Abstract
The primary aim of this study was to evaluate the role of natural killer (NK) cells on antigen-specific adaptive immune responses. After analysing the mechanism of impaired adaptive immune responses of NK-depleted mice, an immune interventional approach was developed to restore adaptive immunity in NK-depleted mice. NK cells were depleted from mice by administration of anti-asialo GM1 antibody (100 μl/mouse), twice, at an interval of 48 h. Hepatitis B surface antigen (HBsAg) was administered intraperitoneally to normal C57BL/6 mice (control mice) and NK-depleted mice. The levels of antibody to HBsAg (anti-HBs) in the sera and HBsAg-specific lymphocytes in the spleen were assessed. The functions of T lymphocytes, B lymphocytes and dendritic cells (DCs) were evaluated in vitro. HBsAg-pulsed DCs were prepared by culturing spleen DCs with HBsAg for 48 h and administered once to NK-depleted mice. The levels of anti-HBs in the sera and HBsAg-specific lymphocytes were significantly lower in NK-depleted mice compared with control mice (P < 0·05). The functions of T and B lymphocytes were similar between control mice and NK-depleted mice. However, the functions of spleen DC and liver DC were significantly lower in NK-depleted mice compared with control mice (P < 0·05). Administration of HBsAg-pulsed DCs, but not HBsAg, induced HBsAg-specific humoral and cellular immune responses in NK-depleted mice. Our study suggests that cross-talk between NK cells and DCs regulates the magnitude of adaptive immunity. In addition, antigen-pulsed immunogenic DCs represent potent immune modulator even if subjects with diminished innate immunity.
Keywords: antigen presentation, dendritic cells, immune therapy, liver immunology, natural killer cells
Introduction
Host defence is maintained by the coordinated actions of both innate and adaptive immunity. Innate immunity is believed to serve as the first line of defence against infection and malignancy. On the other hand, adaptive immunity imposes specificity and ensures that appropriate responses would be mounted against chronic or reoccurring challenges [1]. Natural killer (NK) cells are the primary effector cells of the innate immune system and have a well-established role in tumour rejection and resistance to virus infection [2]. Conversely, the generation and maintenance of adaptive immunity require an extensive array of interactions of microbial agents, tumour cells or allergens with antigen-presenting cells and lymphocytes [3].
Although innate immunity and adaptive immunity are regulated by different pathways, experimental data suggest that the magnitudes of adaptive immunity are not dependent only upon the functional capacities of lymphocytes but cells of innate immunity, such as NK cells, are capable of modulating adaptive immunities [4–6]. In line with this, Jensen et al. have shown that NK depletion diminish tumour-specific B cell responses [7], whereas Byrne et al. have documented that NK depletion is related with dissemination of microbial infection and reduction of antigen-specific immunity [8].
These studies indicate that NK cells are required for induction of adaptive immunity, but the mechanisms underlying this are largely unknown. In addition, most of these studies have checked decreased frequencies of NK cells in lymphoid tissues because of the use of NK-depleting agents. However, almost no information is available regarding frequencies and functions of NK cells in non-lymphoid tissues.
The role of NK cells is especially important in the context of immunity in the liver. In contrast to other organs, the frequencies of NK cells are extremely high in the liver [9]. Moreover, impaired functions of NK cells have been reported in subjects with chronic liver diseases (chronic hepatitis B virus (HBV) infection [10], chronic hepatitis C virus infection [11] and hepatocellular carcinoma [12]). In addition, immune therapy (vaccine therapy) is now used to treat patients with chronic HBV infection. However, the therapeutic efficacy of vaccine therapy is unsatisfactory in these patients [13]. Understanding the role of NK cells in adaptive immunity may allow the development of better therapeutic vaccine against chronic HBV infection.
This study was planned to develop insight into the role of NK cells on adaptive immunity in mice. In the first part of the study, we administered anti-asialo GM1 antibody in mice and checked the frequencies of NK cells in the spleen as well as the liver. The magnitude of innate immunity and hepatitis B surface antigen (HBsAg)-specific humoral and cellular immune responses were assessed in NK-depleted and control mice. In the next part of the study, the mechanisms underlying impaired HBsAg-specific adaptive immune responses of NK-depleted mice were analysed by comparing the functional capacities of different immunocytes, including antigen-presenting dendritic cells (DCs) from the spleen and the liver of NK-depleted mice and control mice. Finally, we developed an immune interventional strategy for induction of adaptive immunity in NK-depleted mice by DCs loaded with HBsAg.
Materials and methods
Mice
Adult male C57BL/6J (H-2Kb) and C3H/HeN (H-2Kk) mice were purchased from Nihon Clea (Tokyo, Japan). Mice were housed in polycarbonate cages in a temperature-controlled room (23 ± 1°C) with a 12-h light/dark cycle in the pathogen-free animal housing facility at Ehime University Graduate School of Medicine. All animals received humane care, and study protocols complied with the institution's guidelines.
Depletion of NK cells and T lymphocytes
Mice were injected in the peritoneum with 100 μl of phosphate-buffered saline (PBS) solution containing 100 μg of anti-asialo GM1 antibody (Wako, Osaka, Japan) on days 0 and 2. Three days after administration of the second dose of anti-asialo GM1 antibody, single-cell suspensions of spleen and liver non-parenchymal cells (NPCs) were isolated, and depletion of NK cells in these organs was confirmed by flow cytometry. T lymphocytes were also depleted from control mice by administration of antibodies against T lymphocytes [anti-mouse CD90·2 (Thy1·2) monoclonal antibody; BD Pharmingen, San Jose, CA, USA]. Control mice were injected twice with PBS in exactly the same manner. NK cells were depleted in vitro from whole spleen cells by culturing with anti-asiolo GM1 and complement (Cerdaline, Hornby, Ontario, Canada). Control spleen cells were cultured with complement only.
Isolation of T lymphocytes, B lymphocytes and DCs
We have previously described in detail the methodologies for isolating spleen cells, T lymphocytes, B lymphocytes and DCs [14,15]. In short, the spleens were removed aseptically, cut into pieces, and incubated at 37°C in 5% CO2 for 30 min in RPMI-1640 (Nipro, Osaka, Japan) supplemented with 1 mg/ml collagenase (type IV; Sigma Chemical, St Louis, MO, USA), and a single-cell suspension of spleen was produced. T lymphocytes and B lymphocytes were purified from single-cell suspensions of spleen by the negative-selection column method using the Mouse Pan T and Mouse Pan B isolation kits respectively (Miltenyi Biotec, Bergisch Gladbach, Germany).
To isolate spleen DCs, single-cell suspensions of spleen were centrifuged at 5000 g for 30 min on a dense albumin column (specific gravity, 1·082) at 4°C and then cultured on a plastic surface for 90 min at 37°C. The adherent cells were cultured for an additional 18 h in culture medium containing RPMI-1640 plus 10% fetal calf serum (Filtron Pty Ltd, Brooklyn, NSW, Australia). Contaminating T and B lymphocytes in the DC population were depleted by antibody-mediated killing [14,15]. Macrophages were discarded by two additional adherent steps on plastic dishes at 37°C. In some experiments, highly purified CD11c+ DCs were isolated from single-cell suspensions of spleen using CD11c+ microbeads of a commercial DC isolation kit (Miltenyi Biotec), exactly as described [16,17].
Liver NPCs were isolated exactly as described previously [16]. In short, liver tissues were homogenized, suspended in 35% Percoll (Sigma) and centrifuged to obtain liver NPCs. Liver DCs were isolated from liver NPCs by positive selection in a magnetic activated cell sorter (CD11c Isolation Kit; Miltenyi Biotec) [16].
Preparation of antigen-pulsed DCs
Based on data from preliminary studies, HBsAg-pulsed DCs were prepared exactly as described previously [18]. Briefly, spleen DCs and HBsAg (Tokyo Institute of Immunology, Tokyo, Japan) were cultured together in complete medium for 48 h. DCs were recovered from the cultures and washed five times with PBS. The final wash solution was preserved to assess whether free HBsAg was present in HBsAg-pulsed DCs. The expression of major histocompatibility complex (MHC) class II and CD86 in HBsAg-pulsed DCs was assessed by flow cytometry. Production of cytokines and T cell stimulatory capacities of HBsAg-pulsed DCs were also assessed in vitro.
Hepatitis B surface antigen-specific memory lymphocytes
Hepatitis B surface antigen-specific memory lymphocytes were prepared as described previously [18]. Briefly, 8-week-old male C57BL/6 mice were immunized twice with 10 μg of HBsAg in adjuvant at an interval of 4 weeks. Serial evaluation revealed that at 7–8 months after immunization, lymphocytes of these mice proliferated after stimulation with DCs and HBsAg but not with HBsAg only, indicating that lymphocytes were in memory state.
Immunization schedule
Control C57BL/6 mice and NK-depleted normal C57BL/6 mice were immunized with a commercially available hepatitis B vaccine containing HBsAg (Heptavax-II, subtype adw; Banyu Pharmaceutical, Tokyo, Japan) or HBsAg-pulsed DCs, intraperitoneally, at different frequencies. Immunization was performed 3 days after depletion of NK cells.
Evaluation of HBsAg-specific humoral immune responses
The levels of antibody to HBsAg (anti-HBs) in the sera were screened by a passive haemagglutinin method and quantified by a chemiluminescence enzyme immunoassay method (Special Reference Laboratory, Osaka, Japan), as described previously [17].
Lymphoproliferative assays
As described previously, we optimized the protocols for assessment of lymphocyte proliferation in response to polyclonal immune modulators, allogenic mixed leucocyte reaction (MLR) and antigen-specific proliferation [14–18].
Lymphocytes from different types of mice were cultured in the absence or presence of HBsAg for 120 h to evaluate HBsAg-specific cellular immune responses.
Hepatitis B surface antigen-specific memory lymphocytes and DCs were cultured with or without HBsAg (1 μg/ml) for 120 h to evaluate the antigen-presenting function of DCs. To assess the functional capacities of HBsAg-pulsed DCs, these DCs were cultured with HBsAg-specific memory lymphocytes for 120 h.
The culture conditions have been described by us previously in detail [14–18]. All cultures were performed in 96-well U-bottomed plates (Corning Incorporated, NY, USA). [3H]-thymidine (1·0 μCi/ml; Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) was diluted in sterile RPMI-1640 and added to the cultures for the last 16 h and harvested automatically by a multiple cell harvester (Labo Mash; Futaba Medical, Osaka, Japan) onto a filter paper (Labo Mash 101–10; Futaba Medical). The levels of incorporation of [3H]-thymidine were determined in a liquid scintillation counter (Beckman LS 6500; Beckman Instruments, Inc., Fullerton, CA, USA) from the level of blastogenesis. The data were expressed as counts per minute. Data were also expressed as a stimulation index. The level of proliferation in control culture was considered to be background proliferation and expressed as a stimulation index of 1·0. A stimulation index of > 2·0 was regarded as significant proliferation.
Production of cytokines and their estimation
Liver NPCs, whole spleen cells and spleen DCs were cultured with concanavalin A, lipopolysaccharides (Sigma) and cytosine–phosphate–guanosine oligodeoxynucleotide (CpG–ODN) (InvivoGen, San Diego, CA, USA), and the culture supernatants were collected. The levels of different cytokines [interferon (IFN)-γ, tumour necrosis factor (TNF)-α, and interleukin (IL)-6 and IL-12] in culture supernatants were estimated using a commercial kit for the cytometric bead array method, as described previously [16]. The levels of cytokines in the culture supernatants were calibrated by computer software from the mean fluorescence intensities of the standard negative control, standard positive control and samples. Data are expressed as pg/ml.
Statistical analysis
Data were analysed by unpaired t-tests if the data were normally distributed and by the Mann–Whitney rank-sum test if they were skewed. Differences were considered significant if P < 0·05. Data were expressed as means ± standard error of mean.
Results
Depletion of NK cells from the liver and the spleen because of administration of anti-asialo GM1 antibody
As reported previously [19], administration of anti-asialo GM1 antibody resulted in depletion of NK cells from the liver and spleen of mice. The frequencies of NK cells in the liver and the spleen of control mice were 11·0 ± 0·5% and 4·6 ± 0·4% (n = 5) respectively. Three days after administration of anti-asialo GM1 antibody, the frequencies of NK cells in the liver and the spleen decreased to 1·07 ± 0·12% and 0·79 ± 0·2% (n = 5) respectively. However, the frequencies of T cells B220+ B cells, CD11b+ macrophages and CD11c+ DCs did not differ significantly between control mice and NK-depleted mice (data not shown). The absolute numbers of T cells in NK-depleted mice and control mice also did not differ significantly (numbers of T cells: control mice versus NK-depleted mice; 2·56 × 107 ± 0·23 × 107versus 2·28 × 107 ± 0·22 × 107, n = 3, P > 0·05). There was no biochemical or histological evidence of liver damage attributable to administration of anti-asialo GM1 antibody (data not shown). The numbers and functions of NK cells mainly reconstituted 2 weeks after NK depletion.
Depletion of NK cells resulted in reduced cytokine production by spleen cells and liver NPCs
We checked the effect of NK depletion on production of innate cytokines by liver NPCs and spleen cells. The amounts of IFN-γ, TNF-α and IL-6 were significantly lower in cultures containing liver NPCs and spleen cells from NK-depleted mice compared with those from control mice (P < 0·05) (Table 1). Decreased levels of various cytokines were also detected when NK cells were depleted in vitro (Table 1).
Table 1.
Decreased cytokine production by liver non-parenchymal cells and spleen cells from natural killer (NK)-depleted mice.
| Mice | Interferon-γ (pg/ml) | Tumour necrosis factor-α (pg/ml) | Interleukin-6 (pg/ml) |
|---|---|---|---|
| (a) Cytokine production by liver nonparenchymal cells depleted of NK cells in vivo | |||
| Control mice | 2018 ± 611 | 248 ± 11 | 164 ± 16 |
| NK-depleted mice | 215 ± 7·3* | 147 ± 12* | 110 ± 24* |
| (b) Cytokine production by spleen cells depleted of NK cells in vivo | |||
| Control mice | 5879 ± 356 | 723 ± 13 | 450 ± 37 |
| NK-depleted mice | 674 ± 57* | 184 ± 12* | 60 ± 3·5* |
| (c) Cytokine production by spleen cells depleted of NK cells in vitro | |||
| Control mice | 2494 ± 207 | 313 ± 10 | 187 ± 10 |
| NK-depleted mice | 1085 ± 52* | 285 ± 3·7 | 111 ± 3·8* |
P < 0·05, compared with control mice. Liver non-parenchymal cells (a) and single-cell suspensions of spleen (b) were isolated from control mice (normal C57BL/6 mice) and NK-depleted C57BL/6 mice, 3 days after depletion of NK cells or administration of phosphate-buffered saline in vivo. (c) NK cells were depleted from spleen cells in vitro. A total of 1 × 106 spleen cells were cultured with concanavalin A for 3 days. The amount of cytokines in culture supernatants was measured by a cytometric bead array method and expressed as pg/ml. Data are shown as mean ± standard error of the mean of five cultures.
Decreased antigen-specific immune responses of NK-depleted mice
To assess the effect of NK cells on HBsAg-specific humoral and cellular immune responses, we immunized NK-depleted and control mice using a commercial vaccine containing HBsAg, as described in Methods. All control normal mice produced anti-HBs as a result of immunization with HBsAg; however, anti-HBs were detected in one of eight (12%), one of four (25%) and two of five (40%) NK-depleted mice after immunization with 1 μg, 2 μg, or 4 μg of HBsAg respectively. In addition, the levels of anti-HBs in the sera were significantly lower in HBsAg-immunized NK-depleted mice compared with HBsAg-injected control mice (P < 0·05) (Fig. 1a).
Fig. 1.
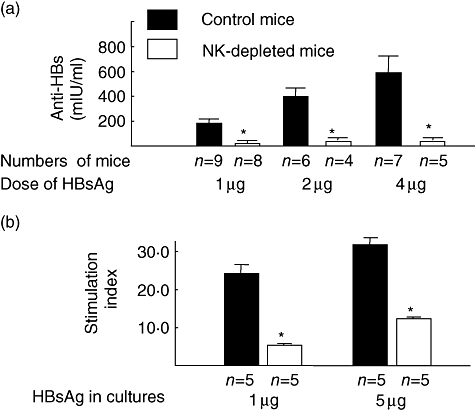
Decreased production of anti-hepatitis B (HBs) in the sera and proliferation of spleen lymphocytes of natural killer (NK)-depleted mice as a result of immunization with a vaccine containing hepatitis B surface antigen (HBsAg). Normal C57BL/6 mice (black bar) and NK-depleted mice (open bar) were immunized once with hepatitis B vaccine containing 1, 2 or 4 μg of HBsAg. The levels of anti-HBs were assessed in the sera 4 weeks after immunization with vaccine containing HBsAg (a). Spleen cells were isolated from these mice 4 weeks after immunization. A total of 2 × 105 lymphocytes were cultured with HBsAg (1 μg and 5 μg) for 5 days. The levels of proliferation of lymphocytes are shown as a stimulation index, as described in Methods. *P < 0·05, compared with control mice; black bar: NK-depleted mice, open bar: control mice; n = numbers of mice.
Also, the levels of lymphocyte proliferation in response to HBsAg were decreased significantly in HBsAg-injected NK-depleted mice compared with those of HBsAg-injected control C57BL/6 mice (Fig. 1b) (P < 0·05).
Effect of NK depletion on functional capacity of different immunocytes
The proliferation of T lymphocytes and B lymphocytes in response to concanavalin A (Table 2a) and lipopolysaccharides (Table 2b), respectively, was not significantly different between NK-depleted mice and control mice.
Table 2.
Functional capacities of different immunocytes from control mice and natural killer (NK)-depleted mice.
| Number of spleen T cells | Sources of T cells | Levels of blastogenesis (counts per minute) |
|---|---|---|
| (a) T cell proliferation by concanavalin A | ||
| 2 × 105 | Control mice | 14 398 ± 897 |
| NK-depleted mice | 15 110 ± 107 |
| Number of spleen B cells | Sources of B cells | Levels of blastogenesis (counts per minute) |
|---|---|---|
| (b) B cell proliferation by lipopolysaccharides | ||
| 2 × 105 | Control mice | 11 929 ± 2738 |
| NK-depleted mice | 9553 ± 1041 |
| Number of liver DCs | Sources of DCs | Levels of blastogenesis (counts per minute) |
|---|---|---|
| (c) Proliferative capacity of liver DCs and spleen DCs | ||
| 1 × 104 | Control mice | 55 458 ± 6578 |
| NK-depleted mice | 32 565 ± 4567* |
| Number of spleen DCs | Source of DCs | Levels of blastogenesis (counts per minute) |
|---|---|---|
| 1 × 104 | Control mice | 70 206 ± 3992 |
| NK-depleted mice | 25 637 ± 2429* |
P < 0·05, compared with control mice. Spleen T cells, spleen B cells, liver dendritic cells (DCs) and spleen DCs were isolated from normal C57BL/6 mice and NK-depleted C57BL/6 mice. Spleen T cells were also isolated from normal C3H/He mice for using as responder of allogenic MLR. Spleen T cells and spleen B cells from control mice and NK-depleted mice were cultured with concanavalin A (a) and lipopolysaccharide, respectively, for 72 h (b). Liver DCs and spleen DCs (1 × 104) from control and NK-depleted C57BL/6 mice were cultured with 2 × 105 T cells from C3H/He mice for 120 h (c). The levels of blastogenesis in the cultures were estimated from incorporation of [3H]-thymidine during the last 16 h of culture and expressed as counts per minute. Mean ± standard error of the mean of five experiments are shown.
The expression of MHC class II, CD80 and CD86 was not different between control mice and NK-depleted mice (data not shown); however, the T cell stimulatory capacities of spleen DCs and liver DCs in allogenic MLR were reduced significantly in NK-depleted mice compared with control mice (Table 2c).
Also, spleen DCs from NK-depleted mice had a significantly lower capacity to stimulate HBsAg-specific lymphocytes compared with those from control mice (P < 0·05) (Fig. 2). This finding was evident with different doses of DCs in the culture.
Fig. 2.
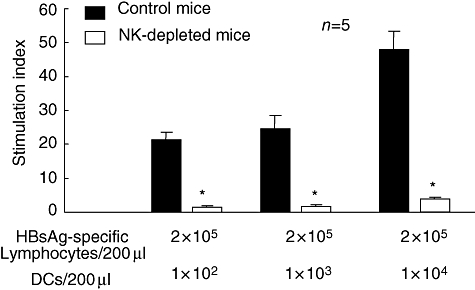
Decreased proliferation of antigen-specific lymphocytes by spleen dendritic cells (DCs) from natural killer (NK) cell-depleted mice. Hepatitis B surface antigen (HBsAg)-specific lymphocytes were prepared by immunization of normal C57BL/6 mice with a vaccine containing HBsAg and serial assessment of their HBsAg-specific cellular immune responses [18]. Spleen DCs from control C57BL/6 mice (black bar) and NK-depleted mice (open bar) were cultured with HBsAg-specific memory lymphocytes in the presence of HBsAg. Data were shown as a stimulation index, as described in the Methods. Mean ± standard error of the mean of five separate experiments are shown. *P < 0·05, compared with control mice.
Impaired antigen processing and presentation capacities of spleen DCs from NK-depleted mice
The T cell stimulatory capacities of DCs of NK-depleted mice were decreased significantly compared with those of DCs from control mice. Another important function of DCs is the processing of antigens. When spleen DCs and antigens are cultured together, DCs process the antigens and express an antigenic epitope. Antigen-pulsed DCs can stimulate antigen-specific lymphocytes in the absence of antigen. In this study, we prepared HBsAg-pulsed DCs from both NK-depleted mice and control mice. HBsAg-pulsed DCs from NK-depleted mice had a significantly lower capacity for stimulating HBsAg-specific lymphocytes compared with HBsAg-pulsed DCs from control mice (DCs from NK-depleted mice versus DCs from control mice; stimulation index 12·2 ± 1·2 versus 2·7 ± 0·2, n = 5, P < 0·05). However, the functional capacities of DCs were not altered significantly because of the depletion of T cells from control mice (stimulation index: control mice versus T-depleted mice, 68·4 ± 9·0 versus 63·9 ± 8·0, n = 3, P > 0·05).
Decreased cytokine production by highly purified CD11c+ spleen DCs from NK-depleted mice
Dendritic cells produce various proinflammatory cytokines, and these immunogenic cytokines are essential for proper DC function. Highly purified CD11c+ spleen DCs were cultured with concanavalin A (Fig. 3a) and CpG–ODN (Fig. 3b) for 72 h, and their cytokine production capacities were evaluated. As shown in Fig. 3, spleen DCs from NK-depleted mice produced significantly lower levels of IL-12p70, TNF-α and IFN-γ compared with those produced by control mice (P < 0·05).
Fig. 3.
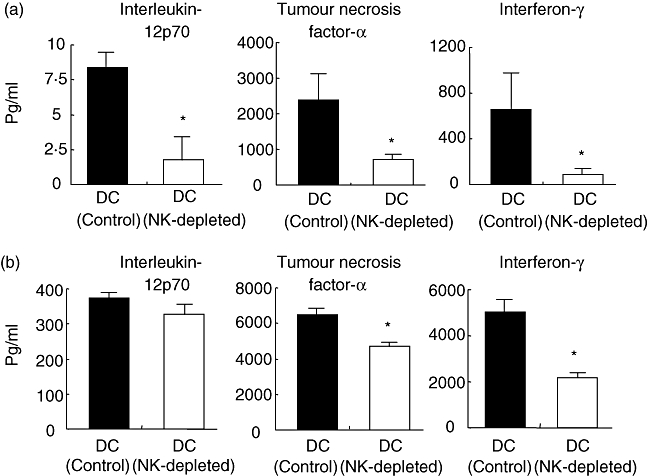
Decreased production of different cytokines by highly purified CD11c+ spleen dendritic cells (DCs) from natural killer (NK) cell-depleted mice. Highly purified spleen DCs were isolated by magnetic cell sorting from control mice and NK-depleted mice. A total of 2 × 105 DCs were cultured with concanavalin A (1 μg/ml) and cytosine–phosphate–guanosine CpG–ODN (1 μg/ml) for 72 h. The levels of cytokines in culture supernatants as a result of stimulation with concanavalin A (a) and CpG–ODN (b) were estimated. The data are shown as pg/ml of cytokines, n = 5. Mean ± standard error of the mean are shown. DC (control): DCs from control C57BL/6 mice. DC (NK-depleted): DCs from NK-depleted mice.
Restoration of HBsAg-specific humoral and cellular immune responses in NK-depleted mice because of administration of HBsAg-pulsed DCs
Finally, we evaluated if an activated form of DC, HBsAg-pulsed DC, can restore HBsAg-specific immune responses of NK-depleted mice. HBsAg-pulsed DCs from control mice expressed significantly higher levels of MHC class II (mean fluorescence intensity; unpulsed DCs versus HBsAg-pulsed DCs, 167 ± 32 versus 378 ± 43, n = 5, P < 0·05) and CD86 (mean fluorescence intensity, unpulsed DCs versus HBsAg-pulsed DCs, 143 ± 23 versus 287 ± 37, n = 5, P < 0·05), and produced increased levels of IFN-γ, TNF-α and IL-6, compared with unpulsed DCs (data not shown). As shown in Fig. 4, anti-HBs and HBsAg-specific lymphocytes were detected in all NK-depleted mice, immunized with HBsAg-pulsed DCs, but not in NK-depleted mice immunized with only vaccine containing HBsAg.
Fig. 4.
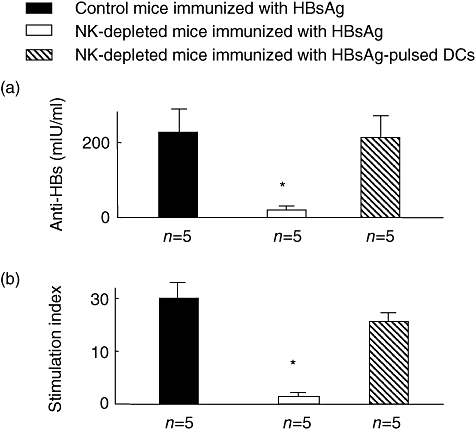
Restoration of hepatitis B surface antigen (HBsAg)-specific immune responses of natural killer (NK) cell-depleted mice because of immunization with HBsAg-pulsed dendritic cells (DCs). Normal C57BL/6 mice (control mice) and NK-depleted mice were immunized once with hepatitis B vaccine containing 1 μg of HBsAg. HBsAg-pulsed DCs (1 × 106 cells) were administered once, intraperitoneally, to NK-depleted mice. The levels of anti-hepatitis B (HBs) were assessed in the sera 4 weeks after immunization with HBsAg or HBsAg-pulsed DCs (a). Spleen cells were isolated from these mice 4 weeks after immunization. A total of 2 × 105 lymphocytes were cultured with HBsAg (1 μg) for 5 days. The levels of proliferation of lymphocytes are shown as a stimulation index, as described in Methods. *P < 0·05, compared with HBsAg-injected control mice. Black bar: normal mice immunized with vaccine containing HBsAg; open bar: NK-depleted mice immunized with vaccine containing HBsAg; hatched bar, NK-depleted mice immunized with vaccine containing HBsAg-pulsed DCs; n = numbers of mice.
Discussion
Experimental evidences indicate that NK cells may have an important role during the induction of antigen-specific adaptive immune responses, in addition to their function in innate immune responses [4–6]. The magnitude of adaptive immune responses is decreased in NK-depleted mice. However, little is known about the underlying mechanisms. Finally, the clinical implications of the role of NK cells in adaptive immunity have not been evaluated properly.
To explore the mechanisms underlying diminished HBsAg-specific immune responses of NK-depleted mice, we checked the functional capacities of T cells, B cells and DCs from NK-depleted mice and control mice. Our data revealed that impaired HBsAg-specific adaptive immune responses of NK-depleted mice can be attributable mainly, if not solely, to DCs. The proliferative capacities of T and B cells were almost similar between NK-depleted mice and control mice (Table 2). However, DCs from NK-depleted mice could not activate allogenic T cells and HBsAg-specific T cells (Table 2, Fig. 2). Also, DCs from NK-depleted mice could not be loaded with HBsAg in vitro. This was evident from the incapability of HBsAg-pulsed DCs from NK-depleted mice to activate HBsAg-specific memory lymphocytes (data shown in Results). It is important to ask why DCs from NK-depleted mice could not act as potent antigen-presenting cells. Investigators have shown that maturation of DCs requires help from NK cells. Also, NK cells trigger the differentiation of monocytes into DCs [20], and NK cell-derived cytokines are required for the proper functioning of DCs [21,22]. In this study, DCs from NK-depleted mice produced significantly lower levels of IFN-γ, TNF-α and IL-12 compared with those produced by DCs of control mice (Fig. 3). Also, indirect roles of other immunocytes on DC function cannot be discarded completely. Although the stimulatory capacities of T cells and B cells were not altered in NK-depleted mice, more elaborate evaluation of their functions may provide more insight about impaired DC functions in NK-depleted mice.
In this study, we showed that depletion of NK cells resulted in reduced functional capacities of DCs. On the other hand, Kassim et al. have reported that depletion of DCs causes diminished NK responses [23]. Interestingly, the functional capacities of DCs and NK cells are diminished in most chronic viral infections and cancers. It is not unlikely that the functions of both of these cells may be down-regulated because of impaired cross-talk between these immunocytes in pathological conditions.
Finally, we showed that adaptive immunity of NK-depleted mice can be restored because of administration of antigen-pulsed DCs. This may have a significant clinical importance. At present, patients with cancers and chronic viral infections are treated by immune therapy to induce anti-viral and anti-tumour adaptive immunity [24]. To accomplish this, various antigens or vaccines or cell-based vaccines are now used. However, the efficacy of these immune interventional strategies is still unatisfactory. Accordingly, there is a need to develop more immunogenic vaccines. Many patients with chronic viral infections and cancers exhibit both impaired innate and adaptive immunity [10–12]. Our study showed that antigen-pulsed DCs are capable of inducing antigen-specific immunity in NK-depleted mice, even when adaptive immunity cannot be induced by traditional vaccination approaches. However, antigen-pulsed DCs have not been effective in many patients with cancers [25]. Although various factors may be responsible for the ineffective therapeutic efficacy of DC-based vaccine in cancers, the immunogenicity of antigen-pulsed DCs should be checked before administration to cancer patients. This is not usually carried out in clinics. If antigen-pulsed DCs are unable to induce both innate and adaptive immunity, then these DCs may not induce adaptive immunity. Recently, we have shown that if antigen-pulsed DCs are immunogenic, they can overcome immune response defects of HB vaccine non-responders [26].
In conclusion, we have shown that depletion of NK cells disrupted antigen-specific immune responses of mice in vivo, and this is mediated mainly through DCs. We have also pointed out that antigen-pulsed DCs may be a potent therapeutic tool for immune therapy, but these DCs must be immunogenic and capable of inducing both innate and adaptive immunity.
Acknowledgments
We would like to thank the Integrated Centre for Science, Shigenobu Station, Ehime University for animal management.
References
- 1.Borghesi L, Milcarek C. Innate versus adaptive immunity: a paradigm past its prime? Cancer Res. 2007;67:3989–93. doi: 10.1158/0008-5472.CAN-07-0182. [DOI] [PubMed] [Google Scholar]
- 2.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–9. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–3. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 6.Kos FJ. Regulation of adaptive immunity by natural killer cells. Immunol Res. 1998;17:303–12. doi: 10.1007/BF02786453. [DOI] [PubMed] [Google Scholar]
- 7.Jensen M, Tawadros S, Sedlacek HH, Schultze JL, Berthold F. NK cell depletion diminish tumour-specific B cell responses. Immunol Lett. 2004;93:205–10. doi: 10.1016/j.imlet.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Byrne P, McGuirk P, Todryk S, Mills KH. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol. 2004;34:2579–88. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 9.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wei H, Sun R, Tian Z. Impaired function of hepatic natural killer cells from murine chronic HBsAg carriers. Int Immunopharmacol. 2005;5:1839–52. doi: 10.1016/j.intimp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–9. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56(+) T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–9. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pol S, Nalpas B, Driss F, et al. for the Multicenter Study Group. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J Hepatol. 2001;34:917–21. doi: 10.1016/s0168-8278(01)00028-9. [DOI] [PubMed] [Google Scholar]
- 14.Akbar SM, Onji M, Inaba K, Yamamura K-I, Ohta Y. Low responsiveness of hepatitis B virus transgenic mice in antibody response to T-cell-dependent antigen: defect in antigen presenting activity of dendritic cells. Immunology. 1993;78:468–73. [PMC free article] [PubMed] [Google Scholar]
- 15.Akbar SM, Inaba K, Onji M. Upregulation of MHC class II antigen on dendritic cells from hepatitis B virus transgenic mice by interferon-gamma: abrogation of immune response defect to a T-cell-dependent antigen. Immunology. 1996;87:519–27. doi: 10.1046/j.1365-2567.1996.516576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasebe A, Akbar SM, Furukawa S, Horiike N, Onji M. Impaired functional capacities of liver dendritic cells from murine hepatitis B virus (HBV) carriers: relevance to low HBV-specific immune responses. Clin Exp Immunol. 2005;139:35–42. doi: 10.1111/j.1365-2249.2005.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niiya T, Akbar SM, Yoshida O, et al. Impaired dendritic cell function resulting from chronic undernutrition disrupts the antigen-specific immune response in mice. J Nutr. 2007;137:671–5. doi: 10.1093/jn/137.3.671. [DOI] [PubMed] [Google Scholar]
- 18.Akbar SM, Abe M, Masumoto T, Horiike N, Onji M. Mechanism of action of vaccine therapy in murine hepatitis B virus-carriers: vaccine-induced activation of antigen presenting dendritic cells. J Hepatol. 1999;30:755–64. doi: 10.1016/s0168-8278(99)80125-1. [DOI] [PubMed] [Google Scholar]
- 19.Ehl S, Nuesch R, Tanaka T, Myasaka M, Hengartner H, Zinkernagel R. A comparison of efficacy and specificity of three NK depleting antibodies. J Immunol Methods. 1996;199:149–53. doi: 10.1016/s0022-1759(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang AL, Colmenero P, Purath U, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–93. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol. 2007;179:590–6. doi: 10.4049/jimmunol.179.1.590. [DOI] [PubMed] [Google Scholar]
- 22.Shu SA, Lian ZX, Chuang YH, et al. The role of CD11c(+) hepatic dendritic cells in the induction of innate immune responses. Clin Exp Immunol. 2007;149:335–43. doi: 10.1111/j.1365-2249.2007.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J Virol. 2006;80:3985–93. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377–413. doi: 10.1080/08830180600992456. [DOI] [PubMed] [Google Scholar]
- 25.Akbar SM, Abe M, Yoshida O, Murakami H, Onji M. Dendritic cell-based therapy as a multidisciplinary approach to cancer treatment: present limitations and future scopes. Curr Med Chem. 2006;13:3113–19. doi: 10.2174/092986706778742882. [DOI] [PubMed] [Google Scholar]
- 26.Akbar SM, Furukawa S, Yoshida O, Hiasa Y, Horiike N, Onji M. Induction of anti-HBs in HB vaccine nonresponders in vivo by hepatitis B surface antigen-pulsed blood dendritic cells. J Hepatol. 2007;47:60–6. doi: 10.1016/j.jhep.2007.02.021. [DOI] [PubMed] [Google Scholar]


