Abstract
Surgical site infections (SSIs) with Staphylococcus aureus are a recognized adverse event of hip and knee replacements. We evaluated the impact of a program to detect S. aureus nasal carriers before surgery with preoperative decolonization (using mupirocin twice daily for 5 days prior to surgery) of carriers. Nasal swab samples were obtained from patients prior to surgery from 8/1/2003 through 2/28/2005. Samples were tested using real-time PCR technology to detect S. aureus. The group that developed S. aureus SSI was compared to a combined concurrent and historical control for one year following the operation. S. aureus caused 71% of SSIs in the combined control groups. Of the 1495 surgical candidates evaluated, 912 (61.0%) were screened for S. aureus; 223 of those screened (24.5%) were positive and then decolonized with mupirocin. Among the 223 positive and decolonized patients, three (1.3%) developed a SSI. Among the 689 screen-negative patients, four (0.6%) developed SSIs for an overall rate of 0.77%. Among the 583 control patients who were not screened or decolonized, 10 (1.7%) developed S. aureus SSIs. SSIs from other organisms were 0.44% and 0.69%, respectively.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Surgical site infections (SSIs) are a major adverse outcome for orthopaedic surgery patients and their surgeons. A leading cause of SSIs is Staphylococcus aureus (S. aureus), and the prevalence of S. aureus as a cause of SSIs continues to increase with it being the only cause of increasing postoperative infections between 2000 and 2005 in a recent multicenter study [1]. During this time period methicillin-resistant S. aureus (MRSA) nearly doubled [1]. Molecular DNA analysis of S. aureus isolates causing SSI reveals a majority of the infecting strains are part of the patient’s resident normal nasal flora [12]. Perl and colleagues report this rate as high as 84.6% [15]. Thus, a reasonable approach to decrease postoperative S. aureus SSI involves eliminating S. aureus nasal carriage from the patient prior to surgery.
This strategy was investigated by Kalmeijer and colleagues [6] in a group of orthopaedic patients undergoing hip, knee, or spinal prosthetic implantation in the Netherlands. Their study showed decolonization with mupirocin ointment beginning 5 days before surgery decreased endogenous S. aureus infections fivefold as compared to patients receiving a placebo, but the results were not statistically significant due to the small sample size of their investigation.
We first wanted to confirm our in-house molecular diagnostic assay [12] could be used as a rapid test for detecting S. aureus in surgical patients and to assess the costs. We then raised three questions: (1) Are S. aureus SSIs the major cause of postoperative infection in our hip and knee surgical population?; (2) Will a program of preoperative surveillance for nasal S. aureus carriage followed by decolonization of those patients found to be positive meaningfully reduce S. aureus SSIs?; (3) What are the costs of infection when it does occur?
Materials and Methods
This study was a retrospective analysis of a prospective infection control initiative designed to lower the risk of postoperative surgical site infection. The key variables were the introduction of preoperative screening for S. aureus using a real-time molecular diagnostic assay (real-time PCR) for S. aureus, preoperative decolonization of nasal S. aureus carriage in those patients found positive, and a 1-year postoperative followup for any SSI. A total of 1495 consecutive patients underwent hip or knee replacement surgery from February 1, 2003 to February 28, 2005. One group of patients (n = 416) operated on between February 1, 2003 and August 1, 2003 served as historical controls. The second group consisted of the intervention group (n = 912), whose surgery occurred between August 1, 2003 and February 28, 2005. We had a third patient set that consisted of 167 concurrent controls who did not receive preoperative surveillance (unscreened) or decolonization therapy and whose surgery occurred at the same time period as the 912 intervention patients (Fig. 1). They were combined with the historical controls for outcome analysis.
Fig. 1.
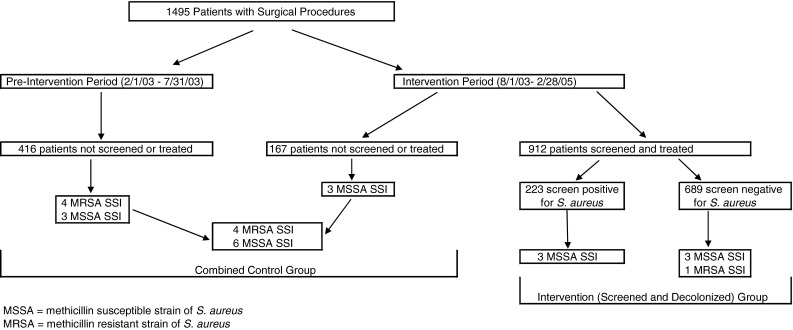
The surgical site infections (SSIs) of patients with and without presurgical nasal screening and nasal decolonization for S. aureus are outlined.
The approach to outcome analysis of the intervention was patterned after that used by Kluytmans et al. [9]. We estimated approximately 1,500 patients would be needed for this evaluation to demonstrate a substantial difference (with a 95% confidence level) if a 4-fold reduction of infection (e.g., from 4% to 1%) was realized in S. aureus carriers (assuming 25% of the subjects would be carriers) who were decolonized versus those who were not. As a secondary endpoint, we also expected S. aureus carriers who were decolonized would not have a higher rate of S. aureus SSI than those persons who were not S. aureus carriers.
There were 39% male and 61% female patients with an average age of 70 years in the unscreened, preintervention period controls, 39% male and 61% female with an average age of 67 years in the unscreened concurrent controls, and 38% male and 62% female with an average age of 69 years in the screened (intervention) patients (Fig. 1). The patient demographic variables were similar in the groups.
The microbiology data and medical records (both inpatient and medical office records) of all included patients operated upon were examined for 1 year after surgery to determine if any developed any infection. The prospective records of the Evanston Northwestern Healthcare Infection Control Department that covered the study period were also reviewed to detect any infections missed by retrospective review. An infection was defined as the presence of a positive culture plus subsequent treatment using an antibiotic directed against the pathogen recovered from that diagnostic culture. The type of infection was noted and recorded as superficial incisional, deep incisional, and organ space/joint, according to definitions established by the Centers for Disease Control and Prevention (CDC, Atlanta, GA) [4].
The organization’s financial administrative database was reviewed for all readmissions that were due to S. aureus infection following major joint replacement surgery during the time of our observation, and we recorded the actual costs (fixed and variable - net margin loss) for readmission for treating the infection.
Patients were screened for S. aureus 2 to 4 weeks before surgery. Nasal samples were collected during the same visit as other scheduled preoperative tests, autologous blood donation, or educational programs. Samples were collected using premoistened, double-headed rayon-tipped swabs (CultureSwab, BBL, Becton Dickinson Inc., Cockeysville, MD). The swabs were premoistened with the Amies liquid media in the sponge at the bottom of the collection tube by simply inserting the swabs into the tube and holding for 5 seconds. During sample collection the swabs were inserted as a pair into one nostril and then the other so that both anterior nares were sampled with both swabs. The inside circumference of each anterior nare was rubbed for 3 to 5 seconds to obtain adequate sampling. One swab was used to perform the test using real-time PCR method for the detection of the femA gene unique to S. aureus [14]. The second swab was used to culture the organism whenever the PCR test was positive in order to perform susceptibility testing to detect methicillin resistance and to detect the ileS-2 gene for demonstrating high-level mupirocin resistance [14]. Patients with a positive nasal screen test were treated with Bactroban Nasal® (mupirocin) ointment (GlaxoSmithKline, Inc., Research Triangle Park, NC) twice daily for 5 days prior to surgery, with the last dose given the night before or the morning of operation. Additionally, hip surgery patients received cefazolin prophylaxis prior to surgery; patients undergoing knee surgery received vancomycin prophylaxis and bathed with Hibiclens® (Regent Medical, LLC, Norcross, GA) on the day of surgery. The choice of preoperative antibiotic prophylaxis agent was permitted as a surgeon’s preference due to the fact that we had documented more than 5% of our inpatients are nasally colonized with MRSA [16]. Other than preoperative surveillance with decolonization of patients positive for S. aureus, no practices changed during the period of this intervention.
To achieve consistency in practice and limit any operator effect on the results all surgical procedures were performed by one hip surgeon (JCK) and one knee surgeon (WJR). The gender and age of the patients who were screened and not screened was recorded to assess comparability of the various groups. Overall, 786 (53%) of the surgical procedures were hip replacements and 709 (47%) were knee replacements.
The intervention group of 912 patients was used to determine the percentage of persons carrying S. aureus in their nose, the rate of SSI in patients who were positive and then decolonized, and the rate of SSI who were screened and found negative for S. aureus. These results were then applied to the (preintervention and concurrent) control group to estimate the rates of S. aureus infection in likely carriers and noncarriers. For data analysis, the rate of persons who developed S. aureus SSI that were negative for S. aureus in their anterior nares and the percentage of persons who were nasal S. aureus carriers was assumed constant in all groups. This permitted an estimation of the number of S. aureus SSIs attributable to S. aureus carriers that were not decolonized in both the historic as well as the concurrent unscreened (control) patients. The overall rates of SSI in the historical and concurrent unscreened groups (controls) including those due to organisms other than S. aureus, were compared to assess the validity of the assumptions made. The significance of any differences in S. aureus SSI between assumed S. aureus carriers who were not decolonized and those who were given decolonization therapy before surgery was assessed using the Chi-square statistic. For results with low frequencies, a Yates’ correction was employed. The Institutional Review Board at our facility approved the outcome analysis of this infection control intervention of preoperative screening and decolonization directed toward S. aureus.
Results
Our experience with the in-house molecular diagnostic assay confirmed it could be used as a rapid test for detecting S. aureus in surgical patients. Test results for the 912 screened patients revealed 223 patients positive with S. aureus (24.5%, 95% CI 21.7–27.4%) and 689 who were negative. The turnaround time for reporting of this test was 2.5 days that provided ample opportunity to prescribe decolonization of those patients found positive. The direct cost of this test for supplies and technical time was $18.76. The 583 patients who comprised the combined control groups were not tested.
We established S. aureus as a major cause of postoperative infection in our hip and knee surgical population: 17 of 25 infections (Table 1). Of the 223 patients recognized as colonized with S. aureus and treated with mupirocin, three (1.3%; 95% CI 0.28–3.88%) developed an S. aureus SSI (Table 1). Of the 689 screen negative patients, four developed S. aureus SSIs (0.58%; 95% CI 0.16–1.48%). Of the 583 unscreened and untreated (historical and concurrent control) patients, 10 (1.7%; 95% CI 0.83–3.13%) developed S. aureus SSIs (Table 1).
Table 1.
Patients with infection (Staphylococcus and other organisms) after hip or knee replacement surgery from February 1, 2003 through February 28, 2005
| Group | Patient | Age | Gender | Screen result | Specimen source | Organism | Deep versus superficial | Time to infection (weeks) | Severe underlying Illness* | Rehospitalized |
|---|---|---|---|---|---|---|---|---|---|---|
| Historical controls | 1 | 90 | M | Not done | Hip | MRSA | Superficial | 3 | No | No |
| 2 | 75 | F | Not done | Hip | MRSA | Superficial | 5 | No | Yes | |
| 3 | 70 | M | Not done | Hip | MSSA | Superficial | 4 | No | No | |
| 4 | 83 | F | Not done | Knee | MSSA | Superficial | 6 | No | No | |
| 5 | 47 | M | Not done | Hip | MRSA | Superficial | 3 | No | No | |
| 6 | 46 | F | Not done | Hip | MSSA | Superficial | 3 | No | No | |
| 7 | 66 | M | Not done | Knee | MRSA | Superficial | 4 | No | No | |
| 8 | 56 | F | Not done | Hip | E. coli, Enterococcus sp. | Superficial | 4 | No | Yes | |
| Concurrent controls | 9 | 64 | F | Not done | Hip | MSSA | Superficial | 3 | Yes | Yes |
| 10 | 60 | M | Not done | Hip | MSSA | Superficial | 6 | No | No | |
| 11 | 90 | M | Not done | Knee | MSSA | Superficial | 12 | No | Yes | |
| 12 | 72 | F | Not done | Hip | P. aeruginosa, Coag neg staphylococci | Superficial | 3 | No | No | |
| 13 | 73 | F | Not done | Hip | Coag neg staphylococci, P. aeruginosa, Enterococcus sp, Group B streptococcus | Superficial | 1 | No | Yes | |
| 14 | 84 | M | Not done | Hip | P. aeruginosa, Coag neg staphylococci | Superficial | 3 | No | Yes | |
| Intervention (screened and decolonized) group | 15 | 79 | F | Positive | Hip | MSSA | Superficial | 3 | No | No |
| 16 | 62 | M | Positive | Hip | MSSA | Superficial | 4 | No | No | |
| 17 | 39 | M | Positive | Hip | MSSA | Superficial | 3 | No | No | |
| 18 | 79 | F | Negative | Hip | MRSA | Deep | 4 | No | Yes | |
| 19 | 66 | F | Negative | Knee | MSSA | Superficial | 4 | Yes | No | |
| 20 | 72 | F | Negative | Knee | MSSA | Superficial | 6 | No | No | |
| 21 | 76 | F | Negative | Hip | MSSA | Superficial | 6 | No | No | |
| 22 | 82 | M | Negative | Hip | E. coli, Enterococus sp and P. mirabilis | Superficial | 2 | No | No | |
| 23 | 74 | F | Positive | Knee | P. aeruginosa | Superficial | 3 | No | No | |
| 24 | 47 | F | Negative | Hip | Coag neg staphylococci | Superficial | 3 | No | No | |
| 25 | 60 | M | Negative | Knee | Coag neg staphylococci | Deep | 2 | No | Yes |
* Severe underlying illness includes malignancy, insulin dependent diabetes or immunosuppressive disease.
The screening and decolonization program prevented at least eight SSIs, demonstrating an S. aureus SSI reduction in decolonized carriers of some fourfold (p ≤ 0.05; 95% CI 2.2–10). Overall, screening and decolonization lowered (but only approached significance of p ≤ 0.1) the SSI rate from S. aureus to seven of 912 (0.77%; CI 0.31%–1.57%) operations in the total intervention group (both S. aureus carriers and non-carriers combined) versus 10 of 583 (1.7%; 95% CI 0.83%–3.13%) in those unscreened (control) patients. (A total population of approximately 7000 would be needed to reach significance of p ≤ 0.05 for this outcome.) Because the expected rate of S. aureus carriage is considered the same in the screened and unscreened groups, 143 (24.5%) of the 583 unscreened patients likely would be colonized with S. aureus and 440 patients would not. Additionally, since the expected rate of SSIs in the S. aureus negative patients should be the same regardless of screening, 2.6 (0.58%) SSIs would have occurred in the 440 S. aureus negative patients. With 10 known S. aureus infections in the 583 unscreened group, and 2.6 likely occurring in the 440 screen-negative patients, 7.4 (5.2%; 95% CI 2.8–8.7%) SSIs would have occurred in the 143 S. aureus-positive, nondecolonized patients. Based on a rate of 5.2% of S. aureus screen-positive patients having an SSI, 11.5 SSIs would have been expected in the 223 S. aureus screen positive patients had they not been decolonized (5.2%) yet only three were found, resulting in a reduction between eight and nine S. aureus SSIs.
The mean inpatient cost for the four patients rehospitalized for treatment of S. aureus infection during our period of observation was $17,122 (range, $3,164–$29,568).
Seventeen S. aureus SSIs developed in the 1495 surgical patients, one being a deep joint space infection with the others considered superficial. The superficial infections included six deep incisional (open incision site but remaining above the fascia), six that involved perisuture infections without extension, three that were superficial cellulitis with minimal drainage, and one undefined. Twelve of the 17 S. aureus infections involved hip replacements including one deep joint space infection, and the remaining five infections occurred in knee replacement patients. Four of these patients were rehospitalized because of their SSI. The lengths of stay for these rehospitalizations were 2, 3, 4, and 9 days. Three of these four patients did not have nasal screens for S. aureus before surgery and the fourth had a negative screen but subsequently developed an infection with MRSA while being cared for in a rehabilitation facility. Eight additional SSIs from other pathogens developed in the 1495 surgical patients, with seven considered superficial and one deep infection (Table 1). Many were mixed flora with two Escherichia coli, three enterococcus species, four Pseudomonas aeruginosa, one Proteus mirabilis, one Streptococcus agalactiae, and five Coagulase-negative staphylococci recovered from the cultures. As expected, the program had no impact on other organisms as there were no differences in the numbers of these nonstaphylococcal infections for any of our patient groupings. Four of these eight patients were in the 583 nonscreened population (0.7%), three were in the 689 patients screened and negative for S. aureus (0.4%), and one was in the 223 subjects (0.4%) who were screened positive and decolonized (all p > 0.5). Four of these eight patients were rehospitalized for 3, 3, 4, and 7 days respectively to treat their SSIs. Two patients who developed an SSI had a history of a serious underlying illness (eg, malignancy, insulin-dependent diabetes, or immunosuppressive disease) (Table 1).
Discussion
Surgical site infections (SSIs) with Staphylococcus aureus are major adverse event of hip and knee replacements. Any program to reduce the incidence would be important. We therefore evaluated a program to detect S. aureus nasal carriers before surgery with preoperative decolonization (using mupirocin twice daily for 5 days prior to surgery) of carriers. We first wanted to confirm our in-house molecular diagnostic assay [12] could be used as a rapid test for detecting S. aureus in surgical patients and to assess the costs. We then raised three questions: (1) Are S. aureus SSIs the major cause of postoperative infection in our hip and knee surgical population?; (2) Will a program of preoperative surveillance for nasal S. aureus carriage followed by decolonization of those patients found to be positive meaningfully reduce S. aureus SSIs?; (3) What are the costs of infection when it does occur?
All data collection was derived from retrospective chart and microbiologic data review, which raises the possibility not all SSIs would have been detected. However, our review of prospective infection control documentation revealed only one missing SSI from our investigation (from a Coagulase-negative staphylococcus), which was added to the data set. The practice of the two surgeons (JCK, WJR) is to routinely culture all postoperative wounds whenever there is the suggestion of any infection where they would prescribe an antimicrobial agent(s) for therapy. This and the fact that our Infection Control Department found only one additional case strongly suggest we have captured the important SSIs. While we did not followup with the patients by phone or a letter to determine if they had developed an infection and were treated at another healthcare facility, the groups of screened and unscreened patients were well-matched and it was expected their followup medical care would be similar. Also, it was expected S. aureus and other organism SSIs would have been missed in similar proportion. Another limitation of our work is the additional cost of using a molecular diagnostic test for performing surveillance testing. This testing was chosen for two reasons. First, and most importantly, the new real-time PCR tests are more sensitive than routine cultures [14], and if the goal is to determine the presence of S. aureus colonization one wants to detect as many carriers as possible. The second reason is that if surgery is to be performed on a somewhat urgent basis, these tests can provide results in a very short time when required (< 0.5 days) [16]. Our cost estimate did not include personnel time for obtaining and transporting the test, nor administrative time for processing or billing; these added costs would affect our estimates of the entire cost of a screening program, but not likely the conclusions regarding its utility.
A recent report on SSI following major joint replacement surgery highlighted the devastating effects of these infections after total hip arthroplasty (THA) on patients, surgeons, and hospitals [2]. That study compared surgical revision due to infection to revision due to aseptic loosening and reported infection was associated with three times the number of repeat hospitalizations and outpatient visits, and nearly four times the number of operations with approximately 22 additional days in the hospital. The total direct costs associated with revision of THA due to infection were 2.8 times higher than the cost associated with revision due to aseptic loosening and 4.8 times higher than primary THA [2]. Importantly, S. aureus is often the cause of deep and superficial infections following joint replacement surgery. Our data demonstrated 17 of 25 SSIs (68%) were caused by S. aureus, well illustrating this pathogen is now the major cause of SSIs in joint replacement surgery, and that reducing these infections provides a substantial improvement in patient safety.
We have shown such testing can be useful in a hospital-wide program for controlling MRSA [16] and have also demonstrated the utility of testing. From an economic standpoint, Kaye and colleagues recently assessed the cost of SSI due to S. aureus, both methicillin-susceptible strains and MRSA [8]. They reported the mean excess hospital charge for a methicillin susceptible SSI was $13,901 while one from MRSA added $41,274 compared to the expense of surgery with no subsequent infection [8]. Others have estimated the cost of SSI for major cardiothoracic surgery to be a mean of $16,878, and suggested decolonization would only add cost to their surgery program if the excess expense for treating a postoperative SSI was less than $245 [17]. Our finding of $17,122 for the mean healthcare cost (loss) of additional hospitalization resulting from S. aureus SSI and the cost of testing only adding $18.76 is certainly consistent with these reports. Had all 1495 patients been screened, the total direct cost to our organization would have been slightly more than $28,000. Thus, our estimated eight fewer S. aureus SSIs would have resulted in two avoided rehospitalizations and a savings of over $34,000, with a likely higher expense if all of the infections had been MRSA, for a net benefit of $6,000 to the program. The financial benefit of this practice if expanded to the entire United States was recently estimated at more than $231 million, should all 7.2 million patients undergoing elective surgery be screened for S. aureus and those positive decolonized before their operation [13]. Additionally important is the emotional, physical and economic burden of repeated doctors’ office visits, additional medication, impaired healing, prolonged therapy and delay in return to normal living that was avoided for eight patients [2].
Further supporting our findings is the recent literature review and meta-analysis that reported in nongeneral surgery (including cardiothoracic surgery, orthopaedic surgery, and neurosurgery, both randomized and before and after trials [such as our report]) perioperative intranasal mupirocin decreased the incidence of SSI and should be considered as routine practice in these settings [5, 10].
The single greatest risk factor for developing a S. aureus SSI in hip or knee replacement surgery is a high-density nasal carriage of S. aureus [7]. A second study suggests the primary source of postoperative S. aureus infection is the patient’s own strain of S. aureus, usually carried in the anterior nares. Here, the rate of endogenous infection with S. aureus was five times lower in a group of patients receiving recommended doses of mupirocin ointment versus a group receiving a placebo [6]. A more recent investigation reported 9.5% of their prosthetic hip or knee surgeries in the 13-month period of January 2003 through January 2004 were infected, and 89% of these infections were caused by S. aureus [11]. This high rate of infection resulted in the interruption of their orthopedic surgery program until the problem could be resolved. For them, 56% of the S. aureus isolates were MRSA that exhibited the community-associated phenotype (CA-MRSA). The pathogenicity of this strain of S. aureus has major implications for prosthetic joint SSIs, thus prevention of infection from S. aureus is increasingly important. Therefore, nasal screening and decolonization prior to surgery has potentially even more merit now in light of CA-MRSA strains that are rising in prevalence across the United States.
The interest in performing S. aureus decolonization before major surgery has been growing during the past decade. In 1996 an unblinded intervention trial using mupirocin for nasal decolonization of all patients (no surveillance for S. aureus was performed) undergoing major cardiothoracic surgery reported a reduction in SSI for in an intervention group compared to historical controls [9]. Subsequently, another paper found similar benefit from S. aureus nasal decolonization in prevention of sternal wound infection for 1846 diabetic and nondiabetic patients undergoing cardiac surgery [3]. More recently, an intervention in the setting of hyperendemic MRSA colonization (point prevalence for MRSA carriage = 38%) and subsequent MRSA infection on four orthopedic surgery wards reported a reduction in (MRSA) infection by using universal mupirocin decolonization and preoperative 2% triclosan bathing [18]. Other reports support our approach by suggesting preoperative surveillance for S. aureus to limit the unnecessary use of mupirocin [10, 12]. Surveillance cultures of anterior nares in those about to undergo cardiothoracic surgery was followed by mupirocin prophylaxis on all patients [12]. When the cultures were completed (at 48 hours) they were able to discontinue mupirocin therapy in 79% of their patients and limit unnecessary mupirocin treatment to 2 days. Using this strategy they observed a reduction of S. aureus SSI from 1.68% to 0.37% over 18 months [12]. We have extended this approach by demonstrating the utility of rapid, real-time PCR molecular diagnostic testing for a preoperative surveillance purpose.
Our nasal screening and decolonization program for S. aureus prior to orthopedic surgery resulted in a fourfold decrease in S. aureus surgical site infections for patients colonized with S. aureus, a favorable outcome. A simple specimen collection technique combined with a currently available molecular microbiology test leading to focused treatment consisting of a 5-day course of nasal mupirocin preoperatively appeared to prevent postsurgical infections with S. aureus that can be considered as a routine addition to any presurgical testing protocol.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:1047–1053. doi: 10.1086/520731. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 3.Cimochowski GE, Harostock MD, Brown B, Bernardi M, Alonzo N, Coyle K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann Thorac Surg. 2001;71:1572–1579. doi: 10.1016/S0003-4975(01)02519-X. [DOI] [PubMed] [Google Scholar]
- 4.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall GC, editor. Hospital Epidemiology and Infection Control. 3. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 5.Kallen AJ, Wilson CT, Larson RJ. Perioperative intranasal mupirocin for the prevention of surgical-site infections: systematic review of the literature and meta-analysis. Infect Control Hosp Epidemiol. 2005;26:916–922. doi: 10.1086/505453. [DOI] [PubMed] [Google Scholar]
- 6.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GA, Stuurman A, van Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: The effect of mupirocin nasal ointment in a double-blind, randomized, placebo controlled study. Clin Infec Dis. 2002;35:353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 7.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere G, Kluytmans J. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21:319–323. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 8.Kaye KS, Engemann JJ, Mozaffari E, Carmeli Y. Reference group choice and antibiotic resistance outcomes. Emerg Infect Dis. 2004;10:1125–1128. doi: 10.3201/eid1006.020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kluytmans JA, Mouton JW, VandenBergh MF, Manders MJ, Maat AP, Wagenvoort JH, Michel MF, Verbrugh HA. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17:780–785. doi: 10.1086/647236. [DOI] [PubMed] [Google Scholar]
- 10.Kluytmans JA, Wertheim HF. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection. 2005;33:3–8. doi: 10.1007/s15010-005-4012-9. [DOI] [PubMed] [Google Scholar]
- 11.Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am J Infect Control. 2005;33:385–391. doi: 10.1016/j.ajic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson MR, Huesman LA. Controlling the usage of intranasal mupirocin does impact the rate of Staphylococcus aureus deep sternal wound infections in cardiac surgery patients. Am J Infect Control. 2006;34:44–48. doi: 10.1016/j.ajic.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Jacobson C, Smulders M, Gemmen E, Bharmal M. Budget impact analysis of rapid screening for Staphylococcus aureus colonization among patients undergoing elective surgery in US hospitals. Infect Control Hosp Epidemiol. 2008;29:16–24. doi: 10.1086/524327. [DOI] [PubMed] [Google Scholar]
- 14.Paule SM, Pasquariello AC, Hacek DM, Fisher AG, Thomson RB, Jr, Kaul KL, Peterson LR. Direct detection of Staphylococcus aureus from adult and neonate nasal swab specimens using real-time polymerase chain reaction. J Molec Diag. 2004;6:191–196. doi: 10.1016/S1525-1578(10)60509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Mupirocin and the risk of Staphylococcus aureus study team. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 16.Peterson LR, Hacek DM, Robicsek A. Case Study: A MRSA intervention at Evanston Northwestern Healthcare. Joint Comm J Qual Patient Safety. 2007;33:732–738. doi: 10.1016/s1553-7250(07)33088-2. [DOI] [PubMed] [Google Scholar]
- 17.VandenBergh MF, Kluytmans JA, van Hout BA, Maat AP, Seerden RJ, McDonnel J, Verbrugh HA. Cost-effectiveness of perioperative mupirocin nasal ointment in cardiothoracic surgery. Infect Control Hosp Epidemiol. 1996;17:786–792. doi: 10.1086/647237. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox MH, Hall J, Pike H, Templeton PA, Fawley WN, Parnell P, Verity P. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54:196–201. doi: 10.1016/S0195-6701(03)00147-6. [DOI] [PubMed] [Google Scholar]


