Abstract
Ulcerative colitis (UC) is a multi-factorial inflammatory disease of the colon and rectum. The present study was undertaken to investigate the effect of taurine, an anti-oxidant amino acid, on oxidative stress and the expression of apoptosis-related proteins, pro-apoptotic Bax and anti-apoptotic B cell lymphoma-2 (Bcl-2) in colon tissue in rats with 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Rats received taurine (1·5% w/v) in drinking water for 15 days before and 15 days after administration of TNBS solution. Then, colonic myeloperoxidase (MPO) activity, malondialdehyde (MDA) and glutathione (GSH) levels, and Bax and Bcl-2 expression were measured. TNBS-induced colitis caused significantly increased MPO activity and MDA levels and decreased GSH levels in colon tissue compared to controls. Increase in Bax expression and decrease in Bcl-2 expression were detected in colon of rats with TNBS-induced colitis. Taurine treatment was associated with amelioration in macroscopic and microscopic colitis scores, decreased colonic MPO activity and MDA levels and increased GSH levels in TNBS-induced colitis. In addition, taurine reduced the expression of Bax and prevented the loss of Bcl-2 proteins in colon tissue of rats with TNBS-induced colitis. The results of this study show that taurine administration may exert beneficial effects in UC by decreasing inflammatory reactions, oxidative stress and apoptosis.
Keywords: apoptosis, oxidative stress, rat, taurine, TNBS-induced colitis
Introduction
Inflammatory bowel disease (IBD) is a chronic condition of the intestine of unknown aetiology involving multiple immune, genetic and environmental factors. IBD includes ulcerative colitis (UC) and Crohn's disease in the clinic. UC is a non-specific inflammatory disorder resulting from transmural infiltration of neutrophils, macrophages, lymphocytes and mast cells, giving rise ultimately to mucosal disruption and ulceration [1,2]. Many factors have been implicated in the pathogenesis of UC, such as neutrophil infiltration and over-production of proinflammatory mediators including cytokines, and reactive oxygen species (ROS). The tissue injury produced by neutrophils and macrophages has been attributed to their ability to release ROS, nitrogen metabolites, cytotoxic proteins, lytic enzymes and cytokines as well as their disrupting effects on epithelial integrity [3–5].
ROS, peroxynitrite and nitric oxide were also reported to induce apoptosis [6,7]. Increasing evidence has shown that the acceleration of epithelial cell apoptosis and inhibition of inflammatory cell apoptosis were associated closely with colonic tissue injury and immunological abnormality in UC [8–11]. Therefore, apoptosis was reported to play an important role in loss of epithelial cells in UC [8–11].
Taurine (2-aminoethanesulphonic acid) is the major intracellular free β-amino acid, which is normally present in most mammalian tissues [12,13]. It has various cytoprotective properties such as anti-oxidation, osmoregulation, membrane stabilization and neurotransmission [12–14]. Taurine was found to be a protective agent against oxidative stress-induced pathologies such as atherosclerosis [15], diabetic complications [12,16], hepatic [17,18] and gastrointestinal [18] damage. In addition, taurine was also reported to have anti-apoptotic properties [18–20]. It has been reported that taurine inhibited oxidative stress-induced apoptosis in several cells, such as hepatocytes [18], cardiomyocytes [19] and epithelial cells [20].
The present study was undertaken to investigate the effect of taurine, an anti-oxidant amino acid, on oxidative stress and apoptosis in colon tissue in rats with 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis. Therefore, lipid peroxide and glutathione (GSH) levels and the expression of apoptosis-related proteins, pro-apoptotic Bax and anti-apoptotic B cell lymphoma-2 (Bcl-2) in the colon tissue were determined.
Methods
Animals
Male Wistar rats weighing 200–250 g were used in the study. They were obtained from the Experimental Medical Research Institute of Istanbul University. All animals were housed in wire-mesh-bottomed cages in a 12-h light/dark cycle and kept in a room at a constant temperature of 22 ± 2°C. They were allowed free access to tap water and standard chow diet. The experimental procedure used in this study met the guidelines of the Animal Care and Use Committee of Istanbul University.
Study design
The rats were divided into four groups. Animals in group 1 were used as the control group (n = 6). Group 2 (Tau; n = 6) received taurine (1.5% w/v) alone in drinking water for 30 days. Group 3 (TNBS; n = 8) had TNBS-induced colitis alone. Group 4 (TNBS + Tau; n = 8) had TNBS-induced colitis and taurine (Sigma, St Louis, MO, USA) treatment for 15 days before and 15 days after administration of TNBS solution.
In this study, the daily amount of taurine received by rats was approximately 250 mg per rat according to average water intake. The given taurine concentration is a pharmacological dose and similar doses were used by several investigators, as reported previously [21–23].
Induction of colitis
Rats were anaesthetized lightly with ether following a 24-h fast. A 5-French polyurethane cannula was inserted into the anus and the tip was advanced to 8 cm proximal to the anal verge. TNBS (Sigma) dissolved in 50% ethanol was instilled into the colon through the cannula (10 mg in a volume of 0.25 ml per rat) to induce colitis. Following instillation of the TNBS solution, rats were maintained in a head-down position for a few minutes to prevent leakage of the intracolonic instillation.
Assessment of macroscopic and microscopic colitis
All animals were killed 15 days after TNBS treatment. The distal 12 cm of the colon was excised free of adherent adipose tissue, rinsed with ice-cold saline and opened longitudinally. The colon was examined visually immediately and damage was scored on a scale of 0–5 [24]. Scoring of macroscopic colon damage in TNBS-induced colitis was as follows: (0) no colonic damage; (1) hyperaemia and no ulcer; (2) linear ulcer and no colonic wall thickening; (3) linear ulcer and colonic wall thickening in one area; (4) colonic ulcer at multiple areas; and (5) major ulcer and perforation.
The samples of colonic tissue were fixed for histopathological examination. Five samples for each group were selected randomly and their paraffin blocks were prepared. At least four paraffin blocks were investigated. The colonic pathological changes were observed and evaluated by two independent researchers with a modified histopathological score formula [25], as follows: (i) infiltration of acute inflammatory cells: 0 none, 1 mild, 2 severe; (ii) infiltration of chronic inflammatory cells: 0 none, 1 mild, 2 severe; (iii) fibrin deposition: 0 negative, 1 positive; (iv) submucosal oedema: 0 none, 1 focal, 2 diffuse; (v) necrosis of epithelial cells: 0 none, 1 focal, 2 diffuse; and (vi) mucosal ulcer: 0 negative, 1 positive.
Myeloperoxidase (MPO) activity
Colonic MPO activity was assayed by the o-dianisidine method [26]. Tissue samples were suspended in 50 mM, pH 6.0, potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide and homogenized. A sample of homogenate was sonicated for 20 s, freeze–thawed three times, sonicated again for 10 s, and centrifuged at 15 000 g for 10 min at 4°C. The reaction was started by mixing and incubating the 100 μl of the supernatant at 25°C with a solution composed of 2.81 μl of 50 mM potassium phosphate buffer, pH 6.0, 30 μl of 20 mg/ml o-dianisidine dihydrochloride and 30 μl of 20 mM H2O2. After 10 min the reaction was terminated by the addition of 30 μl of 2% sodium azide. The change in absorbance was read at 460 nm and the results were calculated by using the molar absorptivity coefficient of oxidized o-dianisidine (1.0062 × 104 M−1 cm−1). MPO activity (1 unit) was expressed as the amount of enzyme necessary for the degradation of 1 μmol of H2O2/min/100 mg tissue at 25°C.
Malondialdehyde (MDA) levels
The levels of MDA in the colonic tissues were measured to assess lipid peroxidation. Samples of colon tissues were homogenized with ice-cold 150 mM potassium chloride for determination of MDA levels. MDA levels were measured spectrophotometrically. Results are expressed as nmol of MDA per mg protein [27].
Glutathione (GSH) levels
Glutathione levels in the colonic homogenates were determined by the method described previously [28]. After precipitation with metaphosphoric acid, supernatants were reacted with 5,5′-dithiobis-2-nitrobenzoic acid. Absorbance was read spectrophotometrically at 412 nm.
Western blot analysis for Bax and Bcl-2
To isolate total colon protein, a portion of frozen tissue (∼ 0.1 g) was placed into a microfuge tube containing cell lysis buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Ipegal 630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 50 mM sodium fluoride (NaF), 1 mM phenylmethylsulphonyl fluoride, 1 mM dithiothreitol, 10 μg/ml aprotinin, 10 μg/ml leupeptin and 10 μg/ml soy bean trypsin inhibitor]. Tissues were homogenized, aspirated through a 21-gauge needle on ice and incubated for 45 min to lyse cells and release proteins. Lysates were collected and microcentrifuged at 12 000 g for 15 min to remove cellular debris [29,30]. The supernatant was processed for determination of total protein concentration by using bicinchoninic acid [31]. SDS-polyacrylamide gel electrophoresis (PAGE) was performed using the Bio-Rad Mini Protean III gel system according to Laemmli's method [32]. Equal amounts of protein (50 μg/well) were loaded onto 12% SDS-PAGE for each sample and proteins were transferred to the polyvinylidine difluoride (PVDF) membranes. Membranes were treated with 5% non-fat dry milk in phosphate-buffered saline containing 0.01% Tween 20 (PBS-T) to block any non-specific antibody binding sites and then incubated for 16 h at 4°C with mouse monoclonal antibody against Bax protein (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse monoclonal antibody against Bcl-2 (1 : 1000; Santa Cruz Biotechnology). Membranes were washed with PBS-T and incubated further for 1.5 h at room temperature with secondary antibody [goat anti-mouse immunoglobulin (IgG) conjugated with horseradish peroxidase (HRP); 1 : 5000; Santa Cruz Biotechnology]. The chemiluminescence signals were visualized by exposing the membranes to Kodak Biomax X-ray film for 1–5 min and the bands were then quantified using the image analysis software.
After imaging, the membranes were stripped of primary and secondary antibodies by incubation in a solution of 0.2 M glycine, 0.05% Tween 20 at pH 2.5 for 15 min at 80°C, then rinsed in PBS-T and reblocked in 5% non-fat dry milk in PBS-T. After blocking, membranes were reprobed using monoclonal anti-β-tubulin antibody as the primary antibody (1 : 1000; Santa Cruz Biotechnology). β-tubulin served as an internal control for equal protein loading.
Immunohistochemistry for Bax and Bcl-2
All specimens were fixed in 10% buffered formalin. Paraffin blocks prepared from routinely processed specimens were cut into 5-μm slices and deparaffinized. Antigen retrieval was performed after this process. After microwave incubation of the peroxyblock, followed by the ultra-V block procedure, primary antibodies Bax mouse monoclonal IgG2b (1 : 100 dilution; Santa Cruz Biotechnology), Bcl-2 alpha Ab-1 (100/D5) MS-123-R7 (ready-to-use Neomarkers) were applied. Following this process biotinylated secondary antibody (goat anti-mouse IgG-HRP; Santa Cruz Biotechnology), streptavidin peroxidase and substrate-chromogen (AEC) solution were applied, respectively. Nuclear stainning was performed with haematoxylin. Staining intensity was defined as a percentage and given a score ranging from 1 to 3: 0 (–), 1–30% (+), 30–60% (++), >60% (+++).
Statistical analysis
All statistical analyses were performed with spss version 11.0 for Windows. The results were expressed as mean ± standard deviation. One-way analysis of variance (anova) followed by Tukey's honestly significant difference post-hoc test was used for equal variances. Kruskal–Wallis test was performed for unequal variances. In all cases, a difference was considered significant when P < 0.05.
Results
Three to 5 days after intracolonic instillation of TNBS, diarrhoea with loose stools and increased stool frequency was observed. None of the rats died during the experimental period. Taurine administration decreased the incidence of diarrhoea by approximately 50%. There were no significant differences in the body weight of animals among the groups at the start of the study. The percentages of the body weight loss in TNBS and TNBS plus taurine groups were 8.07 ± 1.22 and 3.60 ± 1.28, respectively, compared to the control group.
Histopathological findings
Microscopic pictures after haemotoxylin and eosin staining showed wide areas of mucosal necrosis, oedema, desquamated areas and loss of the epithelium in TNBS group. Diffuse polymorphonuclear leucocyte infiltration in lamina propria and muscularis mucosa were seen clearly. In the TNBS plus taurine group, there was marked reduction of the inflammatory infiltrate, mild oedema limited mainly to the lamina propria and minimal ulceration. Normal colon structure was seen in control or taurine-treated rats (Fig. 1a–d).
Fig. 1.
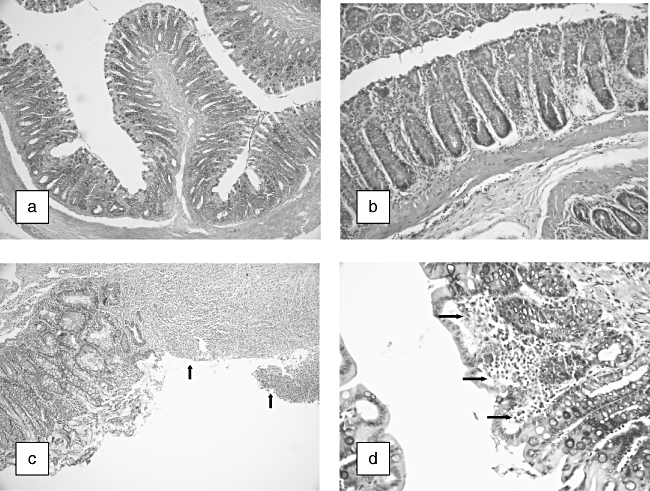
Histopathological appearance of colonic tissue in rats. (a) Normal colonic mucosa of control rats [haematoxylin and eosin (H&E) × 40]. (b) Normal colonic mucosa of taurine-treated rats (H&E × 200). (c) Colonic mucosa of 2,4,6-trinitrobenzene sulphonic acid (TNBS)-treated rats. Large ulcerations (arrows) (H&E × 100). (d) Colonic mucosa of TNBS + taurine-treated rats. Moderated inflammatory cell infiltration (arrows) (H&E × 400).
Macroscopic colitis score
In rats with TNBS-induced colitis, the macroscopic score was found to be increased significantly compared to the control group. Taurine treatment decreased the score significantly in rats with TNBS-induced colitis compared to the TNBS group (Fig. 2).
Fig. 2.
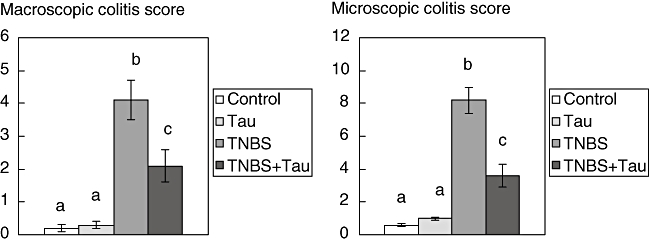
Macroscopic and microscopic colitis scores of control, taurine (Tau)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated rats. Each value is expressed as mean ± standard deviation. Values not sharing a common superscript letter are significantly different by Kruskal–Wallis test (P < 0.05).
Microscopic colitis score
Microscopic pathological score was significantly higher in TNBS-induced colitis rats. Treatment with taurine caused a significant decrease in the pathological scores in TNBS-induced colitis rats compared to the TNBS group (Fig. 2).
MPO activity
The MPO activity of the colonic tissues increased in the TNBS group compared to controls. Treatment with taurine in rats with TNBS-induced colitis decreased MPO levels compared significantly to the TNBS group (Fig. 3).
Fig. 3.
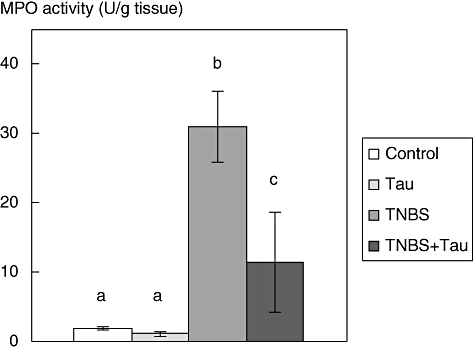
Myeloperoxidase (MPO) activity in the colon tissue of control, taurine (Tau)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated rats. Each value is expressed as mean ± standard deviation. Values not sharing a common superscript letter are significantly different by Kruskal–Wallis test (P < 0.05).
MDA levels
The MDA levels of the colonic tissues were increased significantly following TNBS treatment. Taurine decreased the MDA levels in TNBS-treated rats compared to the TNBS group (Fig. 4).
Fig. 4.
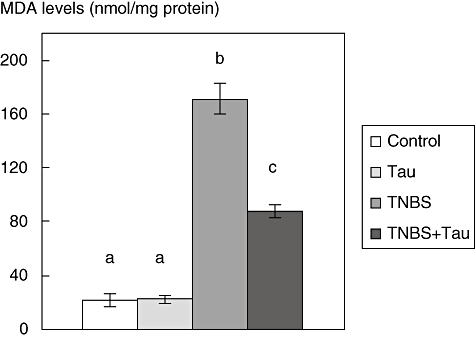
Malondialdehyde (MDA) levels in the colon tissue of control, taurine (Tau)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated rats. Each value is expressed as mean ± standard deviation. Values not sharing a common superscript letter are significantly different by analysis of variance (P < 0.05).
GSH levels
The GSH levels of the colonic tissues were found to be significantly lower in the TNBS group compared to the control group. Taurine treatment caused increases in GSH levels in TNBS-induced colitis rats (Fig. 5).
Fig. 5.
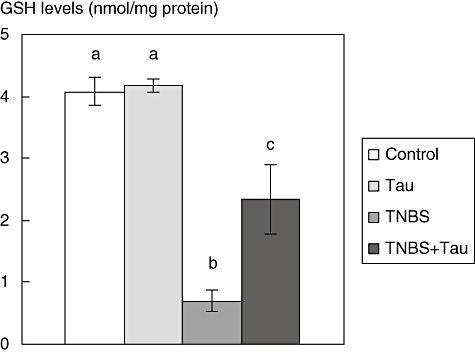
Glutathione levels in the colon tissue of control, taurine (Tau)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated rats. Each value is expressed as mean ± standard deviation. Values not sharing a common superscript letter are significantly different by Kruskal–Wallis test (P < 0.05).
Western blot analysis for Bax and Bcl-2
Although Bax expression was found to be increased in the TNBS group there was a slight, but not statistically significant, decrease in Bcl-2 expression in colonic tissue of TNBS-induced colitis rats. Bax expression in rats with TNBS colitis decreased significantly after taurine treatment. However, there was a slight, but not statistically significant, increase in Bcl-2 expression in rats with TNBS-induced colitis following taurine treatment. However, taurine treatment alone did not alter Bax and Bcl-2 expression in normal rats (Figs 6 and 7).
Fig. 6.
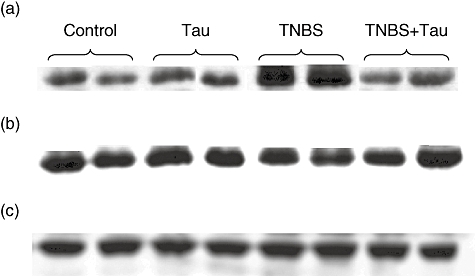
Western blot analysis of colonic Bax (a), B cell lymphoma-2 (Bcl-2) (b) expression and β-tubulin (c) expression as an index of the adequency of sample loading in control, taurine (Tau)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated rats.
Fig. 7.
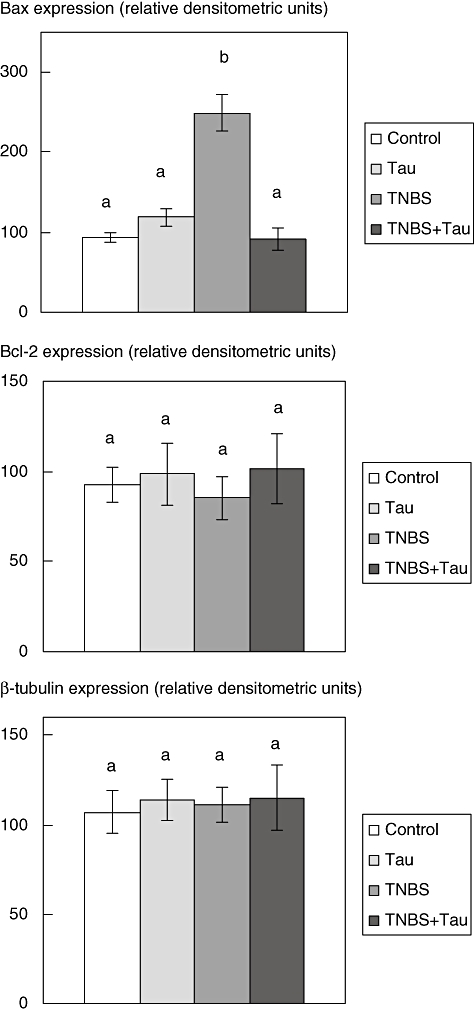
Densitometric quantification of colonic Bax and B cell lymphoma-2 (Bcl-2) and β-tubulin expression in control, taurine (Tau)-, 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated groups. Results are average values of four or six animals. Values not sharing a common superscript letter are significantly different by analysis of variance (P < 0.05).
Immunohistochemistry analysis for Bax and Bcl-2
Bax expression was strongest in the colons of rats with TNBS-induced colitis and this expression was decreased in the taurine-treated colitis group. However, Bcl-2 was weakest in the TNBS group. Bcl-2 expression was observed to increase in the TNBS plus taurine-treated group (Figs 8 and 9, Table 1).
Fig. 8.
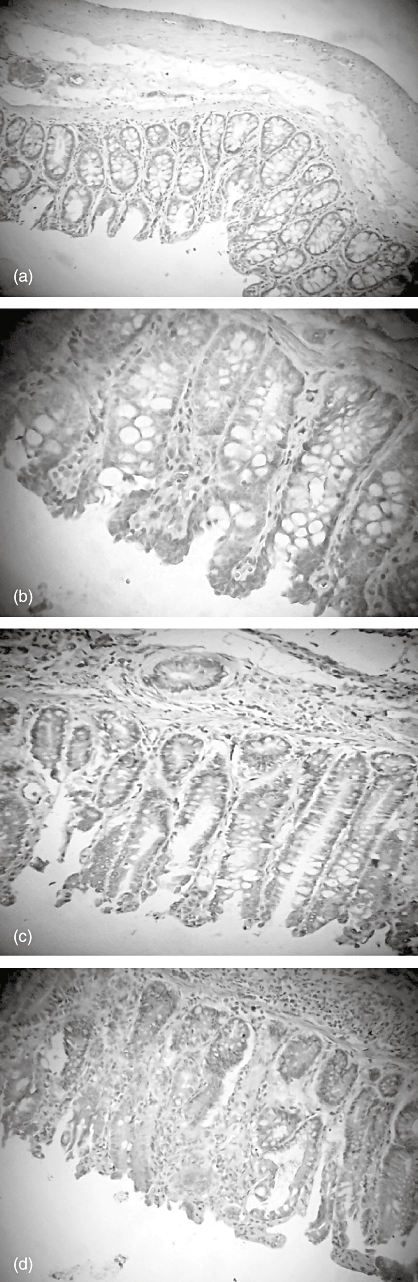
Immunohistochemistry of colonic Bax expression in the colon tissue of rats. (a) Control (× 100); (b) taurine- (× 250); (c) 2,4,6-trinitrobenzene sulphonic acid- (× 100); and (d) TNBS plus taurine-treated groups (× 100).
Fig. 9.
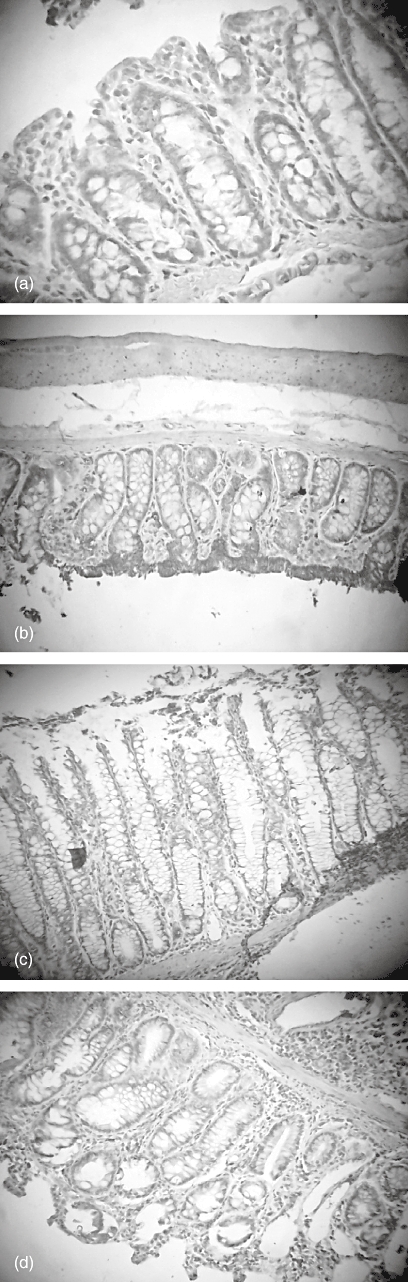
Immunohistochemistry of colonic B cell lymphoma-2 (Bcl-2) expression in the colon tissue of rats. (a) Control (× 250); (b) taurine- (× 100); (c) 2,4,6-trinitrobenzene sulphonic acid (TNBS)- (× 100); and (d) TNBS plus taurine-treated groups (× 100).
Table 1.
Staining intensity of Bax and B cell lymphoma-2 (Bcl-2) in the colon tissue of rats in control, taurine- (Tau), 2,4,6-trinitrobenzene sulphonic acid (TNBS)- and TNBS plus taurine (TNBS + Tau)-treated rats.
| Control | Tau | TNBS | TNBS + Tau | |
|---|---|---|---|---|
| Bax | + | + | +++ | ++ |
| Bcl-2 | ++ | ++ | + | ++ |
Discussion
Induction of colitis by TNBS in the rat is used widely as an experimental model of UC. TNBS-induced colitis involves infiltration of colonic mucosa by neutrophils and macrophages and increased production of inflammatory mediators [33]. MPO is an enzyme found in neutrophils and has been used as a quantitative index of inflammation in the colon [26]. Hypochlorous acid (HOCl) produced by the action of MPO on hydrogen peroxide is also involved in the inflammatory reaction in colitis [3–5]. In our study, MPO activity increased in the colon tissue and an increased number of neutrophils was detected in the colon by microscopic evaluation following TNBS treatment.
Several clinical [34,35] and experimental [36–39] studies have shown that ROS plays an important role in the pathogenesis of UC. Superoxide radicals produced by activated leucocytes or xanthine oxidase pathway and prostaglandin products, or leucotrienes formed by the oxidation of arachidonic acid, constitute the main source of ROS in inflamed mucosa. These toxic products affect cellular membrane stability and promote lipid peroxidation [3–5]. Indeed, in our study, an increase in MDA levels and a decrease in GSH levels were detected in the colon tissue of TNBS-treated rats.
Cell death can follow two distinct pathways: necrosis or apoptosis. Apoptosis or programmed cell death differs from necrosis by distinct morphological and biochemical features [6,7]. There are two classes of regulatory proteins in the Bcl-2 family which have opposite effects on apoptosis: the pro-apoptotic members (Bax, Bcl-XS) promote programmed cell death, whereas the anti-apoptotic members (Bcl-2, Bcl-XL) protect cells against apoptosis [6,7]. Members of the Bcl-2 family play a key role in the apoptosis, as they reduce mitochondrial membrane permeability and prevent the release of cytochrome c into the cytoplasm [6,7]. In this study, a significant increase in the pro-apoptotic Bax protein was found in the colon tissue of TNBS-treated rats. In addition, the expression of anti-apoptotic Bcl-2 protein found a slight but not statistically significant decrease in the TNBS group. Because the ratio of Bax/Bcl-2, a parameter of apoptotic cell death, was increased in colon tissue of TNBS-treated rats, it appears that apoptosis is probably involved in TNBS-induced colitis. These findings are in accordance with other studies [8–11].
There has been one study on the effect of taurine treatment on colitis in the literature [40]. They reported that taurine reduces inflammation and ROS generation in TNBS-induced colitis rats. In our study, the administration of taurine also reduced the inflammation and MPO activity in rats with TNBS-induced colitis. Because MPO activity increased greatly in the colon tissue of colitis rats, high levels of HOCl are generated and taurine is converted to taurine chloramine by reaction with HOCl in inflamed colon tissue. Therefore, the clinical effect of taurine treatment may be partly through taurine chloramines [13].
On the other hand, taurine has been reported to protect cells against oxidative injury [12–18]. Several mechanisms may play a role in taurine-mediated reduction in oxidative stress. Taurine was reported to protect cells by scavenging oxygen free radicals by up-regulating the anti-oxidant defences, by forming chloramines with HOCl, or by binding free metal ions such as Fe2+ by its sulphonic acid group [12–14]. Because cysteine is a precursor of taurine and GSH, taurine supplementation may cause enhancement in GSH levels by directing cysteine into the GSH synthesis pathway [13,23]. Therefore, increased GSH levels after taurine treatment may play an additional role in decreasing oxidative stress. In our study, a significant decrease in colonic MDA levels and an increase in GSH levels were detected in rats with TNBS-induced colitis following taurine treatment.
The ratio of Bax/Bcl-2 is well known to be a parameter of apoptotic cell death [6,7]. When Bax : Bcl-2 ratios were calculated according to Western blotting results, we found that these ratios in TNBS and TNBS plus taurine groups are 2.92 and 0.90, respectively. These results were also supported by our immunohistochemical findings. Therefore, it is speculated that taurine treatment ameliorates TNBS-induced colitis by reducing pro-apoptotic pathway activation and preventing the loss of the anti-apoptotic pathway through inhibition of oxidative stress.
In conclusion, this study appears to be the first in the literature showing the in vivo anti-apoptotic effect of taurine in experimental colitis. Our results show that taurine administration may exert beneficial effects in UC by decreasing inflammatory reactions, oxidative stress and apoptosis.
Acknowledgments
This study was supported by the Research Fund of Istanbul University.
References
- 1.Hendrickson BA, Gokhale R, Cho JH. Clinic aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 4.Pavlick KP, Laroux FS, Fuseler J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 5.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–84. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine AD. Apoptosis: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:191–205. doi: 10.1097/00054725-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–83. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 8.Wu HG, Gong X, Yao LQ, et al. Mechanisms of acapuncture and moxibustion in regulation of epithelial cell apoptosis in rat ulcerative colitis. World J Gastroenterol. 2004;10:682–8. doi: 10.3748/wjg.v10.i5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzon E, Esposito E, Crisafulli C, et al. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J Pineal Res. 2006;41:363–73. doi: 10.1111/j.1600-079X.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 10.Verstege MI, Te Velde AA, Hommes DW. Apoptosis as a therapeutic paradigm in inflammatory bowel diseases. Acta Gastroenterol Belg. 2006;69:406–12. [PubMed] [Google Scholar]
- 11.Karamanolis DG, Kyrlagkitsis I, Konstantinou K, et al. The Bcl-2/Bax system and apoptosis in ulcerative colitis. Hepatogastroenterology. 2007;54:1085–8. [PubMed] [Google Scholar]
- 12.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 13.Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is taurine cytoprotective? Adv Exp Med Biol. 2003;526:307–21. doi: 10.1007/978-1-4615-0077-3_39. [DOI] [PubMed] [Google Scholar]
- 14.Redmond HP, Wang JH, Bouchier-Hayes D. Taurine attenuates nitric oxide- and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg. 1996;131:1280–8. doi: 10.1001/archsurg.1996.01430240034004. [DOI] [PubMed] [Google Scholar]
- 15.Balkan J, Kanbaḡli Ö, Hatipoğlu A, et al. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci Biotechnol Biochem. 2002;66:1755–8. doi: 10.1271/bbb.66.1755. [DOI] [PubMed] [Google Scholar]
- 16.Franconi F, Di Leo MAS, Bennardini F, Ghirlanda G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res. 2004;29:143–50. doi: 10.1023/b:nere.0000010443.05899.2f. [DOI] [PubMed] [Google Scholar]
- 17.Doğru-Abbasoğlu S, Kanbaḡli Ö, Balkan J, Çevikbaş U, Aykaç-Toker G, Uysal M. The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum Exp Toxicol. 2001;20:23–7. doi: 10.1191/096032701673031525. [DOI] [PubMed] [Google Scholar]
- 18.Çetiner M, Şener G, Şehirli AÖ, et al. Taurine protects against methotrexate-induced toxicity and inhibits leucocyte death. Toxicol Appl Pharmacol. 2005;209:39–50. doi: 10.1016/j.taap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Oriyanhan W, Yamazaki K, Miwa S, Takaba K, Ikeda T, Komeda M. Taurine prevents myocardial ischemia/reperfusion-induced oxidative stress and apoptosis in prolonged hypothermic rat heart preservation. Heart Vessels. 2005;20:278–85. doi: 10.1007/s00380-005-0841-9. [DOI] [PubMed] [Google Scholar]
- 20.Casey RG, Gang C, Joyce M, Bouchier-Heyes DJ. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte–endothelial cell interactions and cardiac dysfunction. J Vasc Res. 2007;44:31–9. doi: 10.1159/000097893. [DOI] [PubMed] [Google Scholar]
- 21.Eppler B, Dawson R. Dietary taurine manipulations in aged male Fischer 344 rat tissue: taurine concentration, taurine biosynthesis, and oxidative markers. Biochem Pharmacol. 2002;62:29–39. doi: 10.1016/s0006-2952(01)00647-5. [DOI] [PubMed] [Google Scholar]
- 22.Erman F, Balkan J, Çevikbaş U, Koçak-Toker N, Uysal M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. 2004;27:199–205. doi: 10.1007/s00726-004-0105-5. [DOI] [PubMed] [Google Scholar]
- 23.Hagar HH. The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett. 2004;151:335–43. doi: 10.1016/j.toxlet.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 25.Millar AD, Rampton DS, Chander CL, et al. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–15. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 27.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 28.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissues with Ellmann's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 29.Liu ESL, Ye Y-N, Shin VY, et al. Cigarette smoke exposure increases ulcerative colitis-associated colonic adenoma formation in mice. Carcinogenesis. 2003;24:1407–13. doi: 10.1093/carcin/bgg094. [DOI] [PubMed] [Google Scholar]
- 30.Christensen JG, Romach EH, Healy LN, et al. Altered bcl-2 family expression during non-genotoxic hepatocarcinogenesis in mice. Carcinogenesis. 1999;20:1583–90. doi: 10.1093/carcin/20.8.1583. [DOI] [PubMed] [Google Scholar]
- 31.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 34.Lih-Brody L, Powell SR, Collier KP, et al. Increased oxidative stress and decreased antioxidant defences in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 35.Tüzün A, Erdil A, İnal V, et al. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–72. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 36.Çetinkaya A, Bülbüloğlu E, Kantarçeken B, et al. Effects of 1-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–94. doi: 10.1007/s10620-006-3160-9. [DOI] [PubMed] [Google Scholar]
- 37.Değer C, Erbil Y, Giriş M, et al. The effect of glutamine on pancreatic damage in TNBS-induced colitis. Dig Dis Sci. 2006;51:1841–6. doi: 10.1007/s10620-006-9189-y. [DOI] [PubMed] [Google Scholar]
- 38.Hagar HH, El Medany A, El Eter E, Arafa M. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur J Pharmacol. 2007;554:69–77. doi: 10.1016/j.ejphar.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 39.Giriş M, Erbil Y, Doğru-Abbasoğlu S, et al. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. Int J Colorectal Dis. 2007;22:591–9. doi: 10.1007/s00384-006-0238-y. [DOI] [PubMed] [Google Scholar]
- 40.Son M, Ko JI, Doh HM, et al. Protective effect of taurine on TNBS-induced inflammatory bowel disease in rats. Arch Pharm Res. 1998;21:531–6. doi: 10.1007/BF02975370. [DOI] [PubMed] [Google Scholar]


