement is based on the raw materials of Palacos R-G. Antibiotic release from bone cement is strongly influenced by their concentration, quantity, and structure [2]. The gentamicin concentration in Copal is twice the amount of that of Palacos R-G. Penner et al. suggested this might result in more and larger pores [22] as a result of greater disturbances of the cement matrix after addition of extra antibiotics. However, other generally unknown factors also influence antibiotic release such as the size of the gentamicin sulphate particles added. In addition, the polymer-to-monomer ratio of bone cement greatly affects the antibiotic release rate; increased polymer-to-monomer ratio leads to increased release of antibiotic from the cement [4]. The polymer-to-monomer ratios of Palacos R-G and Copal are 1.82 and 1.92, respectively. A higher ratio can lead to incomplete polymerization and consequently a more porous cement. Prolonged release of antibiotics from bone cements is largely influenced by penetration of dissolution fluids into the polymer matrix, which requires a certain porosity of the cement [16, 17, 29]. Although the presence of more pores in Copal cement seems beneficial to antibiotic release, the mechanical strength of this bone cement is negatively affected by the higher quantities of antibiotics. Yet, the mechanical parameters of Palacos R-G and Copal fulfill ISO Standard 5833, but mechanical testing of Palacos R-G indicates better mechanical properties [13].
We found Copal inhibited bacterial growth on agar plates up to 672 hours, whereas with Palacos R-G cement, activity stopped after 48 hours. This may explain why Copal cement was associated with biofilm reduction to below detectable limits between Days 1 and 7, whereas Palacos R-G was associated with biofilm increase during that time. During formation of 7-day–old biofilms, growth medium was refreshed every 2 days (to mimic the in vivo situation and to provide fresh nutrients). This action removes nearly all the gentamicin released during the first 2 days without continued release from Palacos R-G cement, whereas antibiotic release from Copal evidently did continue. Thus, bacteria able to survive the initial gentamicin release from Palacos R-G subsequently are able to grow and form a biofilm, whereas the prolonged high release from Copal cement inhibits bacterial biofilm formation.
Bacterial colony counting of the gentamicin-sensitive S. aureus biofilm on Palacos R-G cement discs resulted in two macroscopically different colonies. Part of the colonies had a normal size with a distinct hemolytic zone, whereas other colonies were smaller and showed no signs of hemolysis. Some authors described this phenomenon for S. aureus and called these small nonhemolytic colonies “small colony variants” (SCVs) [5, 30]. Small colony variants of S. aureus grow slowly and have various other features that are atypical for S. aureus, including increased resistance to aminoglycosides (eg, gentamicin) [23, 30]. The connection of SCVs with persistent and recurrent infections has been defined during the past decade, especially in patients with chronic osteomyelitis, cystic fibrosis, and implant-related infections [5, 24, 30]. In vitro isolation of SCVs is possible in a kinetic model after exposure to antibiotics [14]. The number of S. aureus colonies harvested from the Palacos R and Palacos R-G cement discs after 1 day of growth showed a bacterial growth reduction of one log-unit, indicating a rather small reduction resulting from incorporation of gentamicin in the cement. This is possibly the result of the formation of SCVs, because reduced sensitivity to gentamicin is one of the characteristics of SCVs.
Copal was effective in reducing biofilm to a level that none or only a few bacteria were visible in CLSM. Instead, an increased and heavy distribution of EPS can be seen after Day 7 on Copal. Because EPS production impedes the effect of an antibiotic by protecting the bacteria in the biofilm, it was suggested this could be considered a survival mechanism against an antimicrobial attack as previously described for Pseudomonas aeruginosa [20]. More important, these data suggest effective killing of adhering bacteria adjacent to the cement surface (Palacos R-G; Fig. 2B), whereas the outermost layers of the biofilms remain viable. Systemic antibiotics predominantly attack a biomaterial-related infection through the outermost layers of the biofilm, which is usually ineffective as bacteria continue to grow from the inner layers combined with an increased production of EPS. This constitutes the main reason why infected joint replacements nearly always must be removed to eradicate the infection. Combined use of an antibiotic-releasing bone cement and systemic antibiotics thus sandwiches the biofilm between two antimicrobial attacks with increased chances for resolution of the biofilm, possibly reducing the risk of revision surgery.
Our data suggest antibiotic release from the bone cement surface kills adhering bacteria adjacent to the surface with Copal being more effective in biofilm reduction than Palacos R-G.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. In vitro testing of antimicrobial activity of bone cement. Antimicrob Agents Chemother. 2004;48:4084–4088. doi: 10.1128/AAC.48.11.4084-4088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker AS, Greenham LW. Release of gentamicin from acrylic bone cement: elution and diffusion studies. J Bone Joint Surg Am. 1988;70:1551–1557. [PubMed] [Google Scholar]
- 3.Buchholz HW, Engelbrecht H. Über die Depotwirkung einiger Antibiotica bei Vermischung mit dem Kunstharz Palacos. Chirurg. 1970;41:511–515. [PubMed] [Google Scholar]
- 4.Downes S. Methods for improving drug release from poly(methyl)methacrylate bone cement. Clin Mater. 1991;7:227–231. doi: 10.1016/0267-6605(91)90063-L. [DOI] [PubMed] [Google Scholar]
- 5.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 6.Dunne N, Hill J, McAfee P, Todd K, Kirkpatrick R, Tunney M, Patrick S. In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: effect on mechanical properties, antibiotic release, and biofilm formation. Acta Orthop. 2007;78:774–785. doi: 10.1080/17453670710014545. [DOI] [PubMed] [Google Scholar]
- 7.Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty: review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590–595. doi: 10.1302/0301-620X.79B4.7420. [DOI] [PubMed] [Google Scholar]
- 8.Hendriks JG, Neut D, van Horn JR, van der Mei HC, Busscher HJ. Bacterial survival in the interfacial gap in gentamicin-loaded acrylic bone cements. J Bone Joint Surg Br. 2005;87:272–276. doi: 10.1302/0301-620X.87B2.14781. [DOI] [PubMed] [Google Scholar]
- 9.Jiranek W. Antibiotic-loaded cement in total hip replacement: current indications, efficacy, and complications. Orthopedics. 2005;28(8 suppl):s873–877. doi: 10.3928/0147-7447-20050802-14. [DOI] [PubMed] [Google Scholar]
- 10.Josefsson G, Gudmundsson G, Kolmert L, Wijkstrom S. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty: a five-year survey of 1688 hips. Clin Orthop Relat Res. 1990;253:173–178. [PubMed] [Google Scholar]
- 11.Josefsson G, Kolmert L. Prophylaxis with systematic antibiotics versus gentamicin bone cement in total hip arthroplasty: a ten-year survey of 1,688 hips. Clin Orthop Relat Res. 1993;292:210–214. [PubMed] [Google Scholar]
- 12.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. [PubMed] [Google Scholar]
- 13.Kühn K-D. Bone Cements. Up-to-date Comparison of Physical and Chemical Properties of Commercial Materials. 1. Berlin, Germany: Springer Verlag; 2000. pp. 253–258. [Google Scholar]
- 14.Langford PR, Anwar H, Gonda I, Brown MR. Outer membrane proteins of gentamicin induced small colony variants of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1989;52:33–36. doi: 10.1111/j.1574-6968.1989.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 15.Mc Gowan JE, Jr, Terry PM, Huang TS, Houk CL, Davies J. Nocosomial infections with gentamicin-resistant Staphylococcus aureus: plasmid analysis as an epidemiologic tool. J Infect Dis. 1979;140:864–872. doi: 10.1093/infdis/140.6.864. [DOI] [PubMed] [Google Scholar]
- 16.McLaren AC, McLaren SG, McLemore R, Vernon BL. Particle size of fillers affects permeability of polymethylmethacrylate. Clin Orthop Relat Res. 2007;461:64–67. doi: 10.1097/BLO.0b013e31811f350d. [DOI] [PubMed] [Google Scholar]
- 17.McLaren AC, McLaren SG, Smeltzer M. Xylitol and glycine fillers increase permeability of PMMA to enhance elution of daptomycin. Clin Orthop Relat Res. 2006;451:25–28. doi: 10.1097/01.blo.0000229321.53040.a1. [DOI] [PubMed] [Google Scholar]
- 18.Murray PR, Pfaller MA, Rosenthal KS, Kobayashi G. Antibacterial agents. In: Murray PR, Pfaller MA, Rosenthal KS, Kobayashi G, editors. Medical Microbiology. 3. Philadelphia, PA: Mosby-Year Book, Inc; 1998. pp. 165–168. [Google Scholar]
- 19.Musher DM. The Gram-positive cocci: III. Resistance to antibiotics. Hosp Pract (Off Ed). 1988;23:105–107, 111–112, 117–118 passim. [DOI] [PubMed]
- 20.Neut D, Hendriks JGE, van Horn JR, van der Mei HC, Busscher HJ. Pseudomonas aeruginosa biofilm formation and slime excretion on antibiotic-loaded bone cement. Acta Orthop. 2005;76:109–114. doi: 10.1080/00016470510030427. [DOI] [PubMed] [Google Scholar]
- 21.Neut D, van de Belt H, van Horn JR, van der Mei HC, Busscher HJ. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 2003;24:1829–1831. doi: 10.1016/S0142-9612(02)00614-2. [DOI] [PubMed] [Google Scholar]
- 22.Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939–944. doi: 10.1016/S0883-5403(96)80135-5. [DOI] [PubMed] [Google Scholar]
- 23.Proctor RA, Peters G. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin Infect Dis. 1998;27:419–422. doi: 10.1086/514706. [DOI] [PubMed] [Google Scholar]
- 24.Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis. 2006;43:961–967. doi: 10.1086/507633. [DOI] [PubMed] [Google Scholar]
- 25.Speller DC, Raghunath D, Stephens M, Viant AC, Reeves DS, Wilkinson PJ, Broughall JM, Holt HA. Epidemic infection by a gentamicin-resistant Staphylococcus aureus in three hospitals. Lancet. 1976;1:464–466. doi: 10.1016/S0140-6736(76)91485-9. [DOI] [PubMed] [Google Scholar]
- 26.Stewart PS, Murga R, Srinivasan R, Debeer D. Biofilm structural heterogeneity visualized by 3 microscopic methods. Water Research. 1995;29:2006–2009. doi: 10.1016/0043-1354(94)00339-9. [DOI] [Google Scholar]
- 27.Tunney MM, Dunne N, Einarsson G, McDowell A, Kerr A, Patrick S. Biofilm formation by bacteria isolated from retrieved failed prosthetic hip implants in an in vitro model of hip arthroplasty antibiotic prophylaxis. J Orthop Res. 2007;25:2–10. doi: 10.1002/jor.20298. [DOI] [PubMed] [Google Scholar]
- 28.van de Belt H, Neut D, van Horn JR, van der Mei HC, Schenk W, Busscher HJ. …or not to treat? Nat Med. 1999;5:358–359. doi: 10.1038/7330. [DOI] [PubMed] [Google Scholar]
- 29.van de Belt H, Neut D, Uges DR, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials. 2000;21:1981–1987. doi: 10.1016/S0142-9612(00)00082-X. [DOI] [PubMed] [Google Scholar]
- 30.von Eiff C, Bettin D, Proctor RA, Rolauffs B, Lindner N, Winkelmann W, Peters G. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis. 1997;25:1250–1251. doi: 10.1086/516962. [DOI] [PubMed] [Google Scholar]
- 31.Wahlig H, Dingeldein E. Antibiotics and bone cements: experimental and clinical long-term observations. Acta Orthop Scand. 1980;51:49–56. doi: 10.3109/17453678008990768. [DOI] [PubMed] [Google Scholar]
- 32.Wang JS, Franzen H, Lidgren L. Interface gap after implantation of a cemented femoral stem in pigs. Acta Orthop Scand. 1999;70:234–239. doi: 10.3109/17453679908997799. [DOI] [PubMed] [Google Scholar]
- 33.Wroblewski BM, Esser M, Srigley DW. Release of gentamicin from bone cement: an ex-vivo study. Acta Orthop Scand. 1986;57:413–414. doi: 10.3109/17453678609014759. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt TD, Ferguson WP, Wilson TS, McCormick E. Gentamicin resistant Staphylococcus aureus associated with the use of topical gentamicin. J Antimicrob Chemother. 1977;3:213–217. doi: 10.1093/jac/3.3.213. [DOI] [PubMed] [Google Scholar]