Abstract
Intracellular adhesion molecule-1 (ICAM-1) expression on the thyroid follicular cells of non-obese diabetic (NOD).H2h4 mice is enhanced by iodide treatment, which correlates with autoimmune thyroid disease in genetically susceptible NOD.H2h4 mice. The current study examines the mechanism of iodine-enhanced up-regulation of ICAM-1 on the surface of thyroid cells. We hypothesized that the up-regulation of ICAM-1 is due to a transient increase in production of reactive oxygen species (ROS). ROS may initiate signalling of the ICAM-1 gene promoter, enhancing up-regulated ICAM-1 protein on the cell surface. Single-cell suspensions of thyroid follicular cells from thyroiditis-susceptible NOD.H2h4 or non-susceptible BALB/c mice were treated in vitro with sodium iodide. Extracellular and intracellular ROS were assessed by luminol-derived chemiluminescence and flow cytometry assays respectively. Our results demonstrate that thyroid follicular cells of NOD.H2h4 generate higher levels of ROS compared with cells from non-susceptible strains of mice. Expression of a subunit protein of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, p67phox, was analysed by Western blot immunoassay. A constitutive expression of the p67phox subunit protein was observed in NOD.H2h4 mice prior to iodine treatment. No such expression was found in BALB/c mice. Treatment of NOD.H2h4 thyroid cells with diphenyleneiodium, an inhibitor of NADPH oxidase, reduced generation of ROS and of ICAM-1 protein expression. Thus, thyrocytes from NOD.H2h4 mice produce enhanced levels of ROS that may be mediated by NADPH oxidase. Consequently, in NOD.H2h4 mice the ROS-induced signal for ICAM-1 up-regulation may contribute to mononuclear cellular infiltration of the thyroid gland and the progression of autoimmune thyroid disease.
Keywords: adhesion molecules, autoimmunity, gene regulation, rodent
Introduction
The non-obese diabetic (NOD).H2h4 mouse, expressing the major histocompatibility complex haplotype H-2k on a NOD background, is a well-established model of spontaneous autoimmune thyroiditis (AT) [1–3]. The H-2k haplotype is associated with a strong immune response to thyroglobulin, a major antigen in AT [4]. Progressive infiltration of mononuclear cells into the thyroid gland and thyroid-specific antibody production is accelerated in NOD.H2h4 mice upon dietary supplementation with iodine [2,3,5]. Supplementation of iodine to non-susceptible mice such as BALB/c or B10.A mice does not enhance AT, affirming a genetic predilection to autoimmune disease because of the NOD background [6]. The NOD.H2h4 mouse therefore provides an excellent model to study the mechanisms of iodine-enhanced autoimmune thyroid disease.
We have reported previously that NOD.H2h4 mice express intracellular adhesion molecule-1 (ICAM-1) on their thyrocytes constitutively, and that iodine supplementation in their drinking water up-regulated this ICAM-1 expression [6,7]. In vitro iodine treatment of isolated thyroid follicular cells also demonstrated enhanced expression of ICAM-1 on their surface [6]. Because ICAM-1 has been shown to play a major role in the early stages of inflammatory responses, we predicted that this up-regulation of ICAM-1 on thyrocytes could be one mechanism to explain the accelerated mononuclear cell infiltration into the thyroid glands during the autoimmune process.
The primary goal of this study, therefore, was to examine the mechanism of ICAM-1 expression on the thyroid follicular cells of NOD.H2h4 mice in response to iodine. Iodine is required for the normal functioning of the thyroid gland in humans as well as in all other animals, including mice. It is taken up primarily by thyroid follicular cells and used for the synthesis of thyroid hormones. Reactive oxygen species (ROS) are involved in the biochemical synthesis of thyroid hormones; iodides are oxidized by the enzyme thyroid peroxidase using hydrogen peroxide (H2O2) to form iodinated tyrosyls [8,9]. ROS, as well as many other stimuli, such as cytokines, viruses, bacterial lipopolysaccharide, radiation and retinoic acid, are known to stimulate the ICAM-1 gene promoter [10–12]. In genetically susceptible individuals AT may well be triggered through these diverse stimuli, so that ICAM-1 is over-expressed on the thyroid follicular cells. ICAM-1 has been demonstrated on the thyrocytes of patients with autoimmune thyroid disease both in vivo and in vitro[13–16]. However, whether this increased expression of ICAM-1 was a cause or result of cellular infiltration is not known. To understand if iodine could directly enhance ROS generation that would account for up-regulated ICAM-1 and increased susceptibility to AT, we performed experiments with thyrocytes isolated from NOD.H2h4 mice and compared them with cells from non-susceptible strains of mice.
To pursue this hypothesis we investigated if iodine directly enhances ICAM-1 surface protein on thyroid cells, that is associated with the generation of ROS. Furthermore, using the pharmacological inhibitor diphenyleneiodium (DPI), we examined the role of ROS in ICAM-1-mediated disease pathology in susceptible NOD.H2h4 mice.
Materials and methods
Mice
Non-obese diabetic.H2h4 mice were born and reared at the Johns Hopkins School of Medicine specific pathogen-free housing animal facility. Additionally, B10.A and BALB/c and NOD.H2h4 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The original NOD.H2h4 strain was developed by Dr Linda Wicker (Merck Pharmaceutical Laboratories, Rahway, NJ, USA) by crossing NOD mice with B10.A (4R) followed by back-crossing to the parental NOD [1]. Thus, the NOD.H2h4 mouse expresses the thyroiditis-susceptible I-Ak allele on the NOD background [4]. Both males and females develop thyroiditis in this strain [3,5]. Each experimental group consisted of pooled thyroid cells from B10.A, BALB/c or NOD.H2h4 mice (n = 7–10) approximately 8–10 weeks of age and from both sexes. NOD.H2h4 mice transgenic for interferon (IFN)-γ in their thyroid gland were derived from founder mice developed by Dr Patrizio Caturegli, Department of Pathology, Johns Hopkins University [17]. All protocols were performed under the guidelines of the Animal Care and Use Committee, Johns Hopkins University, Baltimore, MD.
Preparation of single-cell suspension and sodium iodide treatment
Thyroid glands from the different strains of mice were collected following euthanasia. Thyroids from the same mouse strains were pooled and single-cell suspensions were prepared with a slight modification of the Lucas et al. method, as described previously [18]. Briefly, both lobes of thyroid glands were separated from the trachea and kept in Dulbecco's modified Eagle's medium (Gibco brl, Gaithersburg, MD, USA) at 4°C. Thyroids were digested with an enzyme solution of dispase II (1·2 U/ml) and of collagenase II (1 U/ml) [dissolved in 50 mM N-Tris(hydroxymethyl)methyl-2-aminoethanesulphonic acid buffer] (Sigma, St Louis, MO, USA) and incubated in a 37°C water bath for 25 min, shaking occasionally. Cells were released from the tissue by gentle pipetting, and then washed twice with complete phosphate-buffered saline (PBS) (with 0·5 mM MgCl2/0·7 mM CaCl2/0·1% glucose).
Thyrocyte culture
For in vitro experiments, cells obtained after enzymatic digestion as described above were washed twice and cultured overnight in F12 medium with supplements (Sigma). DPI (Sigma) was used at a final concentration of 10 μM, with or without sodium iodide (NaI) for 24 h at 37°C in a 5% CO2 chamber. For the chemiluminescence assay cells were prepared and kept in complete PBS on ice until the evaluation of ROS release. The NaI dose of 0·1 mM was determined to be optimal and used for all in vitro experiments (data not shown).
Reactive oxygen species detection by chemiluminescence
Luminol-derived chemiluminescence was used to assess extracellular H2O2[19]. H2O2 release was detected in 1 × 106 thyroid cells in 2 ml of aerated complete PBS in the presence of 10 μM luminol and 10 μg/ml horseradish peroxidase (HRP) either with 0·1 mM NaI for test reactions or with saline for control reactions. Chemiluminescence was measured continuously for 60 min using a Berthold LB 9505 six-channel luminometer (Pforzheim, Germany).
Reactive oxygen species detection by flow cytometry
Mouse thyroid cells prepared as described above were resuspended in complete PBS. To detect intracellular ROS, hydroethidine (HE) was used, as it penetrates the cell membrane rapidly and enters into the cells. HE becomes fluorescent upon reduction by ROS, which can be detected by fluorescent microscopy or flow cytometry. Cells were first incubated for 5 min with a 0·5 μM HE (Sigma) at 37°C followed by 30 min incubation with 0·1 mM NaI or PBS as control. The reaction was stopped by incubating cells for 10 min on ice followed by two washes with PBS at 300 g. Phorbol 12, 13-dibutyrate (PDBu; 100 ng/ml) was used as a positive stimulator of ROS. ROS was determined within 1 h on a fluorescence-activated cell sorter (FACSCaliber) flow cytometry (BD Biosciences, Sparks, MD, USA) using fluorescent intensity of excitation at 490 nm and emission at 520 nm.
Detection of nicotinamide adenine dinucleotide phosphate oxidase/p67phox by Western blot immunoassay
Single-cell suspensions of thyroids from the different mouse strains were lysed in buffer containing 50 mM Tris–HCl (pH, 7·4), 100 mM NaCl, 50 mM sodium fluoride, 5 mM ethylenediamine tetraacetic acid, 40 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10−4 M phenylmethylsulphonyl fluoride, 10−6 M leupeptin, 10−6 M pepstatin A and 1% (v/v) Triton X-100 (all from Sigma). Lysates were clarified by centrifugation at 13 000 g for 10 min. Samples, each containing 20 μg of cell lysate in loading buffer, were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (12% gel) and proteins were transferred to a polyvinylidene difluoride membrane (Immobilon; Millipore, Bedford, MA, USA). Immunoblotting was performed using monoclonal mouse anti-p67phox (1:1000; BD Transduction Laboratories, San Diego, CA, USA). After washing the blots, secondary antibody bovine anti-mouse IgG HRP (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to the blots. Immunoreactivity was detected by luminol-dependent chemiluminescence reagent plus (Perkin-Elmer, Rockville, MD, USA).
Statistical analysis
Data for in vitro work were obtained from pooled mouse thyroid glands. The experiment was performed at least three separate times. Comparisons of data were made using the non-parametric paired Student's t-test and were considered to be statistically significant at P < 0·05.
Results
Iodine enhances ICAM-1 expression on thyroid cells of NOD.H2h4 mice
We have shown previously that in vitro treatment of NOD.H2h4 thyrocytes with iodine increased the surface expression of ICAM-1 on the thyrocyte cell [6]. To understand the kinetics of iodine-enhanced ICAM-1 expression, single-cell suspensions of thyroid cells from NOD.H2h4 mice were prepared. Thyrocytes were then incubated with 0·1 mM NaI for 24–72 h. Figure 1 shows that ICAM-1 expression increased from an average of 5% of untreated thyrocytes to >20% at 24 h. The increase was transient, as this expression decreased after 48 h and became undetectable after 72 h (data not shown). The transient nature of the phenomenon may be due to unstable mRNA, as suggested by other investigators [20]. A similar transient expression of ICAM-1 was described by Traore et al. when a human myeloid cell line (ML-1 cells) was challenged with a phorbol ester to initiate cell differentiation to macrophages [21].
Fig. 1.
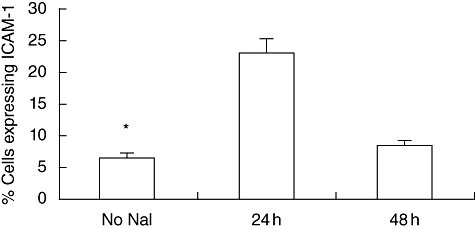
Expression of intracellular adhesion molecule-1 (ICAM-1) on non-obese diabetic.H2h4 mouse cultured thyrocytes. Single-cell suspensions of thyroid glands were prepared and stimulated with 0·1 mM sodium iodide (NaI). Cells were assessed by flow cytometry after 24 and 48 h after iodine treatment and were compared with the no NaI group. Significantly enhanced ICAM-1 expression was noted after 24 h of iodine stimulation.
Iodine-induced ROS in NOD.H2h4 mouse thyroid cells
Detection of extracellular ROS by chemiluminescence
Extracellular ROS from thyroid cells was assessed by the H2O2-dependent luminal-derived chemiluminescence assay, which monitors H2O2 released from the cell. The response of NOD.H2h4 thyrocytes was compared with that of BALB/c. As shown in Fig. 2a, treatment of thyroid cells with iodine resulted in a marked increase in ROS levels in the NOD.H2h4 mice over the basal level of untreated NOD.H2h4 or of BALB/c thyrocytes. Increased H2O2 was detected within minutes of NaI addition. Maximum chemiluminescence was reached at 30 min. The level of ROS became constant in NOD.H2h4 mice and lasted for up to 60 min, when the determination was terminated. Thyrocytes from NOD.H2h4 mice exhibited a basal level of ROS expression, while this was undetectable with cells from the BALB/c mice. ROS levels in thyrocytes from BALB/c mice were undetectable throughout the experiment. Virtually no difference was seen between the untreated and iodine-treated BALB/c thyroid cells.
Fig. 2.
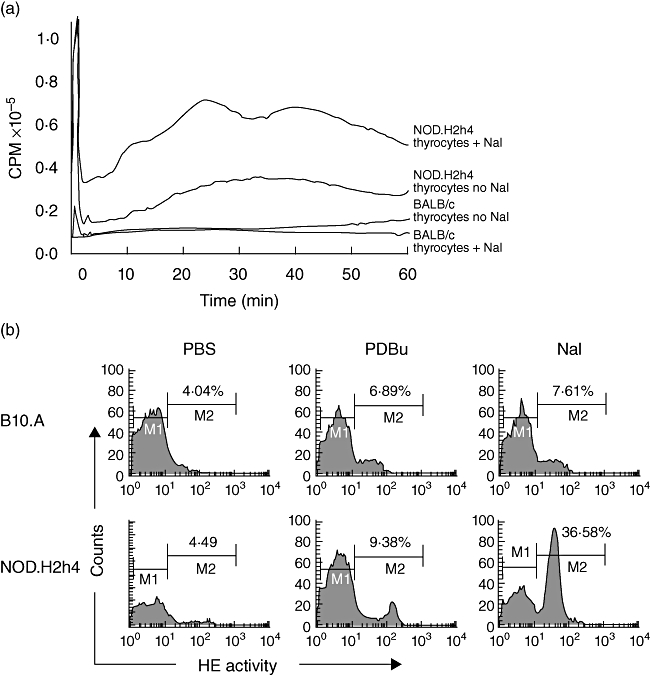
(a) Luminol-derived chemiluminescence of extracellular H2O2 release in BALB/c and non-obese diabetic (NOD).H2h4 mouse thyrocytes. Single-cell suspensions from thyroid glands of 12–14 mice per group were prepared and stimulated with iodine. All samples were run in parallel, and results were compared with the no sodium iodide (NaI) control groups. Constitutively expressed levels of H2O2 release were noted in NOD.H2h4 mice that increased further after iodine addition. In contrast, levels of H2O2 release from BALB/c remained remarkably low at all times (representative of two separate experiments). (b) Intracellular reactive oxygen species (ROS) detection in the thyrocytes of B10.A (upper panel) and NOD.H2h4 mice (lower panel). Thyrocytes were stimulated in vitro with phosphate-buffered saline, phorbol 12, 13-dibutyrate (PDBu) or NaI. Thyrocytes of NOD.H2h4 but not of B10.A mice produced a spike in the level of ROS in response to iodine addition. Shown are 36·58% of thyrocytes from NOD.H2h4 mice and 7·61% of B10.A mice producing ROS (representative of three experiments).
Detection of intracellular ROS by flow cytometry
Because ROS are generated during normal functioning of hormone synthesis in the thyroid cells, we were interested in finding: (i) if this ROS burst occurred directly in response to iodine; and (ii) if thyrocytes of NOD.H2h4 mice produced similar levels of ROS compared with other non-susceptible strains. Single-cell suspensions of thyroid cells from NOD.H2h4 and B10.A mice were stimulated in vitro with iodine. After a brief treatment with HE as the probe (see Methods), intracellular ROS were assessed by flow cytometry.
Figure 2b shows a representative experiment of ROS detection by flow cytometry. NOD.H2h4 mice produced ROS rapidly within 30 min after iodine stimulation. Approximately 36·6% ROS-producing cells from NOD.H2h4 cells were recorded in comparison with 7·6% cells of B10.A mice. As a positive control to measure responses, PDBu was used to stimulate cellular ROS. As expected, approximately an equal number of cells responded to PDBu in both strains of mice, accounting for 6·9% of cells in B10.A and 9·4% in NOD.H2h4. Thus, the number of cells producing intracellular ROS in response to iodide in NOD.H2h4 mice was significantly higher compared with B10.A mice.
Our results demonstrate that thyrocytes of NOD.H2h4 mice generated significantly increased levels of both extracellular and intracellular ROS in direct response to iodine. High levels of ROS were detected in thyrocytes from NOD.H2h4 mice but not in thyrocytes from BALB/c or B10.A mice.
Effect of iodine on p67phox expression, a subunit of nicotinamide adenine dinucleotide phosphate oxidase
Thyroid cells are known to contain nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a well-characterized flavoenzyme [22,23]. A number of phox proteins participate in the active functioning of NADPH oxidase. As p67phox is a critical subunit of NADPH oxidase, we investigated the effect of iodine on the expression of p67phox protein in thyroid cells. Thyroid cells from 8- to 10-week-old NOD.H2h4 or BALB/c mice were isolated and treated for 0 min (control), 15 min and 30 min with 0·1 mM iodine, as was used in the ROS release detection experiments. The cells were then digested and expression of p67phox protein was detected by Western blot assay. As demonstrated in Fig. 3, NOD.H2h4 mice expressed p67phox protein in their thyroid cells prior to iodine treatment. In contrast, BALB/c thyroid cells expressed no p67phox before treatment with iodine, which remained undetectable even after 15 min. However, p67phox was detectable after 30 min of iodine treatment. The 30-min iodine-induced p67phox expression in BALB/c was fairly low and was not comparable with the constitutive expression of p67phox of thyrocytes from the NOD.H2h4 mice.
Fig. 3.
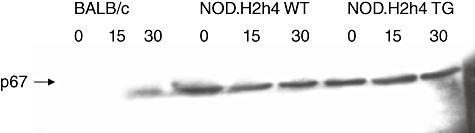
Expression of cellular protein p67phox, a component of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that triggers reactive oxygen species release in cells. Non-obese diabetic (NOD).H2h4 mouse thyroid cells were compared with those of BALB/c. As shown, NOD.H2h4 mice constitutively expressed p67phox prior to iodine treatment. In contrast, BALB/c mice did not constitutively express p67phox.
Cytokines such as IFN-γ are known to affect the outcome of autoimmune thyroid disease [6,24,25]. Therefore we analysed the p67phox expression in the NOD.H2h4 transgenic mice that expresses IFN-γ in their thyroid cells. The IFN-γ transgenic mice, like the parental NOD.H2h4mice, expressed p67phox constitutively. Iodine treatment for 15–30 min did not show any remarkable changes in the expression of p67phox (Fig. 3). Thus, elevated expression of p67phox, a subunit of NADPH oxidase, is associated with the constitutive ICAM-1 expression.
Diphenyleneiodium inhibits iodine-induced ROS and ICAM-1 expression in thyroid cells
Our results demonstrate that iodine treatment enhanced ROS production in thyrocytes from NOD.H2h4 mice but not in any of the non-susceptible strains. However, to confirm that ROS were generated by the enhanced activity of NADPH oxidase in NOD.H2h4 mice, DPI was used to inhibit enzymes that generate ROS [26].
To address this issue, a single-cell suspension of NOD.H2h4 mouse thyroid cells was incubated with 10 μM DPI for 24 h before the addition of NaI and compared with untreated cells. The data in Fig. 4a show a representative experiment illustrating a reduction in the ROS-mediated fluorescence intensity of HE (330 versus 220) in the DPI-treated thyrocytes compared with DPI-untreated cells.
Fig. 4.
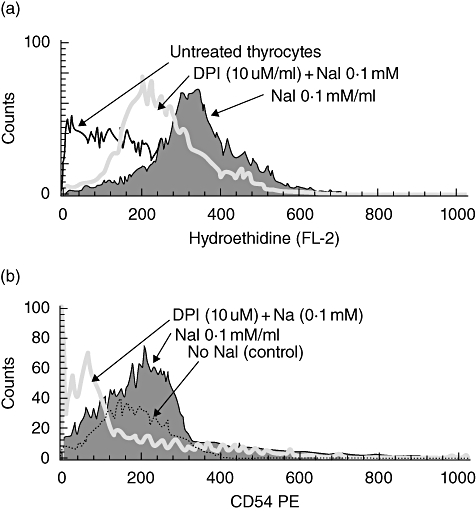
(a) Effect of diphenyleneiodium (DPI) treatment on the reactive oxygen species (ROS) generation by thyrocytes of non-obese diabetic (NOD).H2h4 mice. A remarkable decrease in the intracellular ROS production was noted after treatment with DPI, a pharmacological inhibitor of enzymes that generate ROS. The solid histogram represents the intracellular ROS in response to sodium iodide (NaI) alone, and the open histogram shows the decreased fluorescence intensity (330 versus 220 respectively) after DPI treatment. The broken line histogram represents control (unstimulated and untreated) cells. (b) Effect of DPI treatment on adhesion molecule-1 (ICAM-1) expression on NOD.H2h4 mouse thyrocytes. ICAM-1 expression decreased after treatment with DPI. The solid histogram represents ICAM-1 in response to NaI alone, while the open histogram shows the decreased fluorescence intensity (199 versus 77 respectively) after DPI treatment. The broken line histogram represents control (unstimulated and untreated) cells.
Next, we asked whether ROS inhibition reduced ICAM-1 protein expression on the cell surface. Therefore, we assessed ICAM-1 on thyrocytes treated with DPI as in the ROS experiments. The data presented in Fig. 4b show an almost threefold decrease in the mean fluorescence intensity of ICAM-1 expression (199 versus 74) after overnight treatment with DPI in the presence of NaI. The results illustrated in Fig. 4a and b support our hypothesis that iodine may increase ICAM-1 through the influence of ROS on the cellular signalling system leading to ICAM-1 expression.
Discussion
We have reported previously that the NOD.H2h4 mouse develops AT spontaneously and expresses ICAM-1 constitutively on the thyrocytes. ICAM-1 expression is enhanced further by supplementing the diet with iodine [6]. The experiments performed in this report attempt to understand and explain the mechanisms responsible for this observation. As increased thyroidal ICAM-1 could be a risk factor in the initiation and progression of AT in genetically susceptible individuals, as well as in certain mouse strains, understanding the mechanism by which it occurs could lead to new therapies in patients with Hashimoto's thyroiditis. Elevated levels of ICAM-1 or ICAM-1 mRNA in humans have been shown by several investigators studying thyrocytes from Hashimoto's thyroiditis patients [15,27,28]. While these data suggest that ICAM-1 plays an important role in autoimmune thyroid disease, it is difficult to determine in humans if it is a cause or consequence of disease in patients. Previous work by other investigators using another model of iodine-enhanced AT, the obese strain (OS) chicken, has implicated ROS as a participant in disease pathogenesis [29,30]. While no information about ICAM-1 expression in the OS chicken thyroid is available, the investigators delayed thyroiditis by using anti-oxidants, thus linking the generation of ROS to the pathogenesis of autoimmune thyroid disease [30].
Based on the results of the current study and in conjunction with our previous work, we propose a series of steps following the excess iodine uptake by thyrocytes of the NOD.H2h4 mouse leading to ROS generation and up-regulated expression of ICAM-1. The proposed events of ICAM-1 expression following iodine exposure are summarized in Fig. 5. Iodine is taken up by the thyroid epithelial cell (Fig. 5a). A spike in the ROS generation occurs because of the activity of the already-present enzyme NADPH oxidase (Fig. 5b). While the ROS spike, which includes H2O2 production, promotes organification of the iodine on the thyroglobulin molecule, it may also trigger signal transduction resulting in increased ICAM-1 expression on the surface of the thyrocyte (Fig. 5c) [31]. Thus, an environment is created within the thyroid gland combining a strongly immunogenic antigen, highly iodinated Tg, with an increased adhesion molecule, ICAM-I, favouring the retention of circulating lymphocytes, their activation and subsequent cytokine release which, over time, develops into AT (Fig. 5d) [2,6,32].
Fig. 5.
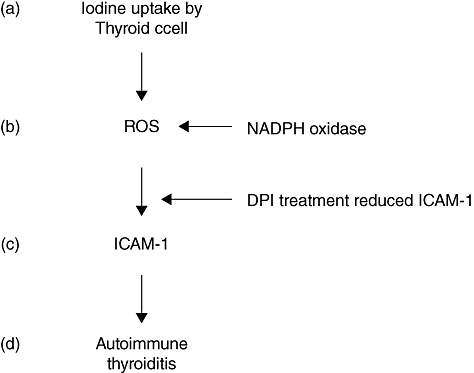
Proposed cascade of iodine-enhanced cellular events leading to adhesion molecule-1 (ICAM-1) up-regulation with subsequent autoimmune thyroiditis. (a) Active uptake of excess iodine. (b) Generation of reactive oxygen species (ROS) induced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [inhibited by diphenyleneiodium (DPI)]. (c) ICAM-1 up-regulation because of the ROS spike (inhibited by DPI). (d) Mononuclear cell infiltration and disease.
Evidence from this study and from previous work supports this model. Within 15–30 min after in vitro exposure of the thyrocyte to iodine there is an increase of ROS activity (shown in Fig. 2) because of a constitutive expression of NADPH oxidase (Fig. 3). These changes were evident only in thyrocytes of the NOD.H2h4 mouse (Fig. 5b and c). This, by itself, is an unusual event, as thyroid epithelial cells are normally protected from acute exposure to large amounts of iodine by the Wolff–Chaikoff effect.
This effect relates to an inhibition of iodide uptake and organification after exposure to high iodine levels [33]. We observed this effect in the BALB/c but not in the NOD.H2h4 mouse (Fig. 2a). Thus, based on our observation, we suggest that the Wolff–Chaikoff effect appears to be entirely ineffective or has a different threshold in the NOD.H2h4 mouse.
Nicotinamide adenine dinucleotide phosphate oxidase expression has been recognized in thyroid cells for some time [22] but its role, although indirect, in thyrocyte ICAM-1 up-regulation has not been described previously. NADPH oxidase functioning is regulated by the assembly of several members of the phox protein family. The initiating subunit p47phox is located in the resting cells and upon stimulation mediates translocation of p67phox to the cell membrane in association with the cell cytoskeleton [34,35]. These levels of the p67phox subunit of NADPH oxidase serve as a surrogate for the entire enzyme complex and may relate directly to the increased ROS generation following excess iodine exposure. The increased ROS leads to increased ICAM-1 expression. It was interesting to note that, like constitutive ICAM-1 expression, the p67phox expression was also found to be expressed constitutively prior to iodine stimulation, but only in the susceptible NOD.H2h4 mice and not in the non-susceptible BALB/c mice. Several species of ROS are generated during the metabolic functioning of normal thyroid cells; however, it is not clear if only one species is enough to induce the up-regulation of ICAM-1 or more than one species are required. While we have no evidence that NADPH oxidase is defective, the constitutive expression of this enzyme within the thyrocyte prior to iodine stimulation in vitro suggests an abnormality somewhere along this pathway.
Another point to consider when comparing the susceptible NOD.H2h4 mouse with the non-susceptible BALB/c mouse is a potential toxic effect of the higher level of H2O2 on the NOD.H2h4 thyrocyte. A direct response to iodine was demonstrated by the luminol-derived chemiluminescence experiment (see Fig. 4a), where thyroid cells were assayed for generation of extracellular H2O2. This may damage the cell further and lead to release of degraded thyroglobulin, as described by Duthoit et al. [36], potentiating the autoimmune response further because of its increased iodinated epitopes [32].
Our data demonstrate clearly that iodine by itself can induce ICAM-1 in NOD.H2h4 mouse thyrocytes following ROS generation. As is evident from these data, none of the non-susceptible mouse strains following iodine exposure expressed such elevated levels of ROS. It is not a question of non-responsiveness of the thyrocytes altogether, as we showed that thyrocytes from BALB/c mice generate ROS in response to PDBu as an alternate stimulator. Thus, while thyrocytes from BALB/c mice produce ROS equally to the NOD.H2h4 after PDBu stimulation, increased ROS because of excess iodine exposure is not seen (Fig. 2b). It was shown only in the NOD.H2h4 mouse, in which both ICAM-1 and NADPH oxidase were present constitutively. The poor ROS generation in the non-susceptible BALB/c mice correlates well with the low ICAM-1 expression, as we have shown previously that dietary supplementation of iodine for several weeks did not enhance ICAM-1 expression in BALB/c mouse thyrocytes {3596}.
The association between ROS generation and ICAM-1-enhanced expression was supported further by the experiments using DPI. DPI inhibits ROS generation mediated by NADPH oxidase {3439}. Our results using DPI showed a remarkable decrease in the fluorescent intensity of ROS and the subsequent expression of ICAM-1.
In the present study we also show that DPI, an inhibitor of enzymes that generate ROS, decreased the surface ICAM-1 protein markedly on the thyroid cells of NOD.H2h4 mice. Pretreatment with other inhibitors specific for other mediatory proteins may also be useful in understanding the signalling cascades of ROS-mediated up-regulation. The ROS-dependent up-regulation of ICAM-1 pathway provides one potential mechanism for promoting the autoimmune process leading to AT. While the up-regulated ICAM-1 may not be the sole mechanism that regulates disease progression, it is definitely an important disease initiating factor. The use of blocking antibodies in humans may not be practical so utilizing another pathway, such as blocking thyroidal ROS, to achieve the same end may be a suitable alternative approach. ICAM-1 inhibition with anti-oxidants, or other compounds that inhibit the enzymes generating ROS, could lead to new therapies that delay or reverse disease.
Acknowledgments
This work was supported in part by NIH Grants ES07141 and ES 03819.
References
- 1.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. I-E+ nonobese diabetic mice develop insulitis and diabetes. J Exp Med. 1993;178:793–803. doi: 10.1084/jem.178.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–92. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 3.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12:157–65. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 4.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis: relation to histocompatibility (H-2) type. Science. 1971;174:1137–9. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 5.Bonita RE, Rose NR, Rasooly L, Caturegli P, Burek CL. Kinetics of mononuclear cell infiltration and cytokine expression in iodine-induced thyroiditis in the NOD-H2h4 mouse. Exp Mol Pathol. 2003;74:1–12. doi: 10.1016/s0014-4800(03)80002-3. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RB, Alegria JD, Talor MV, Rose NR, Caturegli P, Burek CL. Iodine and IFN-gamma synergistically enhance intercellular adhesion molecule 1 expression on NOD.H2h4 mouse thyrocytes. J Immunol. 2005;174:7740–5. doi: 10.4049/jimmunol.174.12.7740. [DOI] [PubMed] [Google Scholar]
- 7.Bonita RE, Rose NR, Rasooly L, Caturegli P, Burek CL. Adhesion molecules as susceptibility factors in spontaneous autoimmune thyroiditis in the NOD-H2h4 mouse. Exp Mol Pathol. 2002;73:155–63. doi: 10.1006/exmp.2002.2470. [DOI] [PubMed] [Google Scholar]
- 8.Dunn JT, Dunn AD. Update on intrathyroidal iodine metabolism. Thyroid. 2001;11:407–14. doi: 10.1089/105072501300176363. [DOI] [PubMed] [Google Scholar]
- 9.Deme D, Pommier J, Nunez J. Kinetics of thyroglobulin iodination and of hormone synthesis catalysed by thyroid peroxidase. Role of iodide in the coupling reaction. Eur J Biochem. 1976;70:435–40. doi: 10.1111/j.1432-1033.1976.tb11034.x. [DOI] [PubMed] [Google Scholar]
- 10.Czech W, Krutmann J, Budnik A, Schopf E, Kapp A. Induction of intercellular adhesion molecule 1 (ICAM-1) expression in normal human eosinophils by inflammatory cytokines. J Invest Dermatol. 1993;100:417–23. doi: 10.1111/1523-1747.ep12472082. [DOI] [PubMed] [Google Scholar]
- 11.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai N, Fukuda K, Fujitsu Y, Lu Y, Chikamoto N, Nishida T. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46:114–20. doi: 10.1167/iovs.04-0922. [DOI] [PubMed] [Google Scholar]
- 13.Weetman AP, Cohen S, Makgoba MW, Borysiewicz LK. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J Endocrinol. 1989;122:185–91. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]
- 14.Tolosa E, Roura C, Marti M, Belfiore A, Pujol-Borrell R. Induction of intercellular adhesion molecule-1 but not of lymphocyte function-associated antigen-3 in thyroid follicular cells. J Autoimmun. 1992;5:119–35. doi: 10.1016/s0896-8411(05)80056-3. [DOI] [PubMed] [Google Scholar]
- 15.Bagnasco M, Pesce GP, Caretto A, et al. Follicular thyroid cells of autoimmune thyroiditis may coexpress ICAM-1 (CD54) and its natural ligand LFA-1 (CD11a/CD18) J Allergy Clin Immunol. 1995;95:1036–43. doi: 10.1016/s0091-6749(95)70105-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, An MA, Jeon JS, et al. Circulating intercellular adhesion molecule-1 (ICAM-1) in sera of patients with Graves' disease and Hashimoto disease. Korean J Intern Med. 1995;10:10–15. doi: 10.3904/kjim.1995.10.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barin JG, Afanasyeva M, Talor MV, Rose NR, Burek CL, Caturegli P. Thyroid-specific expression of IFN-gamma limits experimental autoimmune thyroiditis by suppressing lymphocyte activation in cervical lymph nodes. J Immunol. 2003;170:5523–9. doi: 10.4049/jimmunol.170.11.5523. [DOI] [PubMed] [Google Scholar]
- 18.Jeker LT, Hejazi M, Burek CL, Rose NR, Caturegli P. Mouse thyroid primary culture. Biochem Biophys Res Commun. 1999;257:511–15. doi: 10.1006/bbrc.1999.0468. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–23. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 20.Ford GS, Barnhart B, Shone S, Covey LR. Regulation of CD154 (CD40 ligand) mRNA stability during T cell activation. J Immunol. 1999;162:4037–44. [PubMed] [Google Scholar]
- 21.Traore K, Sharma RB, Burek CL, Trush MA. Role of ROS and MAPK in TPA-induced ICAM-1 expression in ML-1 cells. J Cell Biochem. 2007;100:1010–21. doi: 10.1002/jcb.21101. [DOI] [PubMed] [Google Scholar]
- 22.Leseney AM, Deme D, Legue O, et al. Biochemical characterization of a Ca2+/NAD(P)H-dependent H2O2 generator in human thyroid tissue. Biochimie. 1999;81:373–80. doi: 10.1016/s0300-9084(99)80084-4. [DOI] [PubMed] [Google Scholar]
- 23.De DX, Wang D, Many MC, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–33. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169:3999–4007. doi: 10.4049/jimmunol.169.7.3999. [DOI] [PubMed] [Google Scholar]
- 25.Tang H, Sharp GC, Peterson KP, Braley-Mullen H. IFN-gamma-deficient mice develop severe granulomatous experimental autoimmune thyroiditis with eosinophil infiltration in thyroids. J Immunol. 1998;160:5105–12. [PubMed] [Google Scholar]
- 26.Lee YS, Kang YS, Lee SH, Kim JA. Role of NAD(P)H oxidase in the tamoxifen-induced generation of reactive oxygen species and apoptosis in HepG2 human hepatoblastoma cells. Cell Death Differ. 2000;7:925–32. doi: 10.1038/sj.cdd.4400717. [DOI] [PubMed] [Google Scholar]
- 27.Pesce G, Fiorino N, Riccio AM, et al. Different intrathyroid expression of intercellular adhesion molecule-1 (ICAM-1) in Hashimoto's thyroiditis and Graves' disease: analysis at mRNA level and association with B7.1 costimulatory molecule. J Endocrinol Invest. 2002;25:289–95. doi: 10.1007/BF03344004. [DOI] [PubMed] [Google Scholar]
- 28.Zheng RQ, Abney ER, Grubeck-Loebenstein B, Dayan C, Maini RN, Feldmann M. Expression of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-3 on human thyroid epithelial cells in Graves' and Hashimoto's diseases. J Autoimmun. 1990;3:727–36. doi: 10.1016/s0896-8411(05)80039-3. [DOI] [PubMed] [Google Scholar]
- 29.Bagchi N, Brown TR, Urdanivia E, Sundick RS. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985;230:325–7. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- 30.Bagchi N, Brown TR, Herdegen DM, Dhar A, Sundick RS. Antioxidants delay the onset of thyroiditis in obese strain chickens. Endocrinology. 1990;127:1590–5. doi: 10.1210/endo-127-4-1590. [DOI] [PubMed] [Google Scholar]
- 31.Roebuck KA, Rahman A, Lakshminarayanan V, Janakidevi K, Malik AB. H2O2 and tumor necrosis factor-alpha activate intercellular adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J Biol Chem. 1995;270:18966–74. doi: 10.1074/jbc.270.32.18966. [DOI] [PubMed] [Google Scholar]
- 32.Barin JG, Talor MV, Sharma RB, Rose NR, Burek CL. Iodination of murine thyroglobulin enhances autoimmune reactivity in the NOD.H2h4 mouse. Clin Exp Immunol. 2005;142:251–9. doi: 10.1111/j.1365-2249.2005.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff J, Chaikoff I. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555. [PubMed] [Google Scholar]
- 34.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–15. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 35.El-Benna J, Dang PM, Andrieu V, et al. P40phox associates with the neutrophil Triton X-100-insoluble cytoskeletal fraction and PMA-activated membrane skeleton: a comparative study with P67phox and P47phox. J Leukoc Biol. 1999;66:1014–20. doi: 10.1002/jlb.66.6.1014. [DOI] [PubMed] [Google Scholar]
- 36.Duthoit C, Estienne V, Giraud A, et al. Hydrogen peroxide-induced production of a 40 kDa immunoreactive thyroglobulin fragment in human thyroid cells: the onset of thyroid autoimmunity? Biochem J. 2001;360:557–62. doi: 10.1042/0264-6021:3600557. [DOI] [PMC free article] [PubMed] [Google Scholar]


