Abstract
Although the delayed-type hypersensitivity skin test reaction to tuberculin purified protein derivative (PPD) is used worldwide for tuberculosis (TB) detection, it is incapable of distinguishing Mycobacterium tuberculosis (MTB) infection from bacille Calmette–Guérin (BCG) vaccination or infection with non-tuberculous Mycobacteria. As a result, there is an urgent need for a more specific diagnostic tool for TB. This study reports the skin reactions of guinea pigs and human volunteers to recombinant early secreted antigen target 6 (rESAT6), a secretory protein found only in MTB, M. bovis and few other mycobacterial species. These volunteers had varying histories of BCG vaccination and exposure to MTB, allowing us to determine the specificity of their response to TB exposure. Our results show that 1·0 μg of the purified MTB rESAT6 antigen elicited a positive skin response in both animals and humans exposed to MTB, as well as in animals exposed to M. bovis and M. marinum, all species of Mycobacteria that contain the gene for early secreted antigen target 6 (ESAT6). ESAT6 appears to be more specific to MTB infection than PPD, as demonstrated by the fact that we saw no skin responses in the BCG-vaccinated volunteers, nor in the guinea pigs sensitized with BCG vaccine, or with Mycobacteria that do not contain the gene encoding ESAT6. We believe that this is the first report of the use of a rESAT6 protein in a skin test in human volunteers, and that these data support its use in the specific detection of MTB infection.
Keywords: delayed-type hypersensitivity, Mycobacterium tuberculosis, rESAT6 protein, skin test, TB infection
Introduction
It is estimated that, in China, there are 550 million people latently infected with Mycobacterium tuberculosis (MTB), 4·5 million patients with pulmonary tuberculosis (TB) and nearly 2 million patients with smear-positive pulmonary TB [1]. Most latently infected individuals as well as many patients with active TB are smear-negative and/or culture-negative, complicating the diagnosis of TB.
The purified protein derivative (PPD) skin test is based on the delayed-type hypersensitivity (DTH) reaction elicited by a mixture of a large number of MTB antigens, and is used widely as an aid in the detection of MTB infection [2]. However, PPD contains antigens that are also present in non-tuberculous Mycobacteria (NTM) and in M. bovis bacille Calmette–Guérin (BCG), which is used worldwide as a TB vaccine [3]. As a result, the skin test can be falsely positive in those who received the BCG vaccine, which is estimated to be given to 80% of infants at birth [4]. It can also be falsely positive in those who have been exposed previously to NTM, which is a significant confounding factor in many regions of the world. Because of this poor specificity, the PPD skin test cannot be used to definitively identify MTB infection [5]. A more specific skin test using MTB-specific antigens has the potential to greatly improve the current methods of TB prevention, diagnosis and disease control.
The RD1 genetic region is present in the genomes of MTB and M. bovis, but is absent from all strains of M. bovis BCG, as well as most NTM [6–8]. The early secreted antigen target 6 (ESAT6) gene that is encoded by RD1 has been investigated extensively, and has been shown to have great potential in the specific in vitro diagnosis of MTB infection in humans [9–11]. Although its promise for use in a skin test has been demonstrated in animal models [12], ESAT6 has not been tested as a skin test antigen in humans. The primary objective of this study was to evaluate the sensitivity and specificity of a recombinant ESAT6 (rESAT6) protein as a stimulating antigen in a skin test for differential diagnosis of MTB infection.
Materials and methods
Bacterial strains and products
Escherichia coli strain BL21 (DE3) (Invitrogen, Carlsbad, CA, USA) was grown in standard liquid and solid media. Mycobacterium reference strains including MTB (H37Rv), M. bovis, M. bovis BCG Danish (chosen because it is used widely in China), M. avium[American Type Culture Collection (ATCC) 25291], M. intracellulare (ATCC 13950), M. nonchromogenicum (TMC1481), M. marinum (TMC1218), M. smegmatis (ATCC 19420), M. fortuitum (ATCC 6841), M. phlei (ATCC 11758), M. gilvum (ATCC 43909) and M. triviale (TMC1453) were obtained from the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Stock cultures of MTB (H37Rv), M. bovis, M. bovis BCG Danish, M. avium, M. intracellulare, M. nonchromogenicum and M. triviale were grown on Lowenstein–Jensen slants at 37°C for 4 weeks, and then transferred to Sauton liquid medium at 37°C for 4 weeks. Stock cultures of M. fortuitum, M. phlei and M. gilvum were grown on Lowenstein–Jensen slants at 37°C for 1 week, and then transferred to Sauton liquid medium at 37°C for 1 week. Stock cultures of M. marinum were grown on Lowenstein–Jensen slants at 28°C for 4 weeks, and then transferred to Sauton liquid medium at 28°C for 4 weeks. Stock cultures of M. smegmatis were grown on Lowenstein–Jensen slants at 45°C for 1 week, and then transferred to Sauton liquid medium at 45°C for 1 week [13]. PPD produced from MTB (50 IU/ml) was purchased from Beijing Gaoke Life and Technology Inc., China.
Gene cloning, expression and protein purification
The procedures for MTB ESAT6 cloning, expression and purification have been described previously [14,15]. Briefly, the gene encoding the MTB ESAT6 protein was amplified by polymerase chain reaction (PCR). PCR products were digested by the restrictive enzymes NheI and XhoI. The resulting fragments were ligated with T4 DNA ligase into an NheI- and XhoI-digested pET-24b (Novagen, San Diego, CA, USA) plasmid vector and transferred into E. coli BL21 (DE3). The resultant transformants were plated on LB plates containing kanamycin (50 μg/ml), IPTG (isopropyl-b-D-thiogalactopyranoside) and X-Gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside). rESAT6 was expressed as a C-terminal 6× histidine-tagged protein in inclusion bodies and purified by metal chelate affinity chromatography under denaturing conditions according to the purification procedure provided by the manufacturer (Novagen).
Determination of molecular mass and purity
The molecular mass of purified rESAT6 protein was determined by 15% sodium dodecyl sulphate-polyacrylamide gel elecrtophoresis (SDS-PAGE) in the presence of 6 M urea, as described previously [14,15]. The molecular masses of the protein standards ranged from 6 to 70 kDa (Sigma, St Louis, MO, USA). Gels were stained with Coomassie blue. The molecular mass and purity of rESAT6 protein were analysed and calculated with Biologic Software TotalLab version 1·11. The molecular mass of purified rESAT6 protein was also determined by mass spectrometry in the Instrument Test Center, Institute of Military Medical Science, Beijing, China.
Analysis of amino acid sequence
Fifteen amino acid residues at the N-terminus of the purified ESAT6 protein were determined at AuGCT Biotechnology (Beijing, China) in order to demonstrate the identity of the protein.
Determination of isoelectric point
Samples of purified ESAT6 were submitted to isoelectric focusing using methods described by Guo [16]. The isoelectric point (pI) value was analysed and calculated by the comparison of the migration of the purified rESAT6 to the ampholine standards (Amersham Pharmacia, Piscataway, NJ, USA) on the same SDS-PAGE gels with Biologic Software TotalLab version 1·11.
Guinea pig sensitization and skin tests
Ninety-eight NIH white guinea pigs weighing 250–300 g were obtained from the Institute of Epidemic and Microbiology Research (Academy of Chinese Preventive Medicine, Beijing, China), and maintained under specific pathogen-free conditions. There was a female : male ratio of 1:1. In the first experiment, 36 guinea pigs were sensitized by a hypodermic in the groin with 5 mg autoclaved MTB in 0·1 ml of saline once-weekly for 4 weeks. In the second experiment, six guinea pigs were sensitized by peritoneal injection of 100 live MTB in 0·5 ml of saline and then kept in a P2 animal house for 5 weeks before skin testing. In the third experiment, 48 guinea pigs were divided into 12 groups and sensitized by the hypodermic in the groin with 0·1 ml of live BCG-Danish vaccine, or 5 mg of one of the 11 autoclaved mycobacterial species per group (see Table 3) in 0·1 ml of saline once-weekly for 4 weeks.
Table 3.
Skin reactivity to recombinant early secreted antigen target 6 (rESAT6) in guinea pigs sensitized with a variety of killed Mycobacteria.
| Skin reactions in guinea pigs sensitized with mycobacterial species (mean ± s.d. mm in diameter) | ||||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||
| Groups | PPD | rESAT6 | PPD | rESAT6 | PPD | rESAT6 |
| Mycobacterium bovis | 8·25 ± 0·6 | 8·25 ± 0·3 | 6·38 ± 0·4 | 6·33 ± 0·4 | 4·38 ± 0·4 | 4 ± 0·6 |
| M. marinum | 8·13 ± 0·5 | 7·25 ± 0·8 | 6·38 ± 0·4 | 5·5 ± 0·6 | 4·5 ± 0·4 | 3·13 ± 0·5 |
| M. avium | 8·75 ± 1·1 | 1·00 ± 1·7 | 7 ± 0·8 | 0 | 5 ± 0·8 | 0 |
| Live BCG vaccine | 9·08 ± 3·0 | 0 | 8·08 ± 2·4 | 0 | 6·00 ± 1·5 | 0 |
| Dead M. bovis BCG | 8·33 ± 0·8 | 0 | 6·5 ± 0·7 | 0 | 4·17 ± 0·6 | 0 |
| M. intracellulare | 7·75 ± 0·3 | 0 | 6·13 ± 0·2 | 0 | 4·25 ± 0·3 | 0 |
| M. nonchromogenicum | 8·25 ± 0·8 | 0 | 6·50 ± 0·8 | 0 | 4·50 ± 0·5 | 0 |
| M. gilvum | 7·25 ± 0·6 | 0 | 5·75 ± 0·6 | 0 | 4·25 ± 0·6 | 0 |
| M. fortuitum | 6·83 ± 0·8 | 0 | 5·00 ± 0·8 | 0 | 2·33 ± 1·7 | 0 |
| M. smegmatis | 8·00 ± 0·4 | 0 | 6·00 ± 0·4 | 0 | 4·00 ± 0·4 | 0 |
| M. phlei | 7·33 ± 0·8 | 0 | 5·50 ± 0·7 | 0 | 3·33 ± 0·6 | 0 |
| M. triviale | 8·75 ± 0·8 | 0 | 4·50 ± 0·5 | 0 | 2·50 ± 0·5 | 0 |
PBS, phosphate-buffered saline; PPD, purified protein derivative; s.d., standard deviation; BCG, bacille Calmette–Guérin.
For skin testing, guinea pigs were shaved on the back and injected intradermally with 0·1–1·0 μg of the purified rESAT6 protein in 0·1 ml of phosphate-buffered saline (PBS) or 0·1 ml (5 IU) of PPD as a control 4–8 weeks following sensitization. The diameters of both axes of skin induration were measured and recorded at 24, 48 and 72 h after antigen injection. Results were expressed as means of diameters (in mm) of induration ± standard deviations.
Human participants and skin tests
Ten human volunteers were medical workers or Master's degree students from the Institute for TB Research (the Second Affiliated Hospital of Chinese PLA General Hospital, Beijing 100091, China). Informed consent was obtained from all participants. The ages of the participants ranged from 24 to 52 years, with a median age of 34. Their BCG status and past MTB exposure history are shown in Table 4. All participants were injected intradermally in the left forearm with 0·1 ml of 5 IU PPD and in the right forearm with 1 μg rESAT6 antigen in 0·1 ml ESAT6 solution (Mantoux technique). The diameters of both axes of skin induration were measured and recorded by a certified doctor at 72 h after antigen injection. Results were expressed as the means of the diameters of induration in mm. A positive result was defined as an induration greater than 5 mm in diameter.
Table 4.
Skin test reaction of recombinant early secreted antigen target 6 (rESAT6) antigen in the volunteers.
| Diameter (mm) of two axes of skin reactions | ||||||
|---|---|---|---|---|---|---|
| No. of volunteer | Sex | Age | TB exposure history | BCG vaccination | PPD | rESAT6 |
| 1 | Female | 52 | Yes | Yes | 22 × 21 (had blister) | 20 × 25 |
| 2 | Female | 28 | Yes | Yes | 22 × 23 | 17 × 23 |
| 3 | Female | 51 | Yes | No | 17 × 15 | 11 × 12 |
| 4 | Female | 30 | Yes | Yes | 22 × 20 | 10 × 8 |
| 5 | Female | 39 | Yes | Yes | 11 × 12 | 5 × 5 |
| 6 | Female | 31 | Yes | Yes | 16 × 15 | 2 × 3 |
| 7 | Male | 27 | No | Yes | 15 × 15 | 0 × 0 |
| 8 | Male | 28 | No | No | 5 × 5 | 0 × 0 |
| 9 | Female | 24 | No | No | 0 × 0 | 0 × 0 |
| 10 | Female | 30 | No | No | 0 × 0 | 0 × 0 |
PPD, purified protein derivative; BCG, bacille Calmette–Guérin; TB, tuberculosis.
Results
Purification and analysis of rESAT6
The rESAT6 protein showed only one band when analysed by 15% SDS-PAGE, with a purity of > 90% (Fig. 1). Fifteen amino acid residues at the N-terminus of the purified ESAT6 protein were ASMTEQQWNFAGIEA. The average molecular mass obtained from multiple rESAT6 samples as determined by SDS-PAGE was 9·262 kDa. By mass spectrometry, the molecular mass of purified rESAT6 protein was determined to be 11·119 kDa. Finally, samples of purified rESAT6 from three batches were analysed by isoelectric focusing, and found to have an average pI of 4·69.
Fig. 1.
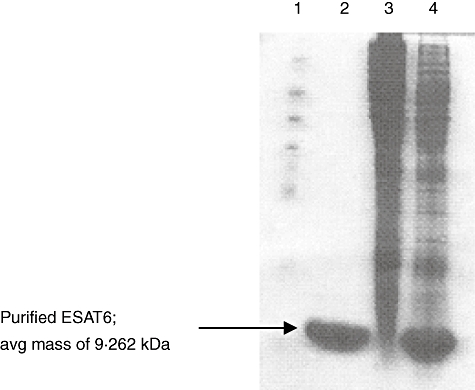
Purification of recombinant early secreted antigen target 6 (rESAT6) protein as determined by sodium dodecyl sulphate-polyacrylamide gel elecrtophoresis electrophoresis. Lane 1, molecular weight protein standards (Sigma). Lane 2, the purified rESAT6 protein. Lane 3, Escherichia coli extracts before isopropyl-b-D-thiogalactopyranoside induction. Lane 4, E. coli extracts 4 h after induction. The gel was subjected to electrophoresis followed by Coomassie blue staining.
Delay-type hypersensitivity reactivity to rESAT6 in guinea pigs sensitized with killed MTB
Different doses of rESAT6 protein were tested for their ability to produce DTH responses in guinea pigs sensitized with killed MTB. Thirty-six guinea pigs were divided into six groups, and each group received a unique dose of rESAT6. These doses included 0·1 μg, 0·2 μg, 0·4 μg, 0·6 μg, 0·8 μg and 1·0 μg rESAT6 protein, as well as 0·1 ml PBS as a negative control and 0·1 ml (5 IU) PPD as a positive control. All six rESAT6 doses tested elicited positive DTH responses at 24 and 48 h after injection, as defined by an indurated response greater than 5 mm in diameter (Table 1). When at least 0·4 μg of the rESAT6 antigen was administered, it elicited similar or stronger DTH responses when compared with PPD. The two lower rESAT6 doses, 0·1 μg and 0·2 μg, displayed weaker activity than PPD. The positive responses measured at the 24-h time-point were generally stronger than that at 48 h for all groups, although there was no statistically significant difference. By 72 h, all reactions, including those to PPD stimulation, decreased to less than 5 mm in diameter and were therefore considered negative.
Table 1.
Skin reactivity to different doses of recombinant early secreted antigen target 6 (rESAT6) in guinea pigs sensitized with killed Mycobacterium tuberculosis (MTB).
| Skin reactions in guinea pigs sensitized with dead MTB (mean ± s.d. mm in diameter) | ||||
|---|---|---|---|---|
| Groups | Doses | No. of guinea pigs | 24 h | 48 h |
| PBS | 36 | 0 | 0 | |
| PPD | 5 IU | 36 | 8·25 ± 1·73 | 7·41 ± 1·22 |
| rESAT6 | 0·1 μg | 6 | 6·67 ± 0·81 | 5·33 ± 0·44 |
| 0·2 μg | 6 | 6·83 ± 0·75 | 5·67 ± 0·67 | |
| 0·4 μg | 6 | 8·33 ± 1·03 | 7·16 ± 0·56 | |
| 0·6 μg | 6 | 9·33 ± 2·25 | 7·40 ± 0·88 | |
| 0·8 μg | 6 | 9·33 ± 1·75 | 8·00 ± 1·67 | |
| 1·0 μg | 6 | 9·67 ± 1·21 | 8·00 ± 0·67 | |
PBS, phosphate-buffered saline; PPD, purified protein derivative; s.d., standard deviation.
Skin reactivity to rESAT6 in guinea pigs sensitized with live MTB
Six guinea pigs were sensitized by a peritoneal injection of 100 live MTB in 0·5 ml of saline. Five weeks after this challenge, each of the six animals was injected intradermally at six sites with PBS, PPD (5 IU) and four doses of rESAT6 (0·4, 0·6, 0·8 and 1·0 μg). All four doses of rESAT6 and the PPD control elicited DTH responses with similar intensities at 24 h and 48 h after injection (Table 2). The results were similar to those observed in the animals sensitized with dead MTB.
Table 2.
Skin reactivity to different doses of recombinant early secreted antigen target 6 (rESAT6) in the same guinea pig sensitized with live Mycobacterium tuberculosis (MTB).
| Skin reactions in guinea pigs sensitized with the live MTB (mean ± s.d. mm in diameter) | ||||
|---|---|---|---|---|
| Groups | Doses | No. of animals | 24 h | 48 h |
| PBS | 6 | 0 | 0 | |
| PPD | 5 IU | 6 | 10·00 ± 2·09 | 7·92 ± 1·77 |
| rESAT6 | 0·4 μg | 6 | 9·42 ± 1·63 | 7·17 ± 2·14 |
| 0·6 μg | 6 | 10·17 ± 2·32 | 7·97 ± 2·67 | |
| 0·8 μg | 6 | 10·92 ± 3·12 | 8·50 ± 2·81 | |
| 1·0 μg | 6 | 10·50 ± 2·17 | 7·67 ± 2·18 | |
PBS, phosphate-buffered saline; PPD, purified protein derivative; s.d., standard deviation.
Specificity of the skin reaction elicited by rESAT6
Forty-eight guinea pigs were divided into 12 groups of four animals per group, half male and half female. One group was sensitized with the live BCG vaccine, and the remaining 11 groups were sensitized with a variety of killed Mycobacteria, as listed in Table 3. Each guinea pig was injected intradermally in three separate sites on the backs of the animals with 0·1 ml of PBS as a negative control, 0·1 ml (5 IU) of PPD as a positive control and 0·1 ml of rESAT6 antigen at 10 μg/ml (1 μg total) respectively. The diameters of reactivity in guinea pigs at 24, 48 and 72 h are shown in Table 3. Twenty-four hours following injection, all guinea pigs had a positive reaction to PPD, while only the guinea pigs sensitized by M. bovis and M. marinum, both of which express ESAT6, reacted positively to ESAT6. Forty-eight hours following injection, the results were similar to that at 24 h, but the size of the skin responses had decreased. Seventy-two hours following injection, most of the guinea pigs had negative reactions (less than 5 mm) to PPD, rESAT6 and the PBS control. The exceptions were the animals sensitized to M. avium and live BCG vaccine; these still had weak positive reactions to PPD, but all were negative to ESAT6 and the negative control.
Skin reactions to rESAT6 in human volunteers
Ten human volunteers were injected intradermally in the left forearm with 0·1 ml of 5 IU PPD and in the right forearm with 1 μg of the rESAT6 antigen. The results of the skin tests were measured by a certified doctor 72 h after antigen injection and expressed as the diameters of both axes of skin induration in millimeters (Table 4). A positive result was defined as an induration greater than 5 mm in average diameter.
Six volunteers were considered to have had regular contact with MTB, as all had worked in the TB centre for many years. During their time at the TB centre, these subjects had significant contact with TB patients and/or infected clinical samples. All six of those volunteers reacted positively to PPD, while five responded to ESAT6. The remaining four volunteers had no history of TB exposure, and all had negative reactions to ESAT6, regardless of their BCG vaccination status. One volunteer had been BCG-vaccinated, but had no history of TB exposure; this volunteer had a positive reaction to PPD but no response to ESAT6. Among the 10 volunteers, the PPD test caused a blister in one subject, while in all other subjects there were no significant side effects to either antigen.
Discussion
In this study, a rESAT6 protein from MTB as purified and characterized. The average mass of rESAT6 was determined to be 9·262 kDa by SDS-PAGE and 11·119 kDa by mass spectrometry. This mass of rESAT6 was higher than that of native ESAT6, as well as the deduced molecular mass [8], and the pI of rESAT6 was also slightly higher than that of the native protein [17]. These results are likely to be related to the presence of the tag on the recombinant protein. Finally, we demonstrated the identity of the purified protein by showing that amino acid residues 3–15 in the rESAT6 protein were identical to those in the native ESAT6 protein; the first and second amino acid residues (Ala and Ser) in rESAT6 protein differed ESAT6 by design, in order to maximize expression of the recombinant protein.
We found that this rESAT6 antigen has great potential as a skin test reagent, as demonstrated by our results in both guinea pigs and humans. Specifically, 1·0 μg of purified rESAT6 antigen of MTB produces a similar intensity of reaction in the skin test to the PPD antigens, and we did not find any side effects in either animals or humans. There are some differences in the time of maximum responses between the animal model and humans. The maximum response in the guinea pig model was between 24 and 48 h after antigen injection, while in humans the optimal time is 48–72 h following the injection; this is consistent with other studies of DTH in humans and animals [12,18,19].
The rESAT6 protein as a skin test antigen appeared to be very sensitive to TB infection. The rESAT6 protein was recognized strongly in all TB-infected guinea pigs. Five of the six volunteers who had a history of TB exposure produced a positive response. This is perhaps a little surprising, given that we were testing only a single antigen. Some studies using ESAT-6 as a skin test antigen in guinea pigs infected with TB and cattle infected with M. bovis found that a certain number of animals did not respond to ESAT-6 [20,21]. ESAT6 is expressed constitutively during both bacterial proliferation and non-proliferation, and is expressed at levels 60–360 times higher than other genes during the non-proliferation phase [22]. One explanation for this phenomenon may be that among different animal species, genetic variability plays a role in the ability to respond to ESAT6. These exposed volunteers may be infected latently with TB that is expressing the ESAT6 protein at high levels, thereby eliciting the required cellular immune responses to produce DTH. One volunteer with a history of TB exposure as well as BCG vaccination was negative to ESAT6 but positive to PPD. This could be a false negative result, or it could be that this volunteer has not been infected by MTB, even given the history of exposure, and that the positive PPD response is a result of BCG vaccination or exposure to NTM. It is interesting to note that this volunteer did have a response to ESAT6, but it was not strong enough to be considered positive (2 × 3 mm); it is possible that the criteria for positivity for a single antigen may need to be redefined, as the response may not be as robust as it is to multiple antigens. In a study of the ESAT6 skin test in cattle, the optimal cut-off point was found to be 3 mm [21]. Further studies in a larger human population are necessary to investigate further an appropriate cut-off value.
One of the main advantages of rESAT6 over PPD is the potential for greatly increased specificity. PPD contains many antigens common among different species of Mycobacteria, and can therefore induce cross-reactions among animals and people with tuberculous infection, non-tuberculous mycobacterial infection and BCG vaccination. However, the gene encoding the ESAT6 protein is located in RD1, which has been found to be present only in the genome of MTB, M. bovis, M. africanum, M. kansasii, M. marinum, M. szulgai and M. flavescens, and is specifically not present in BCG vaccine strains or in 90% of environmental Mycobacteria[6–8]. Previous studies have also investigated the specificity of ESAT6 as a diagnostic antigen in animal models, and the results from these studies are consistent with what we have found. These studies demonstrated that rESAT6 is capable of eliciting a skin test reaction in guinea pigs immunized with MTB and in cattle immunized with M. bovis[20,21], but does not elicit any reaction in the guinea pigs immunized with BCG vaccine or M. avium[20]. Wards and colleagues showed that the rESAT6 antigen elicited a skin test reaction in guinea pigs immunized with M. bovis, but did not elicit any reaction in the guinea pigs immunized with an esat6 gene knock-out mutant of M. bovis[23].
In our experiments, we confirmed the results of previous studies in guinea pigs, and investigated the potential of rESAT6 as a skin test antigen in humans. We found that rESAT6 was capable of eliciting positive skin responses in humans who have been exposed to MTB and in animals sensitized with other Mycobacteria that express ESAT6, but negative responses in BCG vaccinees with no history of TB exposure. We demonstrated its specificity further in our animal model using a broad range of Mycobacteria as sensitizing agents, including M. avium, M. intracellulare, M. nonchromogenicum, M. gilvum, M. fortuitum, M. smegmatis, M. phlei and M. trivial; animals sensitized to these agents did not respond to ESAT6.
In addition to having a greater specificity to TB than PPD, there are many other advantages to developing a skin test that utilizes a single recombinant antigen. First, manufacturing of PPD under good manufacturing practices (GMP) is practically impossible, because of the inconsistent nature of the manufacturing process and the large number of different proteins in the mixture; batch-to-batch variation in the production of PPD at various facilities has been well documented. As a single recombinant protein produced in E. coli, ESAT6 could be produced in an effective and consistent manner that conforms to GMP standards of the World Health Organization or local regulatory agencies, and is therefore likely to produce consistent and more easily interpretable responses when used.
ESAT6 is used currently as a stimulating antigen in the in vitro Quantiferon-Gold test (Cellestis, Australia), and much work has been conducted with that product that confirms the sensitivity and specificity of this protein as a diagnostic agent [24,25]. Use in a skin test could have advantages over Quantiferon in some settings, as a skin test method may be more inexpensive and does not require a laboratory setting and trained laboratory technicians.
In summary, we present here the first data on the use of a rESAT6 protein in a skin test in human volunteers for the detection of tuberculous infection. Our results suggest that further human studies are warranted to evaluate the sensitivity and specificity of rESAT6 antigen skin test to statistical significance.
Acknowledgments
This work was supported by the ‘863’ Innovating Drug and Chinese Traditional Medicine Modernization Special Foundation of China (No. 2004AA2Z3470) and National Nature and Science Foundation of China (No. 30471648).
References
- 1.The Ministry of Health of the People's Republic of China. Report on a nationwide random survey for the epidemiology of tuberculosis. 2001. pp. 9–20.
- 2.American Thoracic Society and Centers for Disease Control. Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis. 1991;142:725–35. doi: 10.1164/ajrccm/142.3.725. [DOI] [PubMed] [Google Scholar]
- 3.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) BCG vaccine. Available at: http://www.who.int/biologicals/areas/vaccines/bcg/en/ [DOI] [PubMed]
- 5.Harboe M. Antigens of PPD, old tuberculin, and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am Rev Respir Dis. 1981;124:80–7. doi: 10.1164/arrd.1981.124.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 7.Behr MA. Comparative genomics of BCG vaccines. Tuberculosis. 2001;81:165–8. doi: 10.1054/tube.2000.0253. [DOI] [PubMed] [Google Scholar]
- 8.Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arend SM, Andersen P, van Meijgaarden KE, et al. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181:1850–4. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- 10.Arend SM, Ottenhoff TH, Andersen P, van Dissel JT. Uncommon presentations of tuberculosis: the potential value of a novel diagnostic assay based on the Mycobacterium tuberculosis-specific antigens ESAT6 and CFP-10. Int J Tuberc Lung Dis. 2001;5:680–6. [PubMed] [Google Scholar]
- 11.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–9. doi: 10.1164/rccm.200402-232OC. [DOI] [PubMed] [Google Scholar]
- 12.Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5:531–6. doi: 10.1128/cdli.5.4.531-536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinese Antituberculosis Association. Chinese laboratory science procedure of diagnostic bacteriology in tuberculosis. 1995. pp. 9–21.
- 14.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Wu XQ, Zhang JX, Shi YC, et al. Expression of ESAT6 protein of Mycobacterium tuberculosis in Escherichia coli and its serodiagnostic value. J Chinese Modern Med. 2001;11:14–17. [in Chinese] [Google Scholar]
- 16.Guo YJ. The experimental technique on protein electrophoresis. China: Science Publishing House; 2001. pp. 161–208. [Google Scholar]
- 17.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–17. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyashchenko K, Manca C, Colangeli R, Heijbel A, Williams A, Gennaro1 ML. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect Immun. 1998;66:3606–10. doi: 10.1128/iai.66.8.3606-3610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romain F, Augier J, Pescher P, Marchal G. Isolation of aproline-rich mycobacterial protein eliciting delayed-type hypersensitivity reactions only in guinea pigs immunized with living mycobacteria. Proc Natl Acad Sci USA. 1993;90:5322–6. doi: 10.1073/pnas.90.11.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elhay MJ, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–6. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock JM, McNair J, Bassett H, et al. Specific delayed-type hypersensitivity responses to ESAT-6 identify tuberculosis-infected cattle. J Clin Microbiol. 2003;41:1856–60. doi: 10.1128/JCM.41.5.1856-1860.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi L, North R, Gennaro ML. Effect of growth state on transcription levels of genes encoding major secreted antigens of Mycobacterium tuberculosis in the mouse lung. Infect Immun. 2004;72:2420–4. doi: 10.1128/IAI.72.4.2420-2424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wards BJ, de Lisle GW, Collins DM. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber Lung Dis. 2000;80:185–9. doi: 10.1054/tuld.2000.0244. [DOI] [PubMed] [Google Scholar]
- 24.Connell T, Bar-Zeev N, Curtis N. Early detection of perinatal tuberculosis using a whole blood interferon-γ release assay. Clin Infect Dis. 2006;42:e82–5. doi: 10.1086/503910. [DOI] [PubMed] [Google Scholar]
- 25.Ravn P, Munk ME, Andersen ÅB, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immun. 2005;12:491–6. doi: 10.1128/CDLI.12.4.491-496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


