Abstract
Ceramic-on-ceramic bearings in THA are a popular alternative to overcome wear concerns in traditional metal-polyethylene bearings. However, squeaking is a potentially worrisome phenomenon in ceramic-on-ceramic THAs which we observed in some of our patients. We reviewed all 42 patients who underwent 43 ceramic-on-ceramic noncemented THAs during the time of the study. Squeaking, defined as a reproducible sound of squeaking, clicking, or grating, occurred in nine of 43 implants (20.9%). Standard radiographs were normal. We used CT imaging to determine cup anteversion and inclination angles, comparing the squeaking hips with those of a randomly selected control group, but found no differences. We then hypothesized specific design features (stem size, cup size, head size, and neck length of the head) would be risk factors for squeaking. We found a difference in neck length between squeaking and nonsqueaking implants. A neck length of −4 mm or shorter resulted in a relative risk of 5.56 (95% confidence interval, 1.14–27.01) for squeaking. We found a high incidence of squeaking in our population, and we believe this phenomenon is an underreported side effect of these types of bearings. A short neck length of the femoral implant was a risk factor for squeaking in ceramic-on-ceramic THA.
Level of Evidence: Level III, therapeutic study.
Introduction
Loosening of total hip implants is the main problem in long-term survival studies of THAs [8]. In most long-term reports of total hip implants, the articulating surface consists of a metal head and a polyethylene liner of a noncemented cup or a polyethylene cemented cup. The wear of these polyethylene contact surfaces generates many polyethylene particles and these wear particles are associated with aseptic loosening and osteolysis [10]. To reduce particulate wear debris, new bearings have been introduced. One option, a ceramic-on-ceramic articulation, has low in vitro wear rates [7] and promising intermediate-term clinical results [4].
We started using a ceramic-on-ceramic bearing in 2002. However, several hips with this bearing started squeaking within the short to middle term after surgery. As this squeaking sound is a worrisome phenomenon with unknown long-term effects, we stopped implanting this type of bearing.
The exact mechanism of the squeaking sound is unknown although there are several theories. One suggestion is metal-on-metal neck-socket impingement, attributable to suboptimal inclination and anteversion of the cup [6, 24]. Other possible mechanisms include focally increased surface roughness, as seen in the case of stripe wear, severe wear, or metal deposit formation on the ceramic femoral head [14–16, 18, 23], lack of lubrication fluid between the articulating surfaces attributable to third-body wear [23], and stick-and-slip mechanisms of the femoral head and liner during microseparation [12, 17, 21, 23]. Squeaking also can occur with bearing failure, such as head fracture or liner fracture and liner dislocation [13, 21, 22, 25].
We first determined the incidence of squeaking in our patients and how long after implantation the onset of squeaking started. We then asked whether squeaking was related to neck-socket impingement attributable to suboptimal inclination and anteversion of the cup. We then asked whether demographic factors (age, gender, body mass index, postoperative complications, and indications) or component features (cup size, head size, neck length, and stem size) would be risk factors for squeaking. Finally, we report the findings during revision surgery of two patients with squeaking.
Materials and Methods
We retrospectively reviewed all 42 patients who underwent 43 noncemented total hip implantations with a ceramic-on-ceramic bearing in 2002 and 2003. Because of our experience, we asked all patients if their hip implants produced reproducible squeaking sounds. We recorded age, gender, body mass index, and possible confounding factors (diagnosis and indication), intraoperative findings, and postoperative complications. We also recorded the cup size, head size, neck length, and stem size to compare between squeaking and normal hips. All patients were available at final review. There were 22 men and 20 women and 16 left hips and 27 right hips. The average age of the patients at surgery was 57.3 years (range, 38–72 years). The diagnoses or indications for surgery were primary osteoarthritis (30), posttraumatic osteoarthritis (four), nontraumatic avascular necrosis (four), developmental hip dysplasia (four), and residuals of septic arthritis (one). Postoperative complications consisted of four cases of heterotopic ossifications (Brooker Class I [one], II [two], and IV [one]), two cases of superficial wound infections, one case of dislocation, and one case of postoperative neuropathy of the femoral nerve with subsequent temporary paresis of the quadriceps muscle. The minimum followup was 39.8 months (average, 47.3 months; range, 39.8–55.9 months).
We used a modified anterolateral approach of Hardinge. The intended cup orientation during surgery was 45° inclination and 0° anteversion. Three surgeons (RMK, JAB, DJW) implanted the THAs. All patients had an ABG II® femoral stem (Stryker, Montreux, Switzerland) with a Trident® acetabular cup providing a ceramic-on-ceramic bearing. The ABG-2 has standardized neck lengths of −5 mm, −4 mm, −2.7 mm, neutral, +4 mm and +5 mm.
At last followup all patients were explicitly asked whether their THA made a squeaking noise. Squeaking was defined as a subjective sensation of a squeaking, clicking, or grating sound originating from the THA during motion. We considered patients as having squeaking only if the noise was reproducible on demand and objectively audible. We also asked all patients how many months postoperatively they first noticed the squeaking.
We reviewed standard postoperative radiographs to determine the presence of liner dislocation and head or liner fracture. Computed tomography (JCK, DJW) was used to measure cup inclination and anteversion angles [9]. Computed tomography scans were available in all but one of the squeaking hips. We obtained CT scans on a random sample of seven consecutive patients with nonsqueaking ceramic-on-ceramic THAs as a control for inclination and anteversion; informed consent was obtained from these patients to use CT instead of conventional hip radiographs at their postoperative followup. The median deviation was calculated from our intended cup values to express the error in cup placement in degrees. Using this method, an error in any direction would be detected.
We also reviewed and described the intraoperative findings and the outcome of the two patients who had revision surgery because of the squeaks in their hips.
Descriptive statistics were calculated for the incidence of squeaking, the onset of squeaking, and the occurrence of liner dislocation and head or liner fracture. Differences in cup placement were compared using the Mann-Whitney U test. Differences in cup size, head size, neck length, and stem size were compared using the Kruskal-Wallis test. Possible confounding factors were compared using the Mann-Whitney U test (age, body mass index, cup anteversion) and the Fisher’s exact test (gender, indications, postoperative complications).
Results
Squeaking occurred in nine of the 42 patients (21%). Squeaking began after an average of 26.4 months (range, 8.3–37.9 months) after surgery. There were no liner dislocations and head or liner fractures in the 43 hips of the study group.
Suboptimal inclination and anteversion did not relate to squeaking as cup position was similar in the squeaking and normal hips. The median deviation from the intended inclination angle of 45° of squeaking hip implants was not different from that of normal hip implants (Table 1). The median deviation from the intended anteversion angle of 0° of squeaking hip implants also was not different from that of normal hip implants.
Table 1.
Comparison of cup positioning between squeaking and normal hips
| Cup positioning | Squeaking | Nonsqueaking | p Value |
|---|---|---|---|
| Number of CT scans of hips | 8 | 7 | |
| Inclination (deviation from 45°)* | 6.65 (3.73) | 5.44 (3.85) | 0.64 |
| Anteversion (deviation from 0°)* | 6.65 (3.17) | 7.60 (6.55) | 0.87 |
*Values are expressed as means, with standard deviations in parentheses.
Demographic features were not risk factors for squeaking: age, gender, and body mass index, and possible confounding factors (postoperative complications and indications) occurred with similar incidence in squeaking and normal hips (Table 2). In the nonsqueaking group, the demographics, indications, postoperative complications, and prosthesis data were similar in patients without and with CT scans suggesting our subset of seven patients represented the larger group (Table 3).
Table 2.
Patient characteristics according to the occurrence of squeaking
| Characteristic | Squeaking | Nonsqueaking | p Value |
|---|---|---|---|
| Number of hips | 9 | 34 | |
| Demographics | |||
| Age (years)* | 59.45 (7.11) | 57.29 (6.78) | 0.32 |
| Gender (male:female) | 5:4 | 17:17 | 1.00 |
| Body mass index* | 26.24 (4.65) | 26.24 (3.88) | 0.99 |
| Perioperative variables | |||
| Indications (primary:secondary arthrosis) | 7:2 | 23:11 | 0.70 |
| Postoperative complications (complicated:noncomplicated) | 7:2 | 28:6 | 1.00 |
*Values are expressed as means, with standard deviations in parentheses.
Table 3.
Comparison of the possible confounding factors between patients with and without CT scans
| Factor | Squeaking | Nonsqueaking | ||||
|---|---|---|---|---|---|---|
| With CT | Without CT | p Value | With CT | Without CT | p Value | |
| Number of hips | 8 | 1 | 7 | 27 | ||
| Demographics | ||||||
| Age (years)* | 59.60 (7.58) | 58.29 | 0.89 | 59.28 (7.30) | 56.77 (6.69) | 0.74 |
| Gender (male:female) | 4:4 | 1:0 | 1.00 | 5:2 | 12:15 | 0.40 |
| Body mass index* | 26.52 (4.89) | 24.00 | 0.89 | 24.97 (4.77) | 26.58 (3.64) | 0.34 |
| Perioperative variables | ||||||
| Indications (primary:secondary arthrosis) | 7:1 | 0:1 | 0.22 | 4:3 | 19:8 | 0.66 |
| Postoperative complications (complicated:noncomplicated) | 1:7 | 1:0 | 0.22 | 1:6 | 5:22 | 1.00 |
| THA measurements | ||||||
| Cup size† | 4.75 | 7.00 | 0.44 | 21.21 | 16.54 | 0.27 |
| Head size† | 5.13 | 4.00 | 1.00 | 16.64 | 17.72 | 0.99 |
| Neck length | ||||||
| Entire group† | 4.69 | 7.50 | 0.44 | 17.21 | 17.57 | 1.00 |
| <−4 mm:> −2.7 mm | 6:2 | 0:1 | 0.33 | 2:5 | 7:20 | 1.00 |
| Stem size† | 5.19 | 3.50 | 1.00 | 19.07 | 17.09 | 0.64 |
*Values are expressed as means, with standard deviations in parentheses; †mean ranks.
We did find an increase (p = 0.046) in squeaking in implants with a neck length shorter than –4 mm (relative risk, 5.56; 95% confidence interval, 1.14–27.01) (Table 4). However, there were no differences in cup size, head size, and stem size between squeaking and nonsqueaking implants (Table 4).
Table 4.
Comparison of specific prosthesis measurements between squeaking and normal hips
| THA measurements | Squeaking | Nonsqueaking | p Value |
|---|---|---|---|
| Number of hips | 9 | 34 | |
| Cup size* | 22.61 | 21.84 | 0.87 |
| Head size* | 24.44 | 21.35 | 0.44 |
| Neck length | |||
| Entire group* | 15.22 | 23.79 | 0.054 |
| <−4 mm:> −2.7 mm | 6:3 | 9:25 | 0.046‡ |
| Stem size* | 26.00 | 20.94 | 0.28 |
*Values are expressed as mean ranks; ‡significant difference; relative risk, 5.56 (95% confidence interval, 1.14–27.01).
Seven of the nine patients with squeaking decided the nuisance of sound was not sufficient to warrant revision surgery. The other two patients decided otherwise and insisted on surgery. We performed a liner exchange in two of the nine patients with a squeaking hip. In the first patient, the original 36-mm ceramic femoral head, with a neck length of −5 mm, and liner were replaced by a new ceramic femoral head and liner of the same size. During revision, there were no signs of neck-socket impingement or metallosis. On removal of the original ceramic head, gray stripes were visible on the surface of the head (Fig. 1). Scanning electron microscopy suggested these stripes were titanium deposits. The postoperative followup was uncomplicated. Unfortunately, squeaking reappeared after 4 months. In the second patient, the 32-mm ceramic femoral head, with a neck length of −4 mm, and liner were replaced by a 28-mm metal head, with a neck length of +4 mm, and a polyethylene liner. Extensive metallosis was seen, which was confirmed by tissue biopsy. During surgery, we observed anterior notching on the femoral neck and acetabular cup, with impingement occurring in 90° flexion. Gray stripes were seen on the dorsal side of the ceramic femoral head, which is suggestive of metal deposits. Squeaking disappeared postoperatively.
Fig. 1.

The photograph shows a 36-mm ceramic femoral head, retrieved during bearing exchange revision for squeaking. Gray stripes are visible on the surface; scanning electron microscopy revealed these stripes were titanium deposits.
Discussion
We observed a high rate of squeaking total hip prostheses after we started using ceramic-on-ceramic bearings in combination with noncemented prostheses. We believe this is important and a potentially worrisome phenomenon that possibly is underreported [14, 21, 22]. We then asked whether squeaking was related to neck-socket impingement owing to suboptimal inclination and anteversion of the cup, to various demographic factors or component features. Clearly, squeaking can be caused by multiple factors. However, we found a short neck was the only significant factor.
Our study has several limitations. Although our followup was complete, we had a small number of patients. We stopped using the ceramic-on-ceramic bearing in this implant after we discovered the high rate of squeaking. Performing more ceramic-on-ceramic THAs would have increased the power but we believe doing so would have been unethical. Computed tomography scans of all but one squeaking hip were obtained. In this hip, MRI was performed instead of CT, which could not be used to study more accurate cup positions. The patients with a nonsqueaking hip who had control CT scans were selected at random. We tried to exclude a possible selection bias by comparing the data of this group of patients with data of patients with squeaking hips and with nonsqueaking hips who did not have a CT scan.
Our intended cup orientation during surgery was 45° inclination and 0° anteversion. We chose this neutral anteversion angle to minimize the risk of anterior dislocations, because we performed the THAs using an anterior approach [11]. Lewinnek et al. [11] proposed a cup orientation of 30° to 50° inclination and 5° to 25° anteversion as a cup safe zone. However, there is no evidence to support this [27]. Admittedly, this neutral anteversion angle limits the range of motion in flexion and adduction and thus increases the risk of anterior metal-to-metal neck-socket impingement [5]. However, the range of motion increases in extension, abduction, and external rotation in this neutral anteversion angle [5]. Therefore it cannot be concluded a priori this neutral anteversion angle increases the absolute risk of impingement.
Although faulty surgical technique could explain the high squeaking rate, the involved surgeons were experienced in using this type of noncemented hip. Before starting to use this new bearing, all had extensive experience with the same noncemented THA in combination with a metal-on-polyethylene bearing.
We identified only neck length as a risk factor for squeaking. Neck-socket impingement may be a part of the explanation. A shorter neck length results in a smaller range of motion before impingement occurs [3]. This smaller range of motion increases the risk of neck-socket impingement in normal daily usage, such as during ambulation or rising from a chair. We found some evidence of neck-socket impingement in one of two patients with squeaking who had a revision. This type of impingement could be more prominent in the ABG II® stem, as the proximal taper diameter widens quickly. The main question then becomes whether the titanium friction in metal-on-metal neck-socket impingement (the so-called titanium squeak [24]) is directly responsible for the squeaking. Indirect mechanisms, such as formation of metal deposits on the ceramic femoral head or third-body wear attributable to intraarticular metal particles, seem more likely [6, 23]. In contrast to the titanium squeak, these mechanisms cannot appear directly postoperatively but need time to develop [23]. Our findings of a delayed onset of squeaking support this theory.
Another possible explanation could be the increased joint laxity with a shorter neck length. This may increase the amount of microseparation [2, 19, 20] and thus be a precursor of the squeaking sound. For instance, increased microseparation makes squeaking as a result of the stick-and-slip mechanism more likely [21]. Microseparation is also a known risk factor for the formation of stripe wear and metal deposits on the ceramic femoral head [15, 18, 26], which in turn led to increased surface roughness, and could be a cause of squeaking.
Both revised cases suggest involvement of a roughened surface of the femoral head attributable to metal deposits. Risk factors for metal deposition are microseparation [15], dislocations [18], surgical errors [1], or contamination of the synovial fluid with metal particles as a consequence of abrasive neck-socket impingement [6]. Dislocations are unlikely causes, as these did not occur in our revised cases and occurred only once in all of our squeaking cases. A surgical error is also an unlikely cause of the squeaking, as the squeaking did not start immediately postoperatively. Squeaking began after an average of 26.4 months (range, 8.3–37.9 months) after surgery and after 8.3 months and 35.0 months, respectively, in the revised cases. Metal-on-metal neck-socket impingement could be the cause of squeaking in the second patient; however, cup anteversion and inclination were within normal limits. More likely causes are the increased surface roughness attributable to metal depositions on the ceramic head or third-body wear attributable to the extensive metallosis. A common finding in both patients who had revision surgery is the short neck length, which is in concordance with the findings in our study. The increased microseparation of these short necks could lead to metal deposition, which could lead to squeaking.
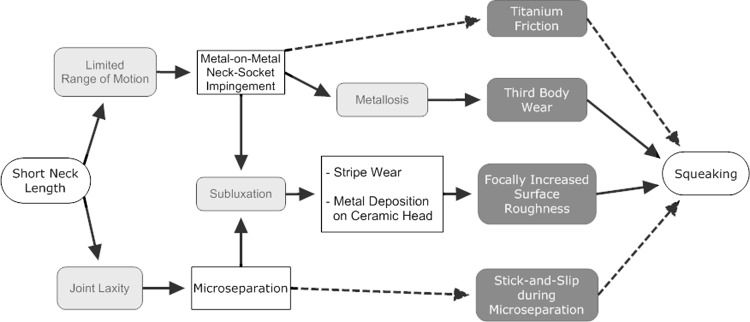
Clearly, there is no single, isolated cause of squeaking. We propose a model of the mechanism of squeaking (Fig. 2). Direct mechanisms of squeaking are titanium friction of neck-socket impingement and a stick-and-slip-mechanism during microseparation and can appear immediately postoperatively. Indirect mechanisms are squeaking attributable to focally increased surface roughness or third-body wear. These mechanisms need time to develop, as they are the result of damage to either the articular surface or the titanium neck or socket [6, 23]. The indirect mechanisms are more likely the cause because of the delayed onset of squeaking. These findings also are reproduced in vitro [23]. Various elements of this model should be tested further in in vivo research to complete the understanding of this awkward phenomenon. We recommend avoiding short neck lengths to reduce the risk of squeaking in ceramic-on-ceramic THAs when using this type of implant.
Fig. 2.
A flowchart illustrates our proposed model of the mechanism of squeaking. White rectangles indicate possible causes or risk factors; gray rounded rectangles indicate mechanisms; and dashed lines indicate immediate causes of squeaking.
Acknowledgments
We thank Jan Hendriks, medical statistician, for advice and Han Bakens, orthopaedic surgeon, for performing some of the THAs.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bal BS, Rahaman MN, Aleto T, Miller FS, Traina F, Toni A. The significance of metal staining on alumina femoral heads in total hip arthroplasty. J Arthroplasty. 2007;22:14–19. doi: 10.1016/j.arth.2006.02.155. [DOI] [PubMed] [Google Scholar]
- 2.Barrack RL, Burak C, Skinner HB. Concerns about ceramics in THA. Clin Orthop Relat Res. 2004;429:73–79. doi: 10.1097/01.blo.0000150132.11142.d2. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28-, 32-, 38-, and 44-mm femoral head sizes. J Arthroplasty. 2005;20:11–19. doi: 10.1016/j.arth.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.D’Antonio J, Capello W, Manley M, Naughton M, Sutton K. Alumina ceramic bearings for total hip arthroplasty: five-year results of a prospective randomized study. Clin Orthop Relat Res. 2005;436:164–171. doi: 10.1097/01.blo.0000162995.50971.39. [DOI] [PubMed] [Google Scholar]
- 5.D’Lima DD, Urquhart AG, Buehler KO, Walker RH, Colwell CW., Jr The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315–321. doi: 10.2106/00004623-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Eickmann T, Manaka M, Clarke I, Gustafson A. Squeaking and neck-socket impingement in a ceramic total hip arthroplasty. Bioceramics 15. 2003;240–2:849–852. [Google Scholar]
- 7.Hannouche D, Hamadouche M, Nizard R, Bizot P, Meunier A, Sedel L. Ceramics in total hip replacement. Clin Orthop Relat Res. 2005;430:62–71. doi: 10.1097/01.blo.0000149996.91974.83. [DOI] [PubMed] [Google Scholar]
- 8.Huo MH, Parvizi J, Gilbert NF. What’s new in hip arthroplasty. J Bone Joint Surg Am. 2006;88:2100–2113. doi: 10.2106/JBJS.F.00595. [DOI] [PubMed] [Google Scholar]
- 9.Kalteis T, Handel M, Herold T, Perlick L, Paetzel C, Grifka J. Position of the acetabular cup: accuracy of radiographic calculation compared to CT-based measurement. Eur J Radiol. 2006;58:294–300. doi: 10.1016/j.ejrad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi A, Freeman MA, Bonfield W, Kadoya Y, Yamac T, Al-Saffar N, Scott G, Revell PA. Number of polyethylene particles and osteolysis in total joint replacements: a quantitative study using a tissue-digestion method. J Bone Joint Surg Br. 1997;79:844–848. doi: 10.1302/0301-620X.79B5.7602. [DOI] [PubMed] [Google Scholar]
- 11.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 12.Lombardi AV, Jr, Mallory TH, Dennis DA, Komistek RD, Fada RA, Northcut EJ. An in vivo determination of total hip arthroplasty pistoning during activity. J Arthroplasty. 2000;15:702–709. doi: 10.1054/arth.2000.6637. [DOI] [PubMed] [Google Scholar]
- 13.Matsuno H, Yudoh K, Kimura T. Failure of the locking mechanisms in cementless acetabular components: a report of 3 cases. SICOT Online Reports. January 2002. Available at: http://www.sicot.org/resources/File/IO_reports/01-2002/1-01-2002.php.
- 14.Morlock M, Nassutt R, Janssen R, Willmann G, Honl M. Mismatched wear couple zirconium oxide and aluminum oxide in total hip arthroplasty. J Arthroplasty. 2001;16:1071–1074. doi: 10.1054/arth.2001.27233. [DOI] [PubMed] [Google Scholar]
- 15.Nevelos J, Ingham E, Doyle C, Streicher R, Nevelos A, Walter W, Fisher J. Microseparation of the centers of alumina-alumina artificial hip joints during simulator testing produces clinically relevant wear rates and patterns. J Arthroplasty. 2000;15:793–795. doi: 10.1054/arth.2000.8100. [DOI] [PubMed] [Google Scholar]
- 16.Nevelos JE, Prudhommeaux F, Hamadouche M, Doyle C, Ingham E, Meunier A, Nevelos AB, Sedel L, Fisher J. Comparative analysis of two different types of alumina-alumina hip prosthesis retrieved for aseptic loosening. J Bone Joint Surg Br. 2001;83:598–603. [PubMed] [Google Scholar]
- 17.Patek SN. Squeaking with a sliding joint: mechanics and motor control of sound production in palinurid lobsters. J Exp Biol. 2002;205:2375–2385. doi: 10.1242/jeb.205.16.2375. [DOI] [PubMed] [Google Scholar]
- 18.Schuh A, Holzwarth U, Kachler W, Goske J, Zeiler G. [Titanium deposits on the ceramic heads of dislocated total hip replacements] [in German] Orthopade. 2004;33:1194–1200. doi: 10.1007/s00132-004-0689-1. [DOI] [PubMed] [Google Scholar]
- 19.Stewart T, Tipper J, Streicher R, Ingham E, Fisher J. Long-term wear of HIPed alumina on alumina bearings for THR under microseparation conditions. J Mater Sci Mater Med. 2001;12:1053–1056. doi: 10.1023/A:1012802308636. [DOI] [PubMed] [Google Scholar]
- 20.Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation. J Biomed Mater Res B Appl Biomater. 2003;66:567–573. doi: 10.1002/jbm.b.10035. [DOI] [PubMed] [Google Scholar]
- 21.Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Severe wear and fracture of zirconia heads against alumina inserts in hip simulator studies with microseparation. J Arthroplasty. 2003;18:726–734. doi: 10.1016/S0883-5403(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Matsubara M, Morita S, Muneta T, Shinomiya K. Fracture of a ceramic acetabular insert after ceramic-on-ceramic THA: a case report. Acta Orthop Scand. 2003;74:101–103. doi: 10.1080/00016470310013752. [DOI] [PubMed] [Google Scholar]
- 23.Taylor S, Manley MT, Sutton K. The role of stripe wear in causing acoustic emissions from alumina ceramic-on-ceramic bearings. J Arthroplasty. 2007;22:47–51. doi: 10.1016/j.arth.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Walter WL, O’Toole GC, Walter WK, Ellis A, Zicat BA. Squeaking in ceramic-on-ceramic hips: the importance of acetabular component orientation. J Arthroplasty. 2007;22:496–503. doi: 10.1016/j.arth.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 25.White SP, Blom AW, Lee M, Smith EJ. The crescent sign: dissociation of the polyethylene liner from a modular acetabular component in total hip arthroplasty. Skeletal Radiol. 2005;34:620–624. doi: 10.1007/s00256-005-0927-6. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Saito M, Ueno M, Hananouchi T, Tokugawa Y, Yonenobu K. Wear analysis of retrieved ceramic-on-ceramic articulations in total hip arthroplasty: femoral head makes contact with the rim of the socket outside of the bearing surface. J Biomed Mater Res B Appl Biomater. 2005;73:301–307. doi: 10.1002/jbm.b.30215. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimine F, Ginbayashi K. A mathematical formula to calculate the theoretical range of motion for total hip replacement. J Biomech. 2002;35:989–993. doi: 10.1016/S0021-9290(02)00040-4. [DOI] [PubMed] [Google Scholar]