Abstract
Previous studies have shown that antibodies from humans exposed continuously to malaria recognize the Plasmodium falciparum asexual blood-stage antigen Pf332. Here we analysed the antibody responses to a C-terminal fragment of Pf332, designated C231, in individuals from Senegal, by measuring the serum levels of immunoglobulin M (IgM), IgG class and subclass and IgE antibodies. IgG antibody reactivity with crude P. falciparum antigen was detected in all the donors, while many of the children lacked or had low levels of such antibodies against C231. The antibody levels increased significantly with age for both crude P. falciparum antigen and C231, and in the older age groups most of the donors displayed antibodies to C231. This was also true for IgM, IgE and IgG subclass reactivity against C231. Moreover, the ratio of IgG1/IgG2 was considerably lower for C231 than for crude P. falciparum antigen, and in age groups 10–14 and 15–19 years the levels of IgG2 against C231 even exceeded that of IgG1. The IgG2/IgG3 ratios suggest that C231 gives similar levels of IgG2 and IgG3, except for children aged 4–9 years, where IgG3 was higher. Raw IgM, IgG class and subclass and IgE antibody levels to C231 tended to be higher in those who did not experience a malaria attack, but following linear multivariate analysis the trends were not significant.
Keywords: antibody responses, antigen/epitopes, immunoglobulins, Plasmodium spp
Introduction
The Plasmodium falciparum asexual blood-stage antigen Pf332 is expressed in trophozoites, and the antigen is translocated to the surface of mature schizonts [1,2]. Antibodies reactive with Pf332 have been shown to be parasite neutralizing in P. falciparum in vitro cultures [3], and they have also been associated with lower parasitaemia [4] and lower numbers of malaria attacks [5], observations forming the basis for considering the antigen a vaccine candidate. Previous analyses of Pf332 have focused on epitopes in the glutamic acid (Glu)-rich repeat region of the antigen, and in particular on EB200, a 157-amino-acid fragment in the central part of Pf332 [6]. EB200 appears to be highly immunogenic in humans, as indicated by the high prevalence of antibody reactivity to it in individuals exposed to malaria [5,7]. However, as antibodies reactive with EB200 are potentially cross-reactive with other Glu-rich P. falciparum antigens [8,9], it is difficult to identify the true target antigen for the antibodies. In order to address this problem, a 231-amino-acid-long C-terminal fragment of Pf332, designated C231, was cloned [10]. The lower content of Glu in the C231 fragment (14%), compared with that in EB200 (33%), is expected to enable monitoring of Pf332-reactive antibodies, showing lower cross-reactivity with other antigens.
The parasite neutralizing effect of antibodies to P. falciparum asexual blood-stage antigens is well documented from both in vitro and in vivo observations. The protective role of immunoglobulin G (IgG) and its subclasses have been investigated for many P. falciparum antigens, but IgM and IgE have not been given the same attention in this context. Anti-malarial IgE antibodies have been reported to be associated with cerebral malaria [11–13] but, in asymptomatic individuals in Tanzania, such antibodies have also been associated with a lower risk of developing a subsequent clinical episode of malaria [14]. Some studies suggest a protective role for IgM in P. falciparum malaria, where non-immune individuals had lower levels of anti-malarial IgM than semi-immune and immunoprotected individuals, and a negative correlation between parasite density and the concentration of IgM antibodies was seen [15]. Also, a correlation between low levels of IgM, high parasitaemia and death has been demonstrated [16]. Several studies show that high titres of malaria-specific IgG are related to protection from severe malaria, and cytophilic subclasses IgG1 and IgG3 have been considered to be the most important antibodies in protection [17]. It has also been indicated that only IgG1 and IgG3 antibodies can mediate efficiently opsonization of infected erythrocytes, and this effect was inhibited by IgG2 and IgG4 antibodies [18]. However, there are some studies that suggest a protective role of IgG2 in vivo[19,20].
In this study we analysed antibody reactivity in individuals from Senegal to C231, compared with that to a crude P. falciparum extract, by measuring the levels of antibodies of IgM, IgG class and subclass and IgE in relation to the number of malaria attacks during the following year.
Materials and methods
Recombinant protein
A His-tagged recombinant protein, C231, was produced in Escherichia coli using the plasmid vector pAff10c [10].
Crude malaria antigen
Strain F32 of P. falciparum was maintained in continuous culture as described previously [21] and kept synchronized by repeated treatment with sorbitol [22]. When the parasitaemia in the cultures was 10% or more, late-stage parasites (late trophozoites and schizonts) were isolated on 60% Percoll, sonicated and used as antigen in the enzyme-linked immunosorbent assay (ELISA) [23].
Study population
Sera from 100 asymptomatic donors aged 4–87 years living in the P. falciparum endemic area of Dielmo, Senegal were collected in March 1994 [5]. Dielmo is located in an area of Sudan-type savanna, where the rainy season extends from the end of June to mid-October. Transmission is intense and perennial and on average each individual receives 204 infected P. falciparum bites per night. All individuals were monitored for clinical attacks of malaria for a period of 36 months prior to the sampling and up to 12 months afterwards. Information pertaining to certain antibody responses, age, sex, haemoglobin, phenotype, G6PD activity and time actually spent in Dielmo by each villager, as well as occurrence of clinically defined malaria attacks, was collected [5,24,25]. A malaria attack was defined as a febrile episode associated with an age-dependent pyrogenic threshold of parasitaemia determined previously in Dielmo village [24,25]. Sera from 10 Swedish donors not exposed to malaria served as controls. All samples were collected with informed consent and the Ministry of National Health in Senegal and the National Ethics Committee in Sweden approved the study.
Enzyme-linked immunosorbent assay
Enzyme immunoassay/radioimmunoassay plates (Costar, Corning, NY, USA) were coated overnight at 4°C with antigens in sodium carbonate buffer (pH 9·6) at concentrations of 10 μg/ml for crude P. falciparum antigen and 5 μg/ml for recombinant C231. After blocking at 37°C with carbonate buffer containing 1% bovine serum albumin (w/v) for 2 h, the plates were washed with saline containing 0·05% Tween 20. Serum dilutions (1:1000 for IgG and IgM, 1:100 for IgE, 1:400 for IgG1 and IgG3 and 1:20 for IgG2 and IgG4) were added to the plates and incubated 1 h at 37°C. IgG and IgM antibodies were detected using alkaline phosphatase conjugated to Fc fragment-specific goat anti-human IgG (Mabtech AB, Nacka, Sweden) and goat anti-human IgM (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) respectively. Antibodies of IgE and IgG1, IgG2, IgG3 and IgG4 subclasses were detected using biotin-conjugated goat or mouse anti-human monoclonal antibodies (IgE: Vector Laboratories, Burlington, CA, USA; IgG1 and IgG2: PharMingen, San Diego, CA, USA; IgG3: Caltag Laboratories, Burlingame, CA, USA; IgG4: Sigma, St Louis, MO, USA) and alkaline phosphatase conjugated streptavidin (Mabtech AB). The assay was developed with p-nitrophenyl phosphate disodium salt (Sigma) as substrate and the optical densities were read at 405 nm in an ELISA plate reader (VmaxTM Kinetic Microplate Reader, Menlo Park, CA, USA). The concentrations of anti-malarial antibodies were calculated from standard curves obtained in a sandwich ELISA with six dilutions of myeloma protein of IgG1-4 isotypes (Serotec, Oxford, UK) or with highly purified IgG, IgM and IgE respectively (IgG and IgM: Jackson ImmunoResearch Laboratories; IgE: NIBSC, Potters Bar, Hertfordshire, UK).
Statistical analyses
Statistical tests were carried out using either Statview 5·0 (®SAS Institute Inc., Cary, NC, USA) or JMP 5·0.1·2 (®SAS Institute Inc.) software. Differences in antibody levels between the different age groups or between individuals with or without a malaria attack were analysed by Mann–Whitney U-test. Spearman's rank correlation test was used to estimate correlations between age and antibody levels as well as between different Ig classes. The relationship between the number of malaria attacks observed during the follow-up, and selected explanatory variables was tested first using a linear multivariate model and then, after elimination of the non-significant variables, by retesting the significant data in a non-linear model with a Poisson distribution. In our analysis, the number of malaria attacks (which follows a Poisson distribution) was modelled as a non-linear function of age and different antibody responses tested. One parameter at a time was removed from the final model formula and the partial sum of squared error (SSE) was recalculated without this parameter and compared with the SSE of the full model. A χ2 value corresponding to the likelihood ratio χ2 test for the hypothesis that a regression parameter is zero was computed. This indication was obtained for the antibody responses found significant previously in the stepwise regression model and then tested for their potential contribution in the non-linear model. When the lack-of-fit χ2 was found significant, the result supported the conclusion that there existed a consistent contribution of the antibody response tested by introducing detectable variation on the regression variable. Bonferroni correction of the P-values was performed when multiple tests were carried out on the same set of data.
Results
Human serum IgG, IgM and IgE reactivity with C231 and crude malaria antigen
In order to investigate the natural antibody response to the antigen Pf332 fragment C231, sera from donors residing in the malaria-endemic village Dielmo in Senegal were assessed by ELISA for their reactivity with recombinant C231 in comparison with their reactivity with crude malaria antigen extract (Figs 1 and 2). The results presented and used for statistical analysis were ELISA units, determined from a standard curve. For univariate statistical analyses, the donors were divided into age groups of 4–9, 10–14, 15–19 and > 20 years.
Fig. 1.
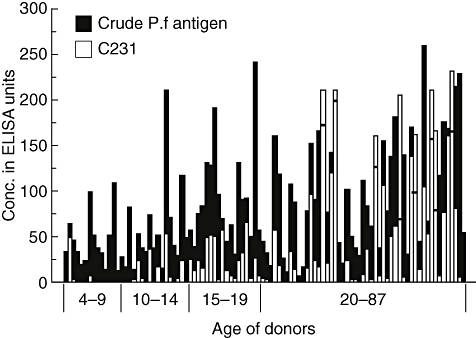
Immunoglobulin G (IgG) reactivity of sera from Senegalese donors with crude malaria antigen and C231. Sera from 100 donors were analysed at a dilution of 1 : 1000 for reactivity with crude Plasmodium falciparum antigen extract as well as with C231 in enzyme-linked immunosorbent assay. The individual donors (4–87 years) are displayed according to age and the different age groups are indicated below the bars. White bars display reactivity with C231 and reactivity with crude malaria antigen is shown as black bars. In cases where the white bars mask the black bars, a horizontal line indicates the height of the black bar.
Fig. 2.
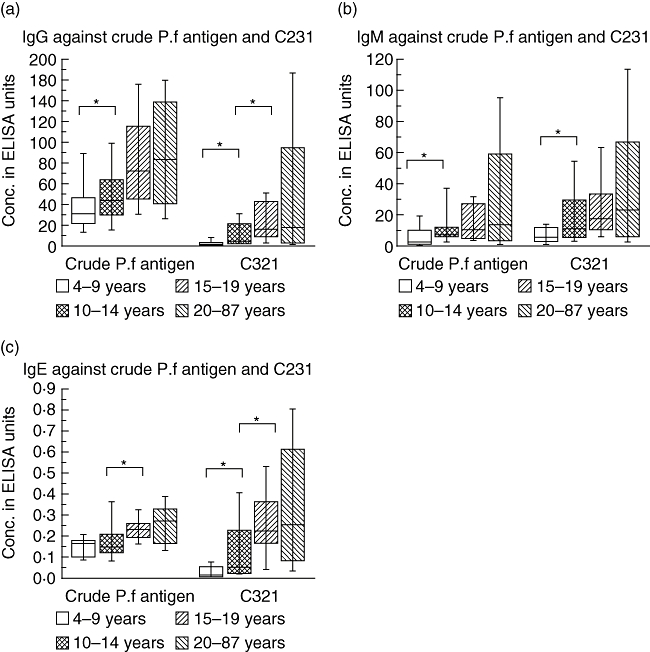
Immunoglobulin G (IgG) (a), IgM (b) and IgE (c) levels to crude Plasmodium falciparum antigen and the recombinant antigen C231, divided into different age groups, 4–9 years (n = 15), 10–14 years (n = 17), 15–19 years (n = 18) and 20–87 years (n = 50). Enzyme-linked immunosorbent assay using sera from Senegalese donors as described in Materials and methods measured antibody reactivity. The boxes represent the values between the 25% to the 75% quartile and the line indicates the median. The whiskers indicate the 10% and 90% quartile. *P < 0·05.
IgG antibody reactivity against crude malaria antigen was considerably higher than against C231, and was detected in the sera from all individuals, increasing with age (R = 0·461; P < 0·0001; n = 100) (Fig. 1). However, when the levels of IgG reactivity were compared between age groups, a significant difference (P < 0·05) was seen between only the two lowest age groups, 4–9 and 10–14 years, while no such difference was seen between the higher age groups (Fig. 2a).
While IgG antibody reactivity to C231 in age groups 4–9 and 10–14 years was mainly low or absent, there was an overall increase by age (R = 0·495; P < 0·0001) (Fig. 1). When the levels of IgG reactivity were compared between age groups, there were significant differences (P < 0·05) between age groups 4–9 and 10–14 years, as well as between 10–14 and 15–19 years (Fig. 2a), while no significant increase was seen in the highest age group. There was a significant correlation between the IgG antibody reactivity to C231 and crude malaria antigen (R = 0·787; P < 0·0001).
As for IgG, the serum IgM antibody reactivity with both crude malaria antigen and C231 increased with age (R = 0·310; P = 0·002 and R = 0·345; P = 0·0006 respectively). When the levels of IgM reactivity were compared between age groups, there was only a significant difference (P < 0·005) between the two lowest age groups, 4–9 and 10–14 years, regarding both antigens (Fig. 2b). While the levels of IgM antibody reactivity were similar against both C231 and crude malaria antigen, the IgM reactivity against C231 showed higher levels than the IgG reactivity against this antigen in all age groups, except for 15–19 years (Fig. 2a,b). With regard to IgG, there was a significant correlation between the IgM antibody reactivity to C231 and crude malaria antigen (R = 0·854; P < 0·0001).
Also the serum IgE antibody reactivity against both crude malaria antigen and C231 showed an increase by age (R = 0·525; P < 0·0001 and R = 0·477; P < 0·0001 respectively). Comparing the levels of IgE antibody reactivity with C231 between the different age groups, there was a significant difference (P < 0·05) between the groups 4–9 and 10–14 years, as well as between the latter group and 15–19 years, while no significant increase in IgE antibody reactivity was seen in the oldest group, 20–87 years (Fig. 2c). Regarding IgE antibodies reactive with crude malaria antigen, the levels differed significantly (P < 0·05) between only the age groups 10–14 and 15–19 years. Also for IgE, a significant correlation between the IgE antibody reactivity to C231 and crude malaria antigen was found (R = 0·553; P < 0·0001).
There was a significant correlation between the levels of IgE and IgG antibodies to both C321 and crude malaria antigen (R = 0·789; P < 0·0001 and R = 0·616; P < 0·0001 respectively), as well as between the levels of IgM and IgG to both antigens (C231: R = 0·776; P < 0·0001; crude malaria antigen: R = 0·728; P < 0·0001).
Immunoglobulin G subclass patterns of antibodies to C231 and crude malaria antigen
The levels of antibodies of the four IgG subclasses, showing reactivity with C231 and crude malaria antigen, were analysed by ELISA. IgG1 dominated the antibodies that were reactive with crude malaria antigen in concentrations, followed by IgG3, IgG2 and IgG4 (Table 1), and an increase of the levels with age was seen for all IgG subclasses, except for IgG4 (IgG1: R = 0·318; P = 0·0015; IgG2: R = 0·466; P < 0·0001; IgG3: R = 0·538; P < 0·0001; IgG4: R = −0·083; P = 0·42). However, when the levels of the IgG subclass reactivity were compared between age groups, significant differences were seen for only IgG1 and IgG3 between some of the age groups (Table 1).
Table 1.
Human serum immunoglobulin G (IgG) subclass reactivity with crude malaria antigen and C231 in different age groups. Values represent enzyme-linked immunosorbent assay units, mean ± standard error.
| Crude malaria antigen (F32) | C231 | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | IgG1 | IgG2 | IgG3 | IgG4 | IgG1 | IgG2 | IgG3 | IgG4 |
| 4–9 (n = 15) | 119 ± 46·6 | 1·1 ± 0·71 | 4·1 ± 1·2 | 0·5 ± 0·92 | 0·37 ± 0·13 | 0·1 ± 0·04 | 1·0 ± 0·91 | 0·08 ± 0·023 |
| P-value | 0·76 | 0·84 | 0·02 | 0·46 | 0·14 | 0·0013 | 0·0097 | 0·64 |
| 10–14 (n = 17) | 109 ± 35·3 | 0·93 ± 0·27 | 9·3 ± 2·3 | 0·56 ± 0·17 | 0·83 ± 0·44 | 1·8 ± 0·69 | 1·7 ± 0·71 | 0·66 ± 0·34 |
| P-value | 0·0091 | 0·067 | 0·013 | 0·87 | 0·016 | 0·075 | 0·0048 | 0·23 |
| 15–19 (n = 18) | 486 ± 194 | 8·8 ± 4·0 | 15·3 ± 1·9 | 0·68 ± 0·18 | 1·1 ± 0·36 | 4·0 ± 2·2 | 4·7 ± 1·2 | 0·72 ± 0·26 |
| P-value | 0·53 | 0·39 | 0·57 | 0·60 | 0·23 | 0·43 | 0·91 | 0·81 |
| 20–87 (n = 50) | 504 ± 108 | 9·9 ± 2·4 | 18·6 ± 1·9 | 0·55 ± 0·1 | 22·1 ± 8·4 | 6·3 ± 1·2 | 6·2 ± 0·98 | 1·2 ± 0·34 |
With regard to antibodies reactive with C231, IgG3 appeared in general at the highest concentrations, followed by IgG1 and IgG2 with largely similar concentrations, and IgG4 with the lowest concentrations (Table 1). The C231-reactive antibodies of all subclasses increased with age (IgG1: R = 0·2389; P = 0·0001; IgG2: R = 0·515; P < 0·0001; IgG3: R = 0·488; P < 0·0001; IgG4: R = 0·302; P = 0·003). However, when the levels of the IgG subclass reactivity were compared between age groups, significant differences were seen mainly for IgG2 and IgG3 between the lower age groups, 4–9 and 10–14 years, and regarding IgG1, IgG2 and IgG3 also between the age groups 10–14 and 15–19 years (Table 1). As with antibodies reactive with crude malaria antigen, nosignificant increase in antibody reactivity against C231 was seen in the highest age group for any of the IgG subclasses.
Comparison of the patterns of IgG subclasses of antibodies to C231 and crude malaria antigen in the different age groups was performed by calculating the ratios of the levels of IgG1/1gG2, IgG1/IgG3 and IgG2/IgG3 respectively (Table 2). IgG4 was omitted in this analysis, as this subclass showed consistently the lowest levels in all instances. While the IgG1/IgG2 ratios of antibodies against crude malaria antigen were high (range 51–108) and declined by age, these ratios for antibodies reactive with C231 were considerably lower (range 0·3–3·6). It is worth noting that, in the age groups 10–14 and 15–19 years, the levels of IgG2 antibodies to C231 exceeded those of IgG1 (Table 2). Also, the IgG1/IgG3 ratios of antibodies to crude malaria antigen were high (range 12–31), and this ratio was lowest in the age group 10–14 years, but was similarly higher in the other age groups. The IgG1/IgG3 ratios antibodies to C231 were low (range 0·2–3·5), and in the three lowest age groups the level of IgG3 exceeded that of IgG1. The IgG2/IgG3 ratios indicated that antibodies to crude malaria antigen show higher IgG3 than IgG2 levels in all age groups (range 0·1–0·5), while the levels of C231-reactive antibodies of these subclasses were similar in all age groups, except in the youngest ones, where IgG3 dominated (Table 2).
Table 2.
Ratios between the levels immunoglobulin G1 (IgG1), IgG2 and IgG3 subclasses of antibodies reactive with crude malaria antigen or C231 in the different age groups.
| Crude malaria antigen | C231 | |||||
|---|---|---|---|---|---|---|
| Age group (years) | IgG1 : IgG2 | IgG1 : IgG3 | IgG2 : IgG3 | IgG1 : IgG2 | IgG1 : IgG3 | IgG2 : IgG3 |
| 4–9 | 108 | 29 | 0·3 | 3·6 | 0·4 | 0·1 |
| 10–14 | 118 | 12 | 0·1 | 0·5 | 0·5 | 1·1 |
| 15–19 | 54 | 31 | 0·6 | 0·3 | 0·2 | 0·9 |
| 20–87 | 51 | 27 | 0·5 | 3·5 | 3·5 | 1·0 |
Correlation between IgG reactivities against C231 and EB200
In a previous report, the levels of IgG antibodies reactive with the Pf332 fragment EB200 were determined in the same serum samples studied herein [5]. Comparison of the IgG antibody reactivity against C231 and EB200 in the individual sera showed that there was a positive correlation (R = 0·560; P < 0·0001) (Fig. 3).
Fig. 3.
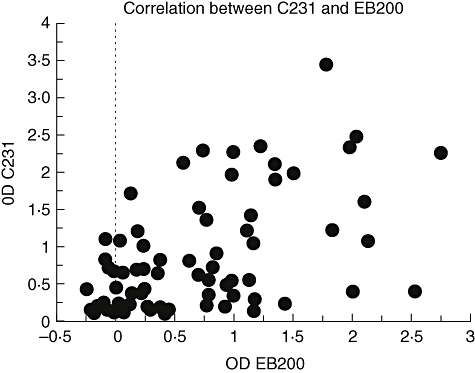
Correlation of enzyme-linked immunosorbent assay optical density values assaying immunoglobulin G (IgG) antibodies against C231 and EB200 in the Senegalese individuals. There is a positive correlation between C231 and EB200 (R = 0·560; P < 0·0001).
Comparison of serological and clinical data
All the donors were monitored for clinical attacks of malaria up to a year after the blood sampling [5]. The number of individuals experiencing one or more attacks was higher in children 4–9 years of age (nine of 17) than in the age groups 10–14 years (five of 17) and 15–19 years (one of 14). Of the 49 individuals older than 20 years of age, three experienced a malaria attack during the year of follow-up [5]. The mean age was higher in the group of individuals that did not experience malaria compared with those who did (28·2 ± 1·8 years and 13·3 ± 3·8 years respectively). Our previous observations indicated an association between reduced risks of malaria attack when IgG anti-crude malaria antigen (F32) or anti-EB200 increased [5]. Our analyses show that individuals who did not experience any malaria attacks during the follow-up period tended to have higher anti-C231 responses (IgM, IgG, IgE and IgG1-4) than the others, although these differences did not reach significance (Table 3). In a multivariate analysis of the IgG responses against C231, and when controlling for age, number of infected bites and parasitaemia, a potential association with protection was found (F ratio = 10·95; P = 0·0013). However, when using a non-linear multivariate model with a Poisson distribution, none of the anti-C231 isotype responses was found associated with protection.
Table 3.
Comparison of the levels of antibodies (geometric means and 95% confidence intervals of enzyme-linked immunosorbent assay units) between individuals with no clinical attack and individuals with malaria attacks detected during the year of follow-up. These comparative analyses were carried out on log-transformed data using non-parametric Mann–Whitney U-tests.
| No attack | Attacks | P-value | |
|---|---|---|---|
| Age (years ± s.d. values) | 28·2 ± 1·8 | 13·3 ± 3·8 | 0·0006 |
| C231 | |||
| IgG | 4·28 (2·49–7·33) | 0·64 (0·20–2·05) | 0·004 |
| IgG1 | 0·701 (0·37–0·77) | 0·18 (0·08–0·38) | 0·013 |
| IgG2 | 1·09 (0·69–1·72) | 0·15 (0·07–0·32) | 0·0002 |
| IgG3 | 1·25 (0·79–2·00) | 0·17 (0·06–0·48) | 0·0004 |
| IgG4 | 0·150 (0·09–0·24) | 0·05 (0·02–0·11) | 0·0517 |
| IgM | 16·20 (11·99–21·88) | 7·87 (4·31–14·39) | 0·0419 |
| IgE | 0·144 (0·11–0·19) | 0·04 (0·02–0·09) | 0·0018 |
| Crude malaria antigen | |||
| IgG | 73·45 (62·52–86·10) | 35·64 (23·99–52·97) | 0·0009 |
| IgG1 | 131 .52 (89·95–191·87) | 27·48 (10·12–74·47) | 0·0023 |
| IgG2 | 1·47 (0·98–2·21) | 0·40 (0·16–0·98) | 0·0012 |
| IgG3 | 11·64 (9·51–14·26) | 2·69 (1·47–4·91) | 0·0001 |
| IgG4 | 0·32 (0·25–0·42) | 0·35 (0·24–0·52) | 0·631 |
| IgM | 11·07 (8·05–15·23) | 5·87 (2·78–12·42) | 0·121 |
| IgE | 0·22 (0·19–0·24) | 0·13 (0·09–0·19) | 0·0008 |
| EB200 | |||
| IgG | 0·373 (0·240–0·579) | 0·036 (0·011–0·124) | 0·0001 |
After Bonferroni adjustment for the range of hypotheses performed simultaneously, a P-value of less than 0·003 for each individual test ensures that the risk of declaring differences in antibody responses by chance is reduced (using this procedure, the overall chance of making such an unjustified claim for the entire family of tests remains at P = 0·05). The values for EB200 (OD values at 1/1000 dilution) were extracted from Ahlborg et al. [5]. Mean ages are given for each subgroup. s.d.: Standard deviation.
The contribution of the isotype responses against crude malaria antigen (F32) in relation to the occurrence of malaria attacks was tested by multivariate analysis, controlling for age, number of infected bites and parasitaemia. The anti-F32 IgG3 responses were increased consistently when the occurrence of malaria attacks decreased (F ratio = 29·43; P < 0·0001). This indication remained valid when using a Poisson regression model to test for the association between anti-F32 IgG3 antibodies and a reduced occurrence of attacks. The potential association between IgG3 responses and protection was tested by including or excluding this explanatory variable from the model with a Poisson distribution (χ2 = 8·36; P = 0·0038). Of note, the same indications were found when testing the ratio of cytophilic (IgG1 + IgG3) to non-cytophilic (IgG2 + IgG4 + IgM) anti-F32 antibodies. In a linear multivariate model, including confounding variables such as age and parasitaemia, the ratio increased when the number of malaria attacks decreased (F ratio = 6·28; P = 0·0139). This association remained valid when tested in a non-linear model (χ2 = 11·47; P = 0·0007).
The potential association between IgE responses and the occurrence of malaria attacks was also tested, first in a stepwise regression model, and second in a Poisson regression model. Whereas in multivariate analysis no consistent association between anti-F32 IgE and protection could be detected, fewer malaria attacks were found when anti-C231 IgE increased (F ratio: 11·09; P = 0·0013). When this association was tested in Poisson regression models including or excluding the IgE responses, a reduced risk of malaria attack was associated only with increased anti-C231 IgE (χ2 = 6·63; P = 0·010).
Discussion
This study confirms and extends previous data, showing that the P. falciparum asexual blood-stage antigen Pf332 is recognized readily by antibodies elicited in humans continuously exposed to malaria [4,5,7,26]. All previous studies on antibody reactivities with Pf332 were performed using recombinant EB200 protein, a 157-amino-acid-fragment of Pf332 [6] or synthetic peptides representing repeat sequences in EB200 [4,27]. As EB200, like the major part of the large Pf332 molecule, is constituted of degenerate repeat sequences, containing regularly spaced pairs of Glu [6,10], antigenic cross-reactivity with other Glu-rich antigens is expected [8,28]. In order to circumvent the problem with cross-reactions, we performed the analyses in this study with recombinant C231 protein, which is derived from the non-repetitive C-terminal region of Pf332, having a considerably lower content of Glu and therefore expected to be less cross-reactive (Vasconselos et al. manuscript in preparation).
While IgG antibodies reactive with crude P. falciparum antigen were detected in the sera of all Senegalese individuals analysed in this study, many of the children displayed no or low reactivity against the C231 antigen. These antibody levels to both crude malaria antigen and C231 increased significantly by age, and most donors above 14 years showed antibodies to C231. The individual pattern of IgG antibody recognition of C231 correlated well with that seen previously in the corresponding sera for antibodies to EB200 [5]. While the antibodies to crude malaria antigen were predominantly of IgG1 subclass in all age groups, the levels of IgG1, IgG2 and IgG3 subclasses were more equal regarding antibodies to C231. For the C231-reactive antibodies the mean levels IgG3 were higher than those of IgG1 in all age groups, except in the oldest group. Also, in the two middle age groups, the mean levels of C231-reactive IgG2 were higher than those of IgG1. Interestingly, a previous study in the same village demonstrated that a high proportion of seropositive individuals showed anti-EB200 antibodies of IgG3 and IgG2 subclass and to a lesser extent IgG1 subclass [7]. The IgG subclass pattern of antibodies to both EB200 and C231 in this area is compatible with the late appearance of these antibodies after repeated exposure to P. falciparum[29].
Most malaria antigens tend to induce either predominant IgG1 or IgG3 antibody responses, the polarization to either subclass being dependent on the antigen or the age of the donor [30,31]. However, in some endemic settings high IgG2 antibody levels to certain P. falciparum antigens have been detected, which correlated with the elevated frequency of the IgG2 binding allele of FcγRIIa [32] in the study population [19,20]. Furthermore, immunofluorescence analysis on the parasite reactivity of sera from malaria endemic areas indicated that IgG2 antibodies bound preferentially to the surface of schizonts [29], which is also the location of Pf332 [1]. In the present study, even when controlling for various confounding factors, anti-P. falciparum IgG3 responses were found to be increased when malaria attacks were reduced. This result corroborates the initial investigations carried out in Dielmo village [17]. In addition, it was possible here to determine the ratio of cytophilic to non-cytophilic anti-P. falciparum antibodies. In agreement with previous observations [33], the ratio of cytophilic to non-cytophilic antibodies was found to be predictive of a decreased risk of malaria attack.
Comparison of serological and clinical data in a previous study with the same Senegalese donors showed that high levels of IgG antibodies to crude malaria antigen and to EB200 were predictive of fewer future clinical attacks of malaria [5]. Although the anti-C231 responses of all isotypes tended to be higher in individuals not experiencing any malaria attack during the follow-up period than those who experienced at least one attack, this difference did not reach statistical significance after controlling for age, number of infected bites and parasitaemia in a multivariate analysis. Thus, although the IgG responses of antibodies reactive with EB200 and C231 correlate well in the study population, they show different power in the association with protection. This difference may be explained possibly by the higher degree of cross-reactivity of anti-EB200 antibodies with other Glu-rich antigens [8,28]. Rabbit antibodies to both C231 and epitopes within EB200 show a similar capacity to inhibit parasite growth in P. falciparum in vitro cultures [3,10,34,35], reflecting a potential parasite neutralizing capacity in vivo of antibodies with these specificities. However, in general there is a poor association between antibody-mediated in vitro inhibition and protection in vivo, due possibly to the presence in naturally primed individuals of a heterogeneous mixture of inhibitory antibodies, blocking antibodies and parasite growth-promoting antibodies (reviewed in [36]).
In conclusion, our study shows that the C231 sequence of Pf332 harbours epitopes recognized by antibodies elicited by natural P. falciparum exposure. The C231-reactive antibodies exhibited a marked bias for IgG2 and IgG3 subclasses over IgG1, a pattern also indicated previously for antibodies reactive with EB200. Whether this subclass pattern reflects intrinsic antigenic properties of Pf332, or also occurs for other malarial antigens, remains to be investigated. Further studies are also needed for assessing possible associations between the bias for IgG2 and expression of the IgG2 binding FcγRIIa allele in the population studied.
Acknowledgments
This work was supported by grants from the Swedish Agency for Research Development with Developing Countries (SIDA, SAREC), the Swedish Medical Research Council (VR) and a grant within the BioMalPar European Network of Excellence (LSMP- CT- 2004-503578).
References
- 1.Hinterberg K, Scherf A, Gysin J, et al. Plasmodium falciparum: the Pf332 antigen is secreted from the parasite by a brefeldin A-dependent pathway and is translocated to the erythrocyte membrane via the maurer's clefts. Exp Parasitol. 1994;79:279–91. doi: 10.1006/expr.1994.1091. [DOI] [PubMed] [Google Scholar]
- 2.Mattei D, Scherf A. The Pf332 gene codes for a megadalton protein of Plasmodium falciparum asexual blood stages. Mem Inst Oswaldo Cruz. 1992;87(Suppl. 3):163–8. doi: 10.1590/s0074-02761992000700026. [DOI] [PubMed] [Google Scholar]
- 3.Ahlborg N, Iqbal J, Björk L, Ståhl S, Perlmann P, Berzins K. Plasmodium falciparum: differential parasite growth inhibition mediated by antibodies to the antigens Pf332 and Pf155/RESA. Exp Parasitol. 1996;82:155–63. doi: 10.1006/expr.1996.0020. [DOI] [PubMed] [Google Scholar]
- 4.Warsame M, Wernsdorfer WH, Perlmann H, et al. A malariometric survey in a rural community in the Muheza district, Tanzania: age profiles in the development of humoral immune responses. Acta Trop. 1997;68:239–53. doi: 10.1016/s0001-706x(97)00100-9. [DOI] [PubMed] [Google Scholar]
- 5.Ahlborg N, Haddad D, Siddique AB, et al. Antibody responses to the repetitive Plasmodium falciparum antigen Pf332 in humans naturally primed to the parasite. Clin Exp Immunol. 2002;129:318–25. doi: 10.1046/j.1365-2249.2002.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattei D, Scherf A. The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene. 1992;110:71–9. doi: 10.1016/0378-1119(92)90446-v. [DOI] [PubMed] [Google Scholar]
- 7.Perraut R, Mercereau-Puijalon O, Diouf B, et al. Seasonal fluctuation of antibody levels to Plasmodium falciparum parasitized red blood cell-associated antigens in two Senegalese villages with different transmission conditions. Am J Trop Med Hyg. 2000;62:746–51. doi: 10.4269/ajtmh.2000.62.746. [DOI] [PubMed] [Google Scholar]
- 8.Mattei D, Berzins K, Wahlgren M, et al. Cross-reactive antigenic determinants present on different Plasmodium falciparum blood-stage antigens. Parasite Immunol. 1989;11:15–29. doi: 10.1111/j.1365-3024.1989.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahlborg N, Berzins K, Perlmann P. Definition of the epitope recognized by the Plasmodium falciparum-reactive human monoclonal antibody 33G2. Mol Biochem Parasitol. 1991;46:89–95. doi: 10.1016/0166-6851(91)90202-h. [DOI] [PubMed] [Google Scholar]
- 10.Vasconselos N. Doctoral degree dissertation. Stockholm University; 2006. Vaccine development strategies applied to the Plasmodium falciparum malaria antigen Pf332. [Google Scholar]
- 11.Perlmann H, Helmby H, Hagstedt M, et al. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria: association of high IgE levels with cerebral malaria. Clin Exp Immunol. 1994;97:284–92. doi: 10.1111/j.1365-2249.1994.tb06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlmann P, Perlmann H, ElGhazali G, Blomberg MT. IgE and tumor necrosis factor in malaria infection. Immunol Lett. 1999;65:29–33. doi: 10.1016/s0165-2478(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 13.Calissano C, Modiano D, Sirima BS, et al. IgE antibodies to Plasmodium falciparum and severity of malaria in children of one ethnic group living in Burkina Faso. Am J Trop Med Hyg. 2003;69:31–5. [PubMed] [Google Scholar]
- 14.Bereczky S, Montgomery SM, Troye-Blomberg M, Rooth I, Shaw MA, Farnert A. Elevated anti-malarial IgE in asymptomatic individuals is associated with reduced risk for subsequent clinical malaria. Int J Parasitol. 2004;34:935–42. doi: 10.1016/j.ijpara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Boudin C, Chumpitazi B, Dziegiel M, et al. Possible role of specific immunoglobulin M antibodies to Plasmodium falciparum antigens in immunoprotection of humans living in a hyperendemic area, Burkina Faso. J Clin Microbiol. 1993;31:636–41. doi: 10.1128/jcm.31.3.636-641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasseur P, Ballet JJ, Druilhe P. Impairment of Plasmodium falciparum-specific antibody response in severe malaria. J Clin Microbiol. 1990;28:265–8. doi: 10.1128/jcm.28.2.265-268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aribot G, Rogier C, Sarthou JL, et al. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa) Am J Trop Med Hyg. 1996;54:449–57. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 18.Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol. 1990;141:529–42. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- 19.Aucan C, Traore Y, Tall F, et al. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun. 2000;68:1252–8. doi: 10.1128/iai.68.3.1252-1258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ntoumi F, Flori L, Mayengue PI, et al. Influence of carriage of hemoglobin AS and the Fc gamma receptor IIa-R131 allele on levels of immunoglobulin G2 antibodies to Plasmodium falciparum merozoite antigens in Gabonese children. J Infect Dis. 2005;192:1975–80. doi: 10.1086/497611. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JB, Trager W. Plasmodium falciparum in culture: establishment of additional strains. Am J Trop Med Hyg. 1978;27:743–6. doi: 10.4269/ajtmh.1978.27.743. [DOI] [PubMed] [Google Scholar]
- 22.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20. [PubMed] [Google Scholar]
- 23.Troye-Blomberg M, Romero P, Patarroyo ME, Bjorkman A, Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. III: proliferative response to antigen in vitro and subset composition of T cells from patients with acute infection or from immune donors. Clin Exp Immunol. 1984;58:380–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Trape JF, Rogier C, Konate L, et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–37. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 25.Rogier C, Tall A, Diagne N, Fontenille D, Spiegel A, Trape JF. Plasmodium falciparum clinical malaria: lessons from longitudinal studies in Senegal. Parassitologia. 1999;41:255–9. [PubMed] [Google Scholar]
- 26.Kulane A, Siddique AB, Sarthou JL, et al. Human immune responses to the highly repetitive Plasmodium falciparum antigen Pf332. Am J Trop Med Hyg. 1999;61:141–8. doi: 10.4269/ajtmh.1999.61.141. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal J, Perlmann P, Greenwood BM, Berzins K. Seroreactivity with the Plasmodium falciparum blood stage antigen Pf332 in adults and children from malaria-endemic regions. Clin Exp Immunol. 1993;94:68–74. doi: 10.1111/j.1365-2249.1993.tb05979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berzins K, Anders R. The malaria antigens. In: Wahlgren M, Perlmann P, editors. Malaria, molecular and clinical aspects. Amsterdam: Harwood Acad Publishers; 1999. pp. 181–216. [Google Scholar]
- 29.Wahlgren M, Perlmann H, Berzins K, et al. Characterization of the humoral immune response in Plasmodium falciparum malaria. III. Factors influencing the coexpression of antibody isotypes (IgM and IgG-1–4) Clin Exp Immunol. 1986;63:343–53. [PMC free article] [PubMed] [Google Scholar]
- 30.Tongren JE, Drakeley CJ, McDonald SL, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257–64. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matondo Maya DW, Mavoungou E, Deloron P, Theisen M, Ntoumi F. Distribution of IgG subclass antibodies specific for Plasmodium falciparum glutamate-rich-protein molecule in sickle cell trait children with asymptomatic infections. Exp Parasitol. 2006;112:92–8. doi: 10.1016/j.exppara.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–43. [PubMed] [Google Scholar]
- 33.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–81. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahlborg N, Flyg BW, Iqbal J, Perlmann P, Berzins K. Epitope specificity and capacity to inhibit parasite growth in vitro of human antibodies to repeat sequences of the Plasmodium falciparum antigen Ag332. Parasite Immunol. 1993;15:391–400. doi: 10.1111/j.1365-3024.1993.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahlborg N, Iqbal J, Hansson M, et al. Immunogens containing sequences from antigen Pf332 induce Plasmodium falciparum-reactive antibodies which inhibit parasite growth but not cytoadherence. Parasite Immunol. 1995;17:341–52. doi: 10.1111/j.1365-3024.1995.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 36.Bolad A, Berzins K. Antigenic diversity of Plasmodium falciparum and antibody-mediated parasite neutralization. Scand J Immunol. 2000;52:233–9. doi: 10.1046/j.1365-3083.2000.00787.x. [DOI] [PubMed] [Google Scholar]


