Abstract
As anti-inflammatory treatments used in rheumatoid arthritis, such as glucocorticoids, often result in secondary detrimental effects on bone health, the objective of this study was to investigate the effects of oestrogen therapy (ET) on the development and activity of collagen-induced arthritis (CIA) in rats, with a focus on assessment of chondroprotective effects using biomarkers of type II collagen degradation. Forty female Lewis rats were allocated into four intervention groups: (i) control + vehicle; (ii) CIA + vehicle; (iii) CIA + ET; and (iv) CIA + prednisolone. During the 28-day intervention period we monitored body weight, time-point of disease onset, incidence of manifest disease and paw volume. Levels of the type II collagen degradation marker (CTX-II) were measured in serum. At euthanasia, hind paws were isolated, extracted for proteins and measured for the concentration of CTX-II. Matrix metalloproteinase (MMP) activity was evaluated using gelatinase zymography. Oestrogen treatment delayed the time-point of disease onset and reduced the incidence and degree of manifest immunoarthritis significantly, assessed by macroscopic evaluation of hind paw inflammation and paw volume. Measures of serum or tissue levels of CTX-II showed significantly reduced type II collagen degradation elicited by oestrogen treatment. In alignment, a decreased activity of MMP-2 and MMP-9 was found in the paw protein extracts. We have demonstrated that the anti-inflammatory effect of ET is linked to chondroprotective effects in an animal model of systemic immunoarthritis. As ET has positive rather than negative effects on bone health in contrast to prednisolone, these observations may be important for potential combination therapy.
Keywords: CIA, type II collagen, CTX-II, oestrogen, prednisolone
Introduction
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease in which progressive destruction of cartilage, and later of bone, causes severe disability and morbidity of the patient [1]. Interestingly, RA is more common in women than in men, with peak incidence coinciding with the menopause and the cessation of ovarian production of oestrogens [2,3]. In conjunction, several investigators have reported favourable effects of oestrogen or hormone replacement therapy on disease activity and the progression of RA in postmenopausal women [4–6]. Collectively, these observations corroborate the notion that oestrogens may exert disease-modifying effects in systemic inflammatory diseases.
Steroid treatment for rheumatoid arthritis, glucocorticoids, is associated with strong negative effects on bone health leading to secondary osteoporosis [7,8]. If, however, an alternative steroid treatment with positive effects on bone was identified, this would benefit a large number of RA patients. The use of another natural steroid, oestrogen, is currently under much debate, in which negative and positive effects are compiled and evaluated [9–11]. An increasing body of evidence suggests positive effects of oestrogens on RA, in addition to the well-known beneficial effects on bone, vasomotor symptoms and a range of other postmenopausal symptoms [12].
Several investigations report that ovariectomy enhances susceptibility to arthritis [13–15], and that oestrogen supplementation suppresses inflammatory processes leading to autoimmunity [16–18], as well as reducing the incidence and degree of arthritic disease [13,15]. Raloxifene, a selective oestrogen receptor modulator, was able to attenuate inflammation and joint tissue damage associated with paw oedema and pleurisy in carrageenan-induced acute inflammation in rats, whereas an oestrogen receptor antagonist resulted in earlier onset of arthritis [19]. Many lines of evidence suggest that oestrogens and oestrogen-like substances may exert anti-inflammatory effects; oestrogen replacement therapy is shown to decrease levels of C-reactive protein and inflammatory cytokines during hormone replacement therapy in animals [20] and patients [21].
Elevated levels of proinflammatory cytokines have direct implications for increased secretion of proteolytic enzymes (e.g. matrix metalloproteinases, MMPs) from stromal cells of the synovium and from chondrocytes which, in turn, play a major role in eliciting cartilage damage [22–24]. Furthermore, experimental observations indicate that oestrogen can inhibit the expression and activity of MMPs and tissue inhibitors of MMPs [12,25]. However, whether the disease-modifying effects of oestrogens in systemic inflammatory joint disease can be assessed using specific markers of type II collagen degradation that reflect cartilage status, and how these relate to onset of disease and inflammation, remains to be investigated in detail in an in vivo animal model.
Therefore, the aims of this study were: to investigate how oestrogen therapy influences (i) the clinical manifestations of local inflammation; (ii) biomarkers of type II collagen degradation in the serum and joint protein extracts; and (iii) and the tissue levels of MMP activity in the rat model of collagen-induced arthritis.
Materials and methods
Animals
Forty female Lewis rats, 9 weeks old, weighing 151–175 g, were obtained from Charles River Laboratories (Sulzfeld, Germany). Two days after arrival, the animals were ear-marked, weighed and stratified into four groups of 10 animals per group (average weight in the range of 165 ± 1·9 g−165 ± 2·2 g). Animals were housed in standard type III H cages with sawdust bedding and nesting material in a Scantainer-plus (Scanbur, Sweden). They were fed with a standard diet (no. 1324, Altromin, Lage, Germany) and had access to MilliQ water ad libitum. Experiments began after 1 week of acclimatization.
All procedures were approved by the Danish Animal Experiments Inspectorate.
Induction of arthritis
Animals were anaesthetized by O2/CO2 and shaved around the root of the tail. On day 0 of the experiment arthritis was induced by intradermal injection at the root of the tail with 450 μg porcine type II collagen dissolved in 0·05 N acetic acid and emulsified 1 : 1 in incomplete Freund's adjuvant, to a total concentration of 1 mg/ml. Seven days after the first immunization, a similar booster injection was performed. Control rats were injected with 0·05 N acetic acid only.
Treatments
Animals were dosed orally twice a day, starting on the day of induction of arthritis, receiving a volume of 1·0 ml/200 g/dose. The animals were allocated into four intervention groups, receiving the following treatment: (i) control group, receiving vehicle [phosphate-buffered saline (PBS)]; (ii) CIA + vehicle, receiving vehicle (PBS); (iii) CIA + ET, implant of oestrogen-pellet (0·18 mg/60 days release) (Innovative Research of America, Sarasota, FL, USA) subcutaneously in the neck on day 0 and receiving vehicle (PBS); and (iv) CIA + prednisolone, receiving 1·5 mg/kg/dose (oral prednisolone fluid, Skanderborg Apotek, Skanderborg, Denmark).
Detection of disease onset and measurement of paw volume
From day 7, the animals were examined daily for signs of disease onset, defined as the first macroscopic evidence of hind paw erythema and oedema. At indicated time-points the hind paw volume was measured by immersion of the paw into a proper-sized container filled with a 10% soap solution of a known density. After retraction of the paw, the container was weighed, subtracted from the start weight and corrected for fluid density. Two people performed detection of disease onset by turns, while a single person recorded paw volume.
Collection of blood and preparation of serum
Blood was sampled from overnight-fasted, CO2/O2-anaesthetized animals exactly 3 h after the morning dosing. Blood was collected from the eye orbital sinus into plain tubes and left to coagulate at room temperature for 30 min. Samples were centrifuged at 1500 g for 10 min and crude serum was transferred to a new plain tube. Centrifugation was repeated and pure serum was harvested and stored at −20°C until use.
Protein extraction from hind paws
Hind paws were isolated following euthanasia, snap-frozen in liquid nitrogen and stored at −80°C until use. Still-frozen paws were submerged in liquid nitrogen and placed in a Bessman tissue pulverizer (Spectrum, Breda, the Netherlands). The sample was crushed and transferred to 3 ml of extraction buffer [50 mM TrisHCL buffer, pH 7·4, containing 0·1 M NaCl, 0·1% Triton X-100, 1 tablet/10 ml complete mini ethylenediamine tetraacetic acid (EDTA)-free protease inhibitor cocktail (Roche, Basel, Switzerland) and 10 μM GM6001 (Biomol, PA, USA)]. Tissues were homogenized (2 × 30 s) using an OMNI homogenizer (OmniInternational, Marietta, GA, USA), speed level 4; the sample was then centrifuged at 1700 g for 10 min, supernatants were harvested and centrifuged again at 10·000 g for 10 min, whereafter supernatants were stored at −20°C.
Enzyme-linked immunosorbent assay (ELISA)
C-terminal telopeptides of type II collagen (CTX-II) were measured in undiluted serum and paw protein extracts using the serum preclinical Cartilaps ELISA (Nordic Bioscience A/S, Herlev, Denmark). All measures were performed in duplicate.
Gelatinase zymography of MMP activity
Paw extracts from two animals in each group were evaluated. In the CIA + vehicle and CIA + ET groups the paws used were all diseased (established macroscopic erythema and oedema). Protein extracts from paw extraction were diluted 1 : 10 in PBS and 10 μl were mixed with 12·5 μl sample buffer [25% 1 M Tris/Base pH 6·8, 40% glycerol, 80 mg/ml sodium dodecyl sulphate (SDS), 0·5 mg/ml bromphenol blue (Merck, Whitehouse Station, NJ, USA)]. A 22·5 μl sample, 5 μl rainbow marker (Amersham, Buckinghamshire, UK) and 5 μl gelatinase zymography standards for MMP-2 and MMP-9 (Millipore, Billerica, MA, USA) were loaded onto a 7·5% SDS-polyacrylamide gel containing 0·5 mg/ml gelatine (Sigma-Aldrich Denmark, Brøndby, Denmark) as a substrate, and proteins were separated. After electrophoresis, gels were washed three times with 2·5% Triton X-100 in water and incubated overnight at 37°C in incubation buffer (0·1% TritonX-100, 5 mM CaCl2, 1 mM ZnCl2, 3 mM NaN3, 50 mM Tris pH 7·4) in a closed container. Gels were stained for 30 min with 0·25% Coomassie R-250 (Sigma-Aldrich) in 10% acetic acid and 45% methanol and destained for 30 min with 20% acetic acid, 20% methanol, 17% ethanol, 0·6% diethylether. Images were obtained by an Olympus C5050 zoom camera (Olympus Denmark, Ballerup, Denmark) and processed in CorelDraw (Corel, Unterschleissheim, Germany).
Data and statistical analysis
All values are expressed as the mean ± standard error of the mean (s.e.m.). All mean values presented in figures represent the entire group, i.e. both symptomatic and non-symptomatic animals. Statistical comparison was performed in prism software using an analysis of variance (anova) test and a Tukey's multiple comparison test. A Fisher's exact test for comparison of small sample sizes was applied for statistical evaluation of the disease incidence data.
Results
Animals
Regardless of whether immunized with vehicle or porcine type II collagen, animals developed superficial wounds from intradermal injections. However, these were not disabling the animals in any way. Disease manifestations, i.e. swelling of the hind paws, was restrictive on mobility, but not to the extent of disabling the animals to move around freely.
One animal in the CIA + ET group died from anaesthesia during blood sampling, while two animals in the CIA + prednisolone group were killed due to too great a weight loss.
Weights of animals
Figure 1depicts the results from monitoring body weight during the study period. At baseline, the average weight of the groups fell into the range of 165 ± 1·9–165 ± 2·2 g. During the experiment, the control group increased body weight continuously over the study period (+19%). After an initial peak non-treated CIA rats showed an even decline, resulting in a final 2·6% weight increase. Oestrogen replacement therapy resulted in an 11·7% weight increase despite a rapid drop at the end of the study period, whereas prednisolone treatment reduced weight by 0·5%.
Fig. 1.
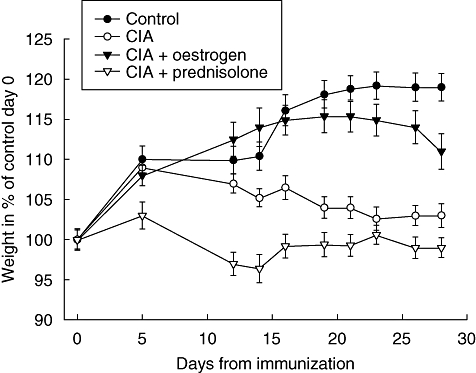
Weights of animals. The graph shows the mean ± standard error of the mean weights of the four groups in percentage of the control group at day 0.
Disease incidence
The time-point of disease onset and percentage of diseased animals during the course of the study period are shown in Fig. 2. Disease onset in the non-treated CIA + vehicle group occurred on day 11 after immunization, and incidence increased rapidly to 100% by day 15. Oestrogen treatment both delayed the average time-point of disease onset and reduced the number of diseased animals to 66%. Prednisolone treatment prevented disease onset completely in the immunized animals. Using Fisher's exact test for small sample numbers, a statistical comparison of disease incidence between the control and CIA groups showed a significant increase (P < 0·05) from day 13 after immunization. Incidence in the CIA + ET group was reduced significantly (P < 0·05) from days 15 to 18 when compared to the CIA group, but from day 19 to the end of the study the difference was non-significant. A significant difference (P < 0·05) between the CIA + ET and CIA + prednisolone groups occurred on day 14 and continued to the end of the study.
Fig. 2.
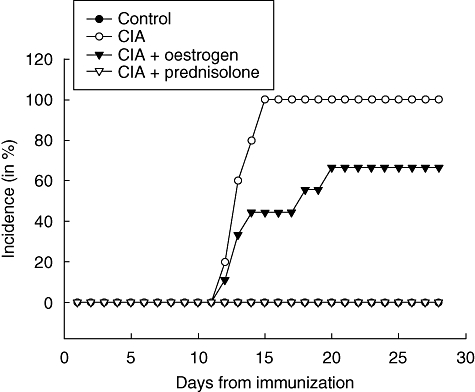
Disease incidence. The graph shows the course of animals with manifest disease in percentage of total animals within the group.
Paw volume
As inflammation is accompanied by interstitial oedema, the paw volume is a frequently used measure of disease activity. As shown in Fig. 3a, no difference in changes in paw volume was seen between groups before disease onset (day 7), whereas on days 14, 21 and 28 the paw volume change was increased considerably in the CIA group. Oestrogen supplementation reduced paw inflammation efficiently and decreased paw volume by 48% (P < 0·01) on day 28, compared to the CIA group (Fig. 3b), while prednisolone treatment caused a negative change in paw volume.
Fig. 3.
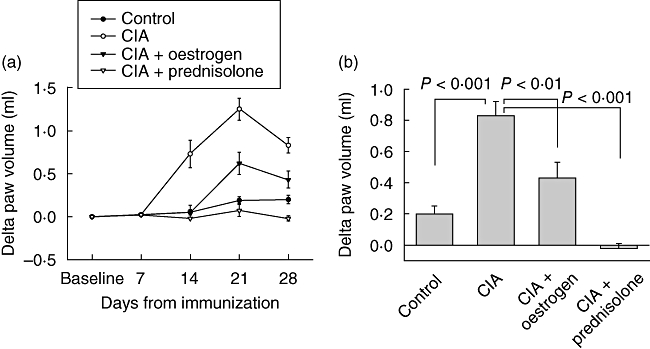
Change in paw volume in ml from baseline within each animal group at different time-points during the course of the study (a), and on day 28 (b). Values are shown as mean ± standard error of the mean changes in paw volume (ml) from baseline within each group.
Serum markers of cartilage degradation
The C-terminal telopeptide of type II collagen (CTX-II) is a biochemical marker of type II collagen degradation. Levels of serum CTX-II were similar between groups at baseline (Fig. 4a); thereafter, CTX-II increased progressively in the non-treated CIA group. Intervention prevented CTX-II release efficiently, so that on day 28 (Fig. 4b) CTX-II in the CIA group was increased to 250% (P < 0·01) compared to control, while oestrogen and prednisolone treatment restored CTX-II levels to 116% (P < 0·01) and 49% (P < 0·001), respectively, compared to control.
Fig. 4.
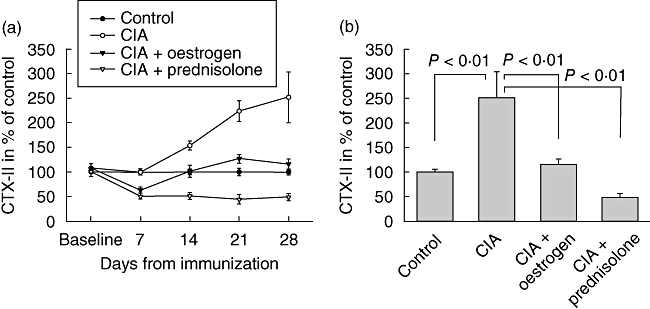
Type II collagen degradation measured in serum. The graph shows mean ± standard error of the mean serum C-terminal telopeptide of type II collagen levels at different time-points during the course of the study in percentage of control on individual days (a) and on day 28 in percentage of control (b).
Measures of cartilage degradation in protein extracts from hind paws
To assess local chondroprotective effects from treatment, we extracted proteins from hind paws collected on day 28 and measured type II collagen degradation. Compared to levels in control joints, the type II collagen degradation marker CTX-II was elevated by 400% (P < 0·001) in joints from the non-treated CIA group (Fig. 5). Oestrogen and prednisolone treatment reduced CTX-II levels efficiently to 112% (P < 0·001) and 21% (P < 0·001), respectively, compared to control.
Fig. 5.
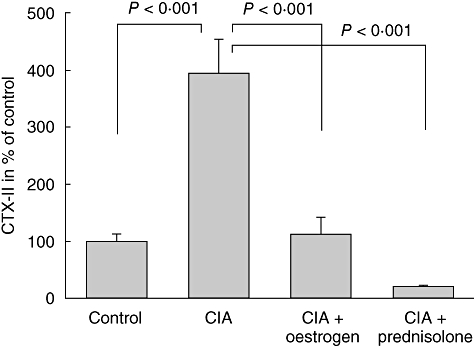
Type II collagen degradation measured in protein extracts from paws. The graph shows mean ± standard error of the mean C-terminal telopeptide of type II collagen levels in percentage of control.
MMP activity in protein extracts from hind paws
Using gelatinase zymography (Fig. 6), we detected marked increases in MMP-2 and MMP-9 activity in paw protein extracts from non-treated CIA rats, identified by clear bands from gelatine degradation. This increased MMP activity associated with disease was countered efficiently by both oestrogen replacement and prednisolone treatment.
Fig. 6.

Matrix metalloproteinase (MMP)-9 and MMP-2 activity in protein extracts from paws, assessed by gelatinase zymography. The figure shows bands corresponding to MMP-9 and MMP-2 from two representative diseased paws within each group. Intervention decreased MMP activity compared to the collagen-induced arthritis group, indicated by a smaller band of degraded gelatin.
Discussion
The main findings of the study were as follows: (i) ET delayed the time-point of disease onset and reduced disease incidence significantly in immunized CIA rats; (ii) increases in paw volume due to arthritic disease were inhibited efficiently by ET; (iii) ET efficiently reduced type II collagen degradation measured in serum and locally in protein extracts from paws; (iv) ET reduced MMP-2 and MMP-9 activity and expression resulting from arthritic disease; and (v) the anti-inflammatory effects of ET were less than, but the effects on reduction of type II collagen degradation similar to, those of prednisolone. Collectively, these observations argue for the notion that the anti-inflammatory effects of ET are associated with decreased collagen type II degradation, and thereby protection of the cartilage in the CIA model, suggesting that this therapy might be a useful adjuvant intervention in the treatment of joint destruction in RA.
Arthritic disease resulted in reduced growth, whereas control animals gained weight as expected. Although prednisolone completely prevented arthritic disease, glucocortcoids are known to possess a general anti-anabolic and catabolic effect on bone and muscle growth, adding to the risk of developing secondary osteoporosis [7,8,26]. In contrast, ET almost restored weights of collagen-challenged animals towards control levels.
ET delayed efficiently the time-point of disease onset and reduced significantly the incidence of manifest arthritis at some time-points. These anti-inflammatory effects of oestrogen are reported by several other studies showing that administration of oestrogens [27–29] or oestrogen receptor agonists [30,31] relieve arthritic disease. Accordingly, an oestrogen receptor antagonist given to CIA mice resulted in the earlier development of arthritis [19]. Indeed, in vitro observations corroborate the notion that the sex steroid may exert an inhibitory effect on the secretion of proinflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6 from stromal cells of the synovium [28] or from macrophages [32], which play a central role in the provocation of local symptoms such as hyperaemia and interstitial oedema. Apparently, ET is able to suppress the overall initial immune responses sufficiently to prevent one-third of the animals from ever developing visual signs of disease, while not ruling out some level of immune response. Oestradiol treatment has also been shown to decrease the production of specific IgG2a anti-collagen type II antibodies and alter the T helper (Th) profile of the autoimmune T cell response towards a poor antibody isotype [33,34]. In addition, the direct effects of oestrogens on articular cartilage health have been proposed recently, and preliminary evidence has been provided [12].
Prednisolone is a strong anti-inflammatory corticosteroid derivative with marked effects on the propagation of white blood cells at sites of immunoreaction. In clinical settings, prednisolone is a standard intervention to suppress acute or fulminate forms of immunoarthritis in RA and other autoimmune diseases [35]. Compared to the effects of prednisolone, ET was also able to exert significant protective effects and prevent the manifestation of the local joint symptoms, suggesting anti-inflammatory properties of oestrogen.
Parallel to the propagation of inflammatory symptoms in affected joints, we observed marked elevation of serum CTX-II in the CIA-group, whereas significantly lower levels were observed in both ET and prednisolone-treated animals, the level being approximately similar to those in control animals. These observations suggest that type II collagen degradation could be prevented effectively by both interventions [12,36]. Interestingly, ET only partly prevented disease incidence, indicating that the cartilage protective properties of oestrogen might originate from direct effects on the cartilage and not solely through modulation of the immune response.
To validate that the protective effects of interventions act on the collagen type II content of affected joints, we extracted proteins from the collected joints and compared CTX-II content between the different groups at different time-points. In accordance with the findings in serum, the local concentration of the biomarker also indicated effective countering of the accelerated type II collagen degradation accompanying the propagation of CIA. Thus, this latter observation made directly in affected joints provides proof for the emerging concept that the anti-inflammatory effects can be linked to cartilage protective effects in animals with immunoarthritis.
Collectively, the observations outlined above support the statement that the anti-inflammatory effects of the interventions tested in this study provide chondroprotective effects, countering effectively the degradation of the collagen type II network, the major component of the extracellular matrix in articular cartilage.
MMPs are pivotal for the generation of CTX-II epitopes from type II collagen [24], and demonstrate clearly that an increase in MMP activity is a major component of disease mechanisms. Proinflammatory cytokines are known stimulators of this enzyme group, as demonstrated by both in vitro and in vivo observations [24,37,38]. We demonstrated by zymography that ET resulted in inhibition of MMP activity and significantly less type II collagen degradation in paw extracts, suggesting that ET works at different levels to improve joint health.
There are important clinical implications as a consequence of the use of different steroids affecting the cholesterol backbone. Whereas glucocorticoids are well known to have deleterious effects on bone health [39,40], oestrogens and oestrogen-like molecules have documented positive effects [41]. Thereby, different steroids that have alternative modes of action may result in different clinical outcomes with highly divergent effects on the bone compartment. Further experimental data and clinical studies are needed to investigate these important implications.
In conclusion, this study demonstrates that ET, similar to corticosteroids, can exert important immunomodulant effects and can be linked to effective protection of type II collagen in an animal model of RA. Underlying mechanisms probably involve the inhibited release of proinflammatory cytokines and other agents which, in turn, results in decreased expression of MMPs and ultimately in decreased type II collagen degradation in affected joints, assessed easily by measures of biochemical markers. Further studies are warranted to test whether ET could be beneficial for reducing the incidence of RA or be used as an adjuvant therapy to promote more effective cartilage protection in destructive joint diseases in patients.
References
- 1.Mili F, Helmick CG, Zack MM. Prevalence of arthritis: analysis of data from the US Behavioral Risk Factor Surveillance System, 1996–99. J Rheumatol. 2002;29:1981–8. [PubMed] [Google Scholar]
- 2.Wluka AE, Cicuttini FM, Spector TD. Menopause, oestrogens and arthritis. Maturitas. 2000;35:183–99. doi: 10.1016/s0378-5122(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 3.Goemaere S, Ackerman C, Goethals K, et al. Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J Rheumatol. 1990;17:1620–2. [PubMed] [Google Scholar]
- 4.D'Elia HF, Larsen A, Mattsson LA, et al. Influence of hormone replacement therapy on disease progression and bone mineral density in rheumatoid arthritis. J Rheumatol. 2003;30:1456–63. [PubMed] [Google Scholar]
- 5.Hall GM, Daniels M, Huskisson EC, Spector TD. A randomised controlled trial of the effect of hormone replacement therapy on disease activity in postmenopausal rheumatoid arthritis. Ann Rheum Dis. 1994;53:112–16. doi: 10.1136/ard.53.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julkunen H. Hormone replacement therapy in women with rheumatic diseases. Scand J Rheumatol. 2000;29:146–53. doi: 10.1080/030097400750002003. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura J, Ikuyama S. Glucocorticoid-induced osteoporosis: pathogenesis and management. J Bone Miner Metab. 2000;18:350–2. doi: 10.1007/s007740070008. [DOI] [PubMed] [Google Scholar]
- 8.Bendele AM. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact. 2002;2:501–3. [PubMed] [Google Scholar]
- 9.Weiner MG, Barnhart K, Xie D, Tannen RL. Hormone therapy and coronary heart disease in young women. Menopause. 2008;15:86–93. doi: 10.1097/gme.0b013e3180413e45. [DOI] [PubMed] [Google Scholar]
- 10.Pines A, Sturdee DW, Birkhauser MH, Society OB. Hormone therapy and cardiovascular disease in the early postmenopause: the WHI data revisited. Climacteric. 2007;10:195–6. doi: 10.1080/13697130701401073. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 12.Oestergaard S, Sondergaard BC, Hoegh-Andersen P, et al. Effects of ovariectomy and estrogen therapy on type II collagen degradation and structural integrity of articular cartilage in rats: implications of the time of initiation. Arthritis Rheum. 2006;54:2441–51. doi: 10.1002/art.22009. [DOI] [PubMed] [Google Scholar]
- 13.Jansson L, Holmdahl R. Oestrogen-induced suppression of collagen arthritis; 17 beta-oestradiol is therapeutically active in normal and castrated F1 hybrid mice of both sexes. Clin Exp Immunol. 1992;89:446–51. doi: 10.1111/j.1365-2249.1992.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmdahl R, Jansson L, Meyerson B, Klareskog L. Oestrogen induced suppression of collagen arthritis. I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin Exp Immunol. 1987;70:372–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito E, Iacono A, Raso GM, et al. Raloxifene, a selective estrogen receptor modulator, reduces carrageenan-induced acute inflammation in normal and ovariectomized rats. Endocrinology. 2005;146:3301–8. doi: 10.1210/en.2005-0375. [DOI] [PubMed] [Google Scholar]
- 16.Kappas A, Jones HE, Roitt IM. Effects of steroid sex hormones on immunological phenomena. Nature. 1963;198:902. doi: 10.1038/198902a0. [DOI] [PubMed] [Google Scholar]
- 17.Mueller MN, Kappas A. Estrogen pharmacology. II. Suppression of experimental immune polyarthritis. Proc Soc Exp Biol Med. 1964;117:845–7. doi: 10.3181/00379727-117-29715. [DOI] [PubMed] [Google Scholar]
- 18.Toivanen P, Maatta K, Suolanen R, Tykkylainen R. Effect of estrone and progesterone on adjuvant arthritis in rats. Med Pharmacol Exp Int J Exp Med. 1967;17:33–42. doi: 10.1159/000137052. [DOI] [PubMed] [Google Scholar]
- 19.Jansson L, Holmdahl R. Enhancement of collagen-induced arthritis in female mice by estrogen receptor blockage. Arthritis Rheum. 2001;44:2168–75. doi: 10.1002/1529-0131(200109)44:9<2168::aid-art370>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Keith JC, Albert LM, Leathurby Y, et al. The utility of pathway selective estrogen receptor ligands that inhibit nuclear factor-kappa B transcriptional activity in models of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R427–38. doi: 10.1186/ar1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsten H. Immune responses and bone loss: the estrogen connection. Immunol Rev. 2005;208:194–206. doi: 10.1111/j.0105-2896.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 22.Malemud CJ, Islam N, Haqqi TM. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cell Tissues Organs. 2003;174:34–48. doi: 10.1159/000070573. [DOI] [PubMed] [Google Scholar]
- 23.Bessis N, Boissier MC. Novel pro-inflammatory interleukins: potential therapeutic targets in rheumatoid arthritis. Joint Bone Spine. 2001;68:477–81. doi: 10.1016/s1297-319x(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 24.Sondergaard BC, Henriksen K, Wulf H, et al. Relative contribution of matrix metalloprotease and cysteine protease activities to cytokine-stimulated articular cartilage degradation. Osteoarthritis Cartilage. 2006;14:738–48. doi: 10.1016/j.joca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Lee YJ, Lim WC, Jin YH, Lee SK. Effects of estrogen receptor and estrogen on the chromatin structure in estrogen receptor stable transfectants. Exp Mol Med. 2002;34:95–9. doi: 10.1038/emm.2002.14. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg Z. Mechanisms of steroid impairment of growth. Horm Res. 2002;58(Suppl. 1):33–8. doi: 10.1159/000064764. [DOI] [PubMed] [Google Scholar]
- 27.Josefsson E, Tarkowski A. Suppression of type II collagen-induced arthritis by the endogenous estrogen metabolite 2-methoxyestradiol. Arthritis Rheum. 1997;40:154–63. doi: 10.1002/art.1780400120. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian S, Tovey M, Afentoulis M, Krogstad A, Vandenbark AA, Offner H. Ethinyl estradiol treats collagen-induced arthritis in DBA/1LacJ mice by inhibiting the production of TNF-alpha and IL-1beta. Clin Immunol. 2005;115:162–72. doi: 10.1016/j.clim.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki D, Enokida M, Okano T, Hagino H, Teshima R. Effects of ovariectomy and estrogen replacement therapy on arthritis and bone mineral density in rats with collagen-induced arthritis. Bone. 2001;28:634–40. doi: 10.1016/s8756-3282(01)00426-4. [DOI] [PubMed] [Google Scholar]
- 30.Badger AM, Blake SM, Dodds RA, et al. Idoxifene, a novel selective estrogen receptor modulator, is effective in a rat model of adjuvant-induced arthritis. J Pharmacol Exp Ther. 1999;291:1380–6. [PubMed] [Google Scholar]
- 31.Harris HA, Albert LM, Leathurby Y, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–9. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 32.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50:1967–75. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 33.Jansson L, Mattsson A, Mattsson R, Holmdahl R. Estrogen induced suppression of collagen arthritis. V. Physiological level of estrogen in DBA/1 mice is therapeutic on established arthritis, suppresses anti-type II collagen T-cell dependent immunity and stimulates polyclonal B-cell activity. J Autoimmun. 1990;3:257–70. doi: 10.1016/0896-8411(90)90145-i. [DOI] [PubMed] [Google Scholar]
- 34.Waksman Y, Hod I, Friedman A. Therapeutic effects of estradiol benzoate on development of collagen-induced arthritis (CIA) in the Lewis rat are mediated via suppression of the humoral response against denatured collagen type II (CII) Clin Exp Immunol. 1996;103:376–83. doi: 10.1111/j.1365-2249.1996.tb08290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landewe R, Geusens P, Boers M, et al. Markers for type II collagen breakdown predict the effect of disease-modifying treatment on long-term radiographic progression in patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1390–9. doi: 10.1002/art.20222. [DOI] [PubMed] [Google Scholar]
- 36.Christgau S, Tanko LB, Cloos PA, et al. Suppression of elevated cartilage turnover in postmenopausal women and in ovariectomized rats by estrogen and a selective estrogen-receptor modulator (SERM) Menopause. 2004;11:508–18. doi: 10.1097/01.wcb.0000121484.18437.98. [DOI] [PubMed] [Google Scholar]
- 37.Leone AK, Chun JA, Koehler CL, Caranto J, King JM. Effect of proinflammatory cytokines, tumor necrosis factor-alpha and interferon-gamma on epithelial barrier function and matrix metalloproteinase-9 in Madin Darby canine kidney cells. Cell Physiol Biochem. 2007;19:99–112. doi: 10.1159/000099198. [DOI] [PubMed] [Google Scholar]
- 38.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–63. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 39.Devogelaer JP. Glucocorticoid-induced osteoporosis: mechanisms and therapeutic approach. Rheum Dis Clin North Am. 2006;32:733–57. doi: 10.1016/j.rdc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–9. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 41.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]


