Abstract
Tissue-type plasminogen activators (tPA) and urokinase-type plasminogen activators (uPA) are involved in liver repair. We examined the potential immunomodulatory actions of uPA, tPA and uPA-receptor (uPAR) in carbon-tetrachloride-induced hepatic fibrosis in wild-type (WT), tPA−/−, uPA−/− and uPAR−/− mice. Carbon-tetrachloride treatment increased fibrosis in four groups but significantly less in three knock-out models. Serum cytokines and intrahepatic T cells elevated significantly following fibrosis process in WT animals but not in the knock-out groups. In culture, uPA increased lymphocyte proliferation significantly in WT and uPA−/− but not uPAR−/− animals. Following uPA exposure in vivo, there was CD8 predominance. To isolate uPA's effect on lymphocytes, WT mice were irradiated sublethally and then reconstituted with WT or uPA−/− lymphocytes. In these animals fibrosis was decreased and T cells were reduced in the uPA−/− recipients. Based on these data we postulate that plasminogen activators affect fibrosis in part by liver-specific activation of CD8 subsets that govern the fibrogenic activity of hepatic stellate cells.
Keywords: CD4, CD8, cytokines, hepatic fibrosis, lymphocytes, plasminogen activators, tPA, uPA, uPAR
Introduction
In the liver, matrix remodelling is directed by cell-associated and extracellular proteases [1,2]. The extracellular proteolytic cascade is composed of plasminogen and plasminogen activators, and plays a central role in liver repair after acute injury [3–8]. The serine proteases, tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) are the principal plasminogen activators in mammals. Activation of the proenzyme plasminogen by tPA or uPA leads to the generation of plasmin [9].
uPA and its receptor (uPAR) have been implicated in cell migration through fibrin and subcellular matrices during angiogenesis, wound repair, inflammation, immunity and tumour metastasis [10,11]. Mice with a genetic deletion of the uPA-gene (uPA−/−) have defects in fibrinolysis [9], wound healing [12], neointima formation [13] and tumour dissemination [14], among others [15]. In uPAR−/− mice, impaired mobilization of leucocytes to sites of inflammation leads to enhanced mortality during infections [16].
Both uPA and tPA are also implicated in vasoreactivity [10], the development of pulmonary fibrosis [17] and in post-stroke neurotoxicity [18] by inducing apoptosis in cortical neurones [19]. Studies of liver repair in mice deficient in one or more components of the plasminogen activation system have shown that in the absence of plasminogen or urokinase, clearance of necrotic cell debris and matrix components are severely impaired [3–5]. These studies indicate that plasminogen activation mediates in part the pleiotropic effects of uPA in liver injury and repair [7].
We have examined the plasminogen activator's contribution to the immune response in liver following injury. This question has become especially relevant in view of our recent finding that the immune system plays a direct role in hepatic stellate cell (HSC) activation and fibrogenesis after liver injury [20].
Hepatic fibrosis is the result of chronic liver injury, regardless of aetiology, during which HSCs proliferate and activate into matrix-producing cells [21]. The activity of HSCs is influenced by an array of cytokines [22]. T helper 1 (Th1) lymphocytes express high levels of anti-fibrotic cytokines such as interferon (IFN)-γ[22], whereas the Th2 lymphocytes express high levels of pro-fibrotic cytokines such as interleukin (IL)-4 [23], such that the Th1 : Th2 ratio can tilt the balance in favour of or against fibrosis [24].
In our previous studies [20] hepatic fibrosis was shown to be CD8-mediated, while CD4 T cells were reduced significantly. However, the mechanisms by which these lymphocyte alterations affect hepatic fibrosis are still unclear.
In this study we used mice with a genetic deletion of the plasminogen activators or uPAR to investigate their potential role in the immunomodulation of hepatic fibrosis. Our data suggest that plasminogen activators contribute to hepatic fibrosis in part by regulating activation and proliferation of lymphocytes, which in turn stimulate HSC activation.
Materials and methods
Animals
Twelve-week-old male wild-type (WT), tPA−/−, uPA−/− and uPAR−/− C57/B6 mice [25] were housed in a barrier facility according to National Institutes of Health guidelines.
Hepatic fibrosis model
Carbon tetrachloride (Sigma, C-5331) fibrosis was induced by intraperitoneal injections at 0·5 μl pure carbon tetrachloride/g body weight, twice weekly for 6 weeks [20]. Hepatic fibrosis was induced in WT, uPA−/−, uPAR−/− and tPA−/− mice, then compared with naive animals from the same four genetic backgrounds. In total, there were eight animal groups, each of which included 10 mice. At the time of death, mice were weighed and anaesthetized intramuscularly with 0·1 ml of ketamine : xylazine : acepromazine (4:1:1) per 30 g of body weight, and blood samples were collected from the inferior vena cava. Determination of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was performed using an automated enzymatic assay with the Vistros Chemistry Systems 950. Whole livers and spleens were harvested and weighed. As there was variability in the total body weights, we calculated the liver weight : body weight ratio to standarize results for each animal. Liver samples were harvested from both liver lobes to reduce sampling variability among experimental and control mice.
Total-body irradiation model
Mice were irradiated sublethally with a single total-body dose of 700 cGy from a dual Cs-source (62 cGy/min) before induction of fibrosis by carbon tetrachloride. Sublethal doses were used to suppress lymphocyte proliferation while maintaining the animal in an apparently healthy state [26].
Liver histology
The posterior third of the liver was fixed in 10% formalin for haematoxylin and eosin (H&E) and Sirius Red staining [20].
Fibrosis quantization
The extent of fibrosis in Sirius Red-stained slides was evaluated using the Bioquant® computerized method [20]. We also assessed the expression of alpha smooth muscle actin (αSMA) and beta actin (β-actin) by immunoblot [20]. Bands were scanned (Hewlett-Packard 3400C) as TIF-files (8 bit, 300 dpi) and quantified (Scion-Image analysis programmer). Corresponding β-actin bands were also scanned and the final result was calculated as a ratio of each protein [27]. To standardize results from different gels, results were expressed as fold change from naive mice in each gel.
Cytokine measurements
To measure serum IL-4, IFN-γ and IL-10 levels, OptEIA-enzyme-linked immunosorbent assay kits (Pharmingen, San Diego, CA, USA) were used according to the manufacturer's protocol. A standard curve was generated using recombinant cytokines and concentrations of samples were determined by a polynomial curve fit analysis.
Lymphocyte isolation, staining and flow cytometric analysis
The spleen and intrahepatic lymphocytes were isolated, washed, counted and stained for fluorescence-activated cell sorter (FACS) analysis [20]. Antibodies used for staining were mouse anti-CD4/CD8/CD3/CD45 and pan-natural killer (NK)-antibodies, conjugated by fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll and allophycocyanin respectively (BD Biosciences, Transduction Laboratories, San Diego, CA, USA). Intracellular staining with anti-IFN-γ and anti-IL-4 antibodies (fluorescein isothiocyanate and phycoerythrin conjugated, respectively) was performed according to the manufacturer's protocol (BD Biosciences).
Direct effect of plasminogen activators on lymphocytes
One million splenocytes from either naive WT, uPA−/− or uPAR−/− C57/Bl6 mice were cultured in vitro for 48 h with 100 nM of either single-chain uPA (scuPA) or tPA. uPA is secreted by the cells as scuPA and converted in the medium (or extracellular space) to two-chain uPA known commonly as uPA. scuPA is converted to uPA after cleavage of a single peptide bond by the proteolytic enzyme plasmin. As a control, lymphocytes from each strain were also stimulated with either 50 ng/ml phorbol 12-myristate 13-acetate, 200 ng/ml ionomycin or with culture medium alone. At each concentration, 10 triplicates from 10 animals were tested. Cells were cultured in 10% fetal calf serum supplemented with RPMI-1640 (Atlantic Biologicals, Norcross, GA, USA), 2-mercaptoethanol (5 × 10−5 M) and 1% penicillin–streptomycin–glutamine (1% BRL; Gibco, Grand Island, NY, USA). Cultured cells were analysed for T cell proliferation. The median result from each triplet was used for statistical analysis.
To assess the direct in vivo effect of plasminogen activators on lymphocytes, eight WT C57/B6 mice were treated by intraperitoneal injection of scuPA, and were compared with eight naive animals. Because of a bleeding diathesis of the treated mice, three scuPA doses were given at 6-h intervals. Animals were killed 2 h following the last scuPA injection and intrahepatic lymphocytes were isolated for FACS analysis of CD4, CD8 and intracellular staining for IFN-γ and IL-4.
Role of plasminogen activators in lymphocyte–HSC interaction
In another in vivo study, 16 C57/B6 male mice were irradiated sublethally and served as recipients. One million splenocytes were transferred intraperitoneally from naive donors to irradiated recipients every week for 5 weeks. Naive donor splenocytes were isolated from WT or uPA−/− animals, and were then reconstituted in the irradiated recipients. Eight recipients were reconstituted with WT lymphocytes and another eight had uPA−/− cells. Beginning in the second week, hepatic fibrosis was induced by carbon tetrachloride for 4 weeks. Recipients were then killed and liver lymphocytes were analysed by FACS and Western blotting.
Statistical analysis
Lymphocyte subsets, serum aminotransferases, fibrosis quantification and cytokine concentrations in animal groups were analysed for statistically significant differences by Student's t-test. Comparison between the three recipient groups was performed according to the Mann–Whitney U-test. Results are presented as mean values ± standard deviation. Standard error was used in the case of Bioquant® analysis, as each group includes 360 readings.
Results
Decreased hepatic fibrosis in tPA−/−, uPA−/− and uPAR−/− mice
All animal groups survived the 6 weeks of observation after carbon tetrachloride treatment. The extent of fibrosis was determined in naive and fibrotic WT, uPA−/−, uPAR−/− and tPAR−/− mice. Fibrotic septa stained with Sirius Red were more established in WT than in −/− animals (Fig. 1a). Based on Bioquant® morphometry, the proportion of the mean liver area with fibrosis (± standard error) in naive WT animals was 0·37 ± 0·068%; this value is similar to that described by us and other groups [20]. The extent of fibrosis in the other naive animals (uPA−/−, uPAR−/− and tPAR−/− mice) was in the same range (data not shown). Six weeks after carbon tetrachloride treatment, fibrosis increased significantly in all animal groups (P = 0·00001, Fig. 1b). Figure 1b shows a greater reduction in the degree of fibrosis in uPA−/− compared with uPAR−/− animals (P = 0·02), suggesting that either another receptor(s) subtype(s) or another uPA-related pathway is involved; this conclusion is supported by our previous findings, which demonstrated that the stimulatory effect of uPA on vascular smooth muscle cells is mediated by the low-density lipoprotein receptor-related protein/alpha(2)-macroglobulin receptor [28]. In WT mice the fibrosis area rose to 1·7 ± 0·08% compared with 1·14 ± 0·09, 0·82 ± 0·12 and 1 ± 0·06% in uPA−/−, uPAR−/− and tPAR−/− animals (P = 0·00001), respectively.
Fig. 1.
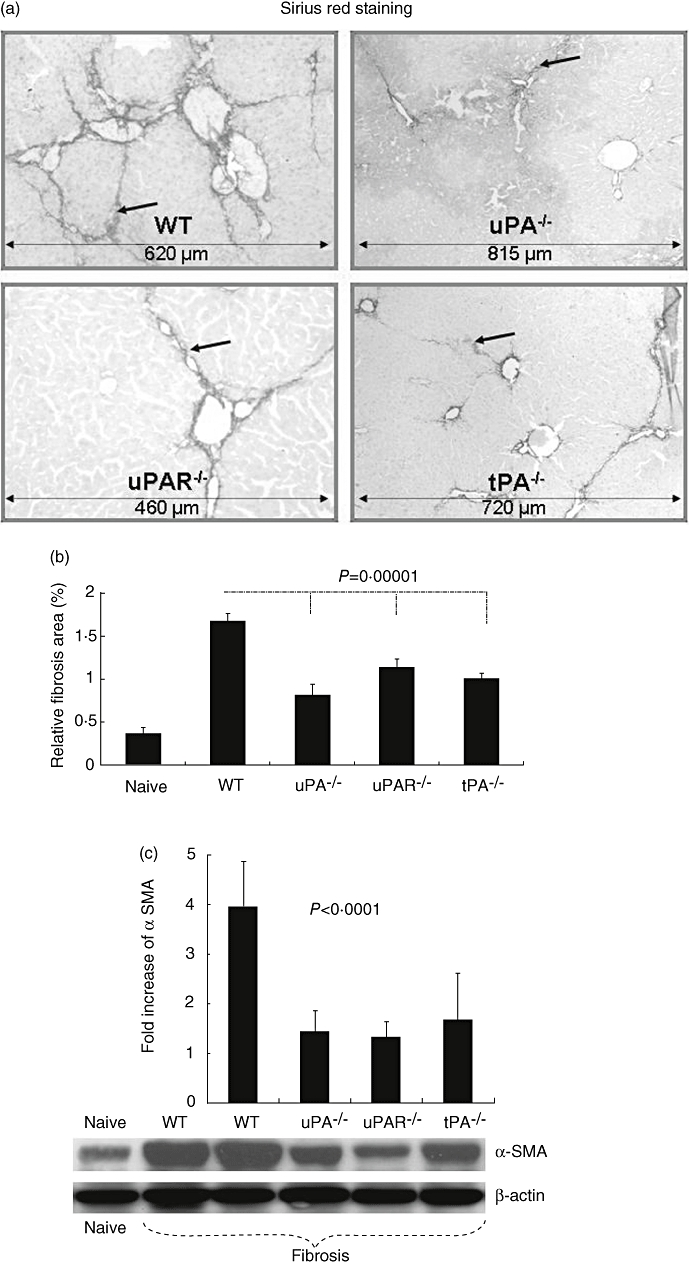
Reduced fibrosis in urokinase type plasminogen activator knockout (uPA−/−), uPA receptor (uPAR−/−) and tissue PA (uPA−/−)versus wild-type (WT) animals in a carbon tetrachloride model of liver injury. Tissue sections were stained with Sirius Red, as described in Materials and methods. Representative tissue sections are shown (a). Fibrotic septa, which are more established in WT than in −/− animals, are highlighted by arrows (scale bars are illustrated in the bottom of each field and presented with μm). Relative fibrosis area, expressed as percentage of total liver area, was assessed by analysing 36 Sirius Red-stained liver sections per animal (b). Each field was acquired at 10× magnification and then analysed using a computerized Bioquant® morphometry system. The relative fibrosis area in the livers of −/− animals was lower than that seen in WT mice. P-values refer to comparisons between WT and –/– animals after carbon tetrachloride. Whole liver protein lysates were extracted and 30 μg total protein was loaded per lane and analysed for alpha smooth muscle actin (αSMA) and β-actin expression (c). Decreased αSMA expression (lower panel) was found in lysates from uPA−/−, uPAR−/− and tPA−/− compared with WT receiving carbon tetrachloride and compared with naive WT animals. To obtain standardizations of β-actin/αSMA expression of all tested wells, bands were scanned and quantified as described in Methods. Results were expressed as fold increase compared with naive mice from each gel and (c, upper panel). The findings are representative of at least four different experiments with the same number of eight to 10 animals in each subgroup.
To support the finding of decreased fibrosis in the mutant mice, the expression of αSMA (a marker of HSC activation) was evaluated in liver protein extracts. Figure 1c shows that the reduced fibrosis in the knock-out mice was associated with diminished expression of αSMA, as assessed by immunoblot (lower panel), indicating decreased HSC activation. Semi-quantitative densitometry of immunoblot bands revealed the same pattern (upper panel). αSMA expression was increased to 4·96 ± 0·9-fold in fibrotic WT compared with naive mice. Compared with fibrotic WT mice, however, αSMA expression to fibrotic knock-out mice was reduced significantly (P < 0·0001) to 1·44 ± 0·41-fold in uPA−/−, 1·33 ± 0·32 in uPAR−/− and 1·68 ± 0·94 in tPAR−/− animals. No other organ impairment was seen in −/− mice following hepatic fibrosis induction (data not shown), including histology of lungs, heart, colon, muscle and kidneys.
Impaired clearance of necrotic tissue in uPA knock-out mice
We found no significant ALT/AST differences among the naive/untreated groups; the serum ALT levels in the naive WT, uPA−/−, uPAR−/− and tPAR−/− mice were 36 ± 12, 58 ± 28, 17 ± 0 and 48 ± 5, and the AST levels were 120 ± 44, 235 ± 206, 101 ± 1 and 137 ± 46 units, respectively (Fig. 2a, upper panel). ALT/AST serum levels increased significantly after fibrosis induction (P < 0·01) to 227 ± 83/299 ± 83, 166 ± 53/146 ± 33, 286 ± 58/513 ± 47 and 260 ± 204/456 ± 308 units, respectively (Fig. 2a, lower panel). Serum AST levels were higher in the uPA−/− group compared with WT and uPAR−/− (P < 0·001), but this was less prominent compared with tPA−/− animals [P = not significant (n.s.)]. ALT serum levels also tended to be elevated in the uPA−/− (tPA) group compared with all others, but this was significant only when compared with the uPAR−/− mice (P = 0·007). These results do not suggest marked differences in hepatic injury, but are more suggestive of impaired clearance of necrotic tissue in the absence of PA or uPAR, as described below.
Fig. 2.
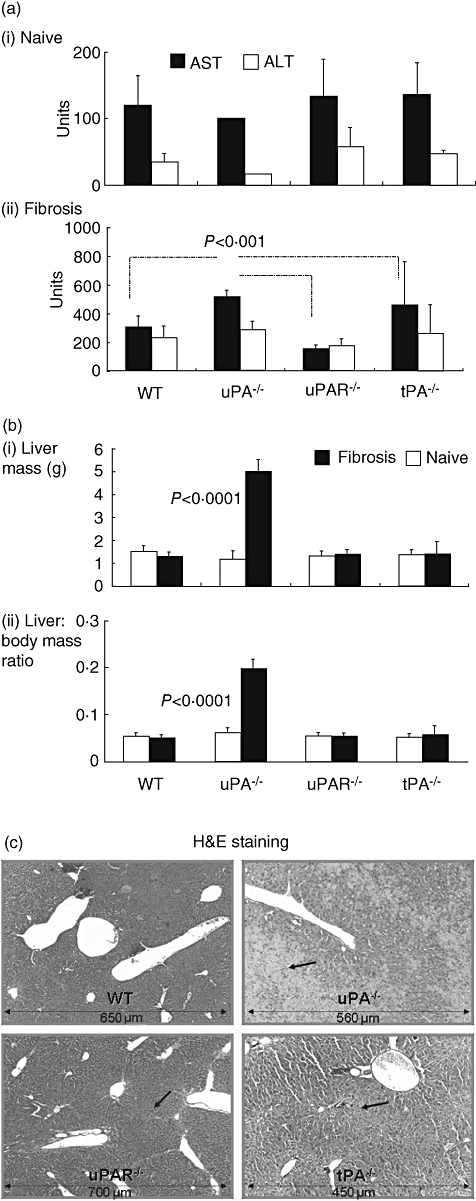
Increased liver injury in urokinase-type-plasminogen activator (uPA)−/− mice (a): serum aspartate (AST, filled bars) and alanine (ALT, plain bars) aminotransferase levels showed no significant differences in naive animals (upper panel). Following fibrosis induction, ALT/AST serum levels increased significantly (P < 0·01) in all groups (lower panel), but specifically AST levels in the uPA−/− group (P < 0·001). Increased ALT/AST values represent the accumulation of non-cleared necrotic tissue. Accumulation caused an increase in liver volume (b). Liver volume, presented as weight (g) (upper panel) or corrected for total body mass, presented as ratios (lower panel), was similar in all naive wild-type (WT), tissue-type (tPA) −/−, uPA−/− and uPA-receptor (uPAR)−/− groups (plain bars). Following induction of fibrosis (filled bars), liver volume in the uPA−/− fibrotic group increased fivefold (P < 0·0001). A repeated experiment showed the same pattern. Haematoxylin and eosin staining (c) demonstrates increased necrosis (dark arraws) mainly in the uPA−/− fibrotic animals (right upper panel) and to a lesser extent in the tPA−/− mice (right lower panel), but is minimal in WT and uPAR−/− fibrotic animals (left upper/lower panels, respectively).
One of the distinctive features of hepatic fibrosis in mice is a decrease in liver size [3–6]. Figure 2b shows that there were no significant differences in the size of the liver between the different naive groups. Figure 2b (upper panel) also displays an increase in liver volume in uPA−/− animals after the induction of fibrosis (from 1·2 ± 0·4 to 5 ± 0·5 g, P < 0·0001); this contrasts with the WT mice, which displayed a tendency towards decreased liver volume. The fourfold increase in liver size in fibrotic uPA−/− was maintained when liver volume was corrected for total body mass, as presented in Fig. 2b (lower panel). This finding is in line with the data published by Bezerra et al. in an acute carbon tetrachloride model [3,4].
The hepatic volume expansion seen in fibrotic uPA−/− mice was due to accumulation of large amounts of necrotic tissue. H&E microscopic analysis of fibrotic livers from uPA−/− mice (Fig. 2c, right upper panel) revealed pericentral necrosis after carbon tetrachloride and pronounced accumulation of non-cleared necrotic cells in the centrilobular area, which were responsible for the pale appearance, with the remaining normal periportal hepatocytes comprising the red foci. These changes were modest in the tPA−/− mice (Fig. 2c, right lower panel) but less prominent in WT and uPAR−/− fibrotic groups (Fig. 2c, left upper and lower panels, respectively). The higher ALT serum levels in the uPA−/− and tPA−/− fibrotic groups are consistent with H&E microscopic analysis. Measurements of spleen mass and number of splenocytes from fibrotic −/− mice did not show significant differences when compared with WT (data not shown).
Plasminogen activators modulate lymphocyte subsets
There is mounting evidence that the immune system plays an important role in the development of liver fibrosis [20,29,30]. On the other hand, uPA is necessary and sufficient for the generation of the monocyte/macrophage maturation promoting fragment mactinin, in vitro and in vivo[31]. uPAR is also a multi-functional molecule involved in migration and adhesion of leucocytes to sites of inflammation [32–34].
Using FACS analysis, lymphocyte subsets were analysed in splenocytes, thymus, lymph nodes (data not shown) and intrahepatic lymphocytes from all animal groups. Significant differences were confined mainly to intrahepatic lymphocytes (Fig. 3). The proportion of CD4 T cells (presented as percentage of CD45+ cells) from naive mice was reduced significantly in all knock-out groups (P < 0·001) compared with WT mice; CD4 T cells were 26 ± 1% of CD45+ cells in naive WT mice and 6·4 ± 1·6, 7 ± 1·2 and 13·5 ± 3·4 in uPA−/−, uPAR−/− and tPA−/− mice, respectively (Fig. 3a). The significant change in CD4+ T cells seen in tPA−/− compared with uPA−/− and uPAR−/− mice also suggests that either another receptor(s) subtype(s) or another tPA-related pathway are involved. This conclusion might be supported indirectly by reports from patients infected with human immunodeficiency virus (HIV)-1 who have low CD4 counts. HIV-1 infection activates or damages the endothelium and significantly increases plasma levels of von Willebrand factor, tumour necrosis factor-α and IL-6 but not tPA [35]. Figure 3a also shows differences in CD4 T cells between WT, uPA and uPAR knock-out mice after induction of fibrosis. WT mice show a significant decrease in CD4 T cells from 26 ± 1% in naive to 13 ± 0·6% in fibrotic mice (P = 0·001), but there was no change in the uPA−/− animals. There was also no significant change in uPAR−/− mice, whereas a change was seen in the tPA−/− subgroup (13·5 ± 3·4, 7 ± 1·8; P = 0·007).
Fig. 3.
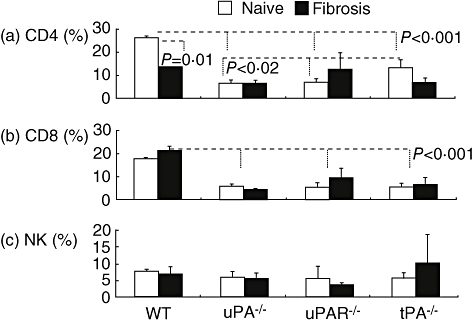
Significant decrease of CD4 and CD8 subsets in tissue-type (tPA)−/−, urokinase-type plasminogen activator (uPA)−/− and uPA receptor (uPAR)−/− mice. Fluorescein isothiocyanate (FACS) analysis of intrahepatic lymphocytes showed significant reduction of CD4+ T cells (P < 0·001) in all naive (plain bars) and fibrotic (filled bars) tPA−/−, uPA−/− and uPAR−/− compared with wild-type (WT) animals (a), expressed as the percentage of CD45+ in the y axis. CD4+ T cells decreased significantly following fibrosis in the WT (P = 0·001) and tPA−/− (P = 0·007) mice. Compared with WT, CD8 (b) from naive and fibrotic mice were reduced significantly in all −/− groups (P < 0·002). They increased in WT animals following fibrosis (P = 0·05) and were not significantly different in the other groups. Natural killer cells were unchanged (c). Similar FACS results were seen in four different experiments.
Similar changes were seen in CD8 T cells. In naive animals the levels were significantly lower in all knock-out mice compared with WT mice. Average CD8 T levels in naive mice were 17·6 ± 0·4% in WT compared with 5·7 ± 1·1%, 5·3 ± 2·1% and 5·6 ± 1·7% in uPA−/−, uPAR−/− and tPA−/− mice, respectively ((P < 0·002 for each) (Fig. 3b). After induction of fibrosis in WT mice, CD8 T cells increased from 17·6 ± 0·4% of total CD45+ lymphocytes in naive mice to 21·3 ± 1·8% in fibrotic mice (P = 0·05); there was no significant difference between naive and all types of fibrotic knock-out mice. Induction of liver fibrosis had no effect on NK cells (Fig. 3c).
Serum cytokine levels were reduced significantly in fibrotic knock-out animals
uPA is known to stimulate the activation of lymphocytes [32,33], and cytokines can modulate liver fibrosis; some cytokines stimulate, whereas others inhibit the fibrotic process [33,36]. Figure 4 shows that IFN-γ concentrations in the naive animals were 6 ± 1·5 pg/ml in WT, 3·4 ± 5 pg/ml in uPA−/− and undetectable in uPAR−/− and tPA−/− mice. In WT animals, induction of fibrosis led to a significant increase in the serum concentration of IFN-γ from 6 ± 15 to 36 ± 21 pg/ml (P < 0·02). In contrast, induction of liver fibrosis in all knock-out mice failed to induce any significant change in the level of serum IFN-γ, suggesting strongly that the reduced fibrosis in livers of −/− mice is not the result of an enhanced anti-fibrotic environment generated by this cytokine.
Fig. 4.
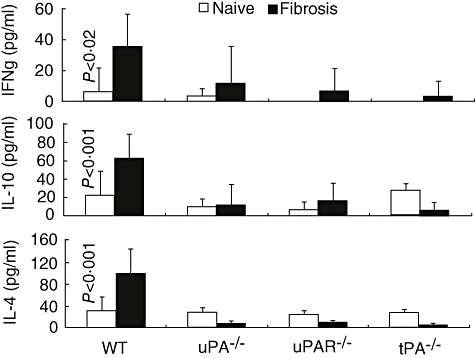
Serum cytokine levels are reduced significantly following fibrosis in tissue-type (tPA)−/−, urokinase-type plasminogen activator (uPA)−/− and uPA receptor (uPAR)−/− compared with wild-type (WT) animals. Serum interferon (IFN) gamma (upper panel), interleukin (IL)-10 (middle panel) and IL-4 (lower panel) levels were determined by enzyme-linked immunosorbent assay in all animals and expressed as pg/ml in naive (palin bars) and fibrotic (dark filled bars) mice (Fig. 4). The fibrotic WT animals had a significant elevation of serum IFN-γ (P < 0·02), IL-10 (P < 0·001) and IL-4 (P < 0·001) compared with all other naive and fibrotic groups that did not show any significant differences. Although serum cytokine levels were similar in all naive animals and among the fibrotic knock-out groups, only IL-4 levels were significantly lower in the fibrotic knock-out animals (P < 0·001). Results were regenerated twice showing same pattern.
No significant differences in IL-4 levels were detected among all four groups of naive mice. The induction of fibrosis in WT mice led to a significant increase of the serum concentration of IL-4 from 31 ± 26 to 100 ± 44 pg/ml (P < 0·001). In the three knock-out groups there were no significant changes in IL-4 levels after induction of fibrosis, suggesting that the decreased fibrosis seen in −/− mice may result, at least in part, from a deficiency in the production of this pro-fibrotic cytokine (Fig. 4).
Expression of IL-10 by HSC increases during cellular activation [37] and is associated with reduced matrix accumulation in culture [38]. Moreover, levels of IL-10 are reduced in progressive human hepatitis C virus fibrosis [39], implying that loss of IL-10 might contribute to increased fibrogenesis. Animal models, including IL-10 knock-out mice [40–42], have also substantiated the anti-fibrotic activity of IL-10. We measured serum IL-10 concentrations and detected no significant differences among the four groups of naive mice. In serum from WT mice, the concentration of IL-10 increased after induction of fibrosis from 31 ± 41·4 to 63·2 ± 25·1 (P < 0·001), similar to the changes in IL-4. In contrast, there were no significant changes in the levels of IL-10 in the serum of uPA−/−, uPAR−/− and tPA−/− mice. Taking these results together, we infer that the increase in IL-10 in WT mice reflects a response to active fibrosis; this assertion is in line with the finding that high transgenic levels of IL-10 decrease fibrosis [20].
Plasminogen activators increase the in vitro proliferation of lymphocytes
Our data demonstrate that knock-out mice had less liver fibrosis than WT animals, which was accompanied by decreased levels of CD4 and CD8 T cells and related cytokines; these observations suggest that plasminogen activators affect the development of liver fibrosis by regulating the expression of cytokines that govern the activity of the HSC, which are directly responsible for liver fibrosis [20]. To examine our hypothesis directly, splenocytes from naive WT and uPA–/– mice were incubated alone or in the presence of scuPA (100 nM). As a positive control, splenocytes were incubated with phorbol 12-myristate 13-acetate or ionomycin.
As expected, phorbol 12-myristate 13-acetate increased cell proliferation of WT, uPA−/− and uPAR−/− mice almost two- to threefold: from 370·6 ± 108·6 counts per min, 367·3 ± 284·4 and 333·1 ± 0·2 (as baseline culture with medium) to 628·4 ± 206·9 (P = 0·006), 1024·5 ± 771 (P = 0·03) and 724 ± 288·2 (P = 0·007), respectively (Fig. 5a). Ionomycin stimulation resulted in a similar pattern wherein proliferation increased to 933 ± 413·3 (P = 0·003), 980·3 ± 326 (P = 0·0006) and 887·7 ± 465·9 (P = 0·008), respectively. No significant changes were observed between all strains following phorbol 12-myristate 13-acetate or ionomycin stimulation. Phorbol 12-myristate 13-acetate and ionomycin proliferation responses therefore appear to be independent from plasminogen activator pathways (Fig. 5a). Figure 5b shows that incubation of splenocytes with scuPA induced a significant increase in WT cell proliferation (P = 0·0002) compared with cells cultured in medium alone. A similar stimulatory effect of scuPA was obtained when lymphocytes from uPA−/− mice were used (Fig. 5b) compared with cells incubated with culture medium alone (Fig. 5a); their proliferation increased to 2822·3 ± 3796 (P = 0·04) in the presence of scuPA. The effect of stimulation with tPA was similar to that seen with scuPA (Fig. 5b), where WT and uPA−/− cell proliferation increased significantly, to 2245 ± 1449/932 ± 658 at 100 nM tPA (P < 0·001). Cells from uPAR−/− animals, however, did not react to either scuPA or tPA administration because of the lack of a receptor, as they were 485·3 ± 330·2 and 514·2 ± 100·2, respectively, similar to those cultured with medium alone (369 ± 267, P = n.s.).
Fig. 5.
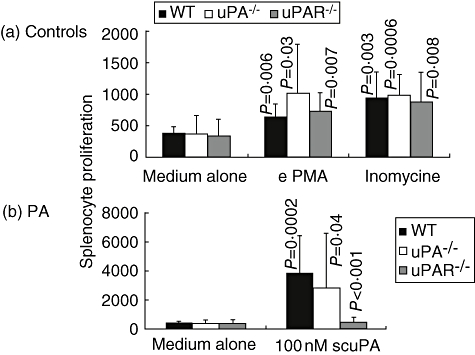
Plasminogen activators (PAs) increase the in vitro proliferation of lymphocytes: 1 million splenocytes from either naive wild-type (WT) (filled bars), urokinase-type plasminogen activator (uPA)−/− (plain bars) or uPA receptor (uPAR)−/− (grey bars) mice were cultured in vitro for 48 h with 100 nM of either single-chain uPA (scuPA) or tissue-type (tPA). As a control, cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) or 200 ng/ml ionomycin, or cultured with medium alone. Cultured cells were analysed for T cell proliferation. Cells from all strains responded significantly to ionomycin and PMA. While scuPA and tPA increased proliferation in WT and uPA−/− mice, they did not affect uPAR−/− derived lymphocytes. Results were similar in two different experiments.
Effect of uPA on lymphocyte subsets and the cytokine profile
To assess the direct effect of uPA in vivo on hepatic lymphocyte subpopulations, WT mice were treated by intraperitoneal injections of scuPA and were compared with naive animals. Intrahepatic lymphocytes were isolated and evaluated by FACS analysis. Figure 6a shows that following scuPA injection CD4 T cells decreased from 19 ± 3 to 16·3 ± 3·5 (P = 0·03), while CD8 subsets increased from 8·8 ± 0·4 to 9·8 ± 1 (P = 0·01). The CD4/CD8 ratio (Fig. 6b) decreased significantly towards CD8 predominance (P = 0·01); from 2·3 ± 0·4 to 1·7 ± 0·4. Intracellular staining for cytokines revealed that CD4 subsets showed a Th1 shift (Fig. 6c), resulting in a significant increase of IFN-γ-secreting CD4 subsets (from 11·5 ± 4·8% to 19·2 ± 5·6% of total CD4 cells) (P = 0·05). A decrease of IL-4 from 11·6 ± 3·8% to 7·4 ± 2·9% (P = 0·04) was also observed. CD8 subsets, however (Fig. 6d), showed an increased secretion of both IL-4 (from 2·6 ± 1% to 6·7 ± 3·4% of total CD8 cells) (P = 0·01) and IFN-γ (from 4·4 ± 2% to 9·2 ± 0·4%) (P = 0·001).
Fig. 6.
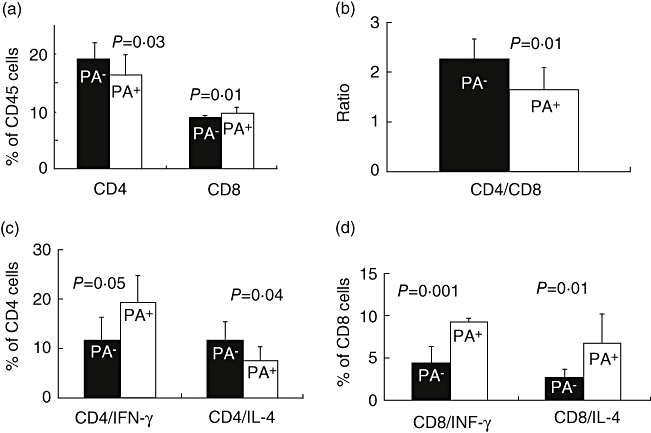
Effect of tissue-type (tPA) on lymphocyte subsets, and the cytokine profile: liver lymphocytes were isolated from wild-type (WT) animals treated by single-chain uPA (scuPA) and compared with naive animals. CD4, CD8 and intracellular staining was performed as described in Methods. While Fig. 5 shows that total proliferation increased in presence of scuPA, (a) shows that intrahepatic CD4 T cells decreased and CD8 subsets increased (left upper panel). The CD4/CD8 ratio, however, decreased (P = 0·01) significantly towards CD8 predominance (b). CD4 subsets (c) showed a T helper 1 shift evident as a significant increase of interferon (IFN)-γ secreting CD4 subsets and a decrease of interleukin (IL)-4 secreting subsets. CD8 subsets, however (d) showed an increased secretion of both IL-4 and IFN-γ. Results were regenerated twice with similar pattern.
Role of plasminogen activators on lymphocytes and HSC interaction
To elucidate further the relationship between plasminogen activators/lymphocytes and liver fibrosis, we examined the effect of uPA on the capacity of lymphocytes to activate HSC. Sublethally irradiated WT mice were reconstituted with WT or uPA−/− naive lymphocytes before and concurrent with induction of fibrosis. Figure 7a shows that uPA−/− lymphocyte failed to increase CD4 as well as many CD8 subsets. CD4 cells decreased from 30·4 ± 6·7% to 24 ± 1·2% in recipients reconstituted with uPA−/− lymphocytes (P = 0·02); CD8 subsets were reduced from 23·5 ± 10·2% to 12·5 ± 2% (P = 0·005). The NK cells remained unchanged. We also found decreased expression (P = 0·002) of αSMA in Western blots of mice reconstituted with uPA–/– lymphocytes (Fig. 7b), indicating decreased HSC activation.
Fig. 7.
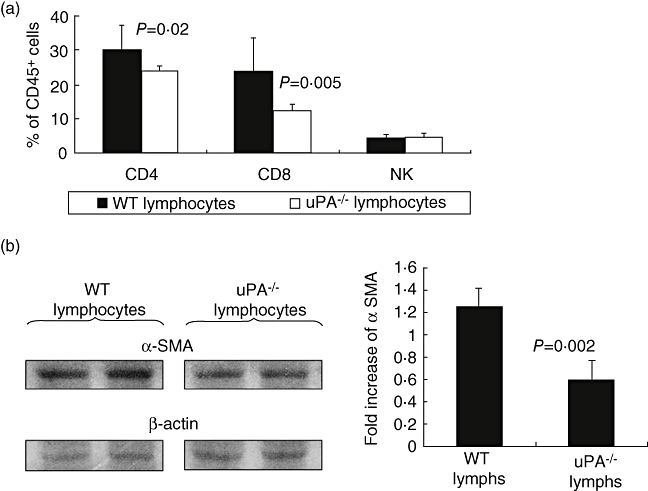
Role plasminogen activators (PAs) on lymphocytes and hepatic stellate cell interaction: to isolate uPA's effect on lymphocytes and their role in fibrogenesis, wild-type (WT) mice were irradiated sublethally and then reconstituted with WT or urokinase-type plasminogen activator (uPA)−/− lymphocytes. Next, fibrosis was induced by carbon tetrachloride; decreased T cells (a) and fibrosis (b) were identified in the uPA−/− recipients. (a) Displays fluorescence activated cell sorter analysis of liver lymphocytes. Western blotting of beta actin and alpha smooth muscle actin in the liver protein extracts are presented in (b). Bands were scanned and quantified as described in Methods. Results were expressed as fold increase compared with naive mice from each gel and (b, right panel). Similar results were replicated in two different experiments.
Discussion
Impaired tissue remodelling has been reported in lungs [43] and livers [4] of mice lacking plasminogen activators or plasminogen [3,5–7]. The results of the present study demonstrate reduced hepatic fibrosis in tPA−/−, uPA−/− and uPAR−/− mice following chronic carbon tetrachloride injury (Fig. 1).
As well as the decrease in liver fibrosis, uPA−/−, uPAR−/− or tPA−/− mice develop an attenuated immune response that may explain the impaired liver remodelling found in these animals. The three groups of knock-out mice used in this report showed a decrease in hepatic T cells, especially of the CD8 subset and decreased levels of IFN-γ and IL-4 in naive animals [20], which did not increase following chronic carbon tetrachloride injury. The link between the modification of the immune response and the absence of the plasminogen activators in knock-out mice is supported by the finding that treatment of WT naive (non-fibrotic) mice with scuPA shifted hepatic T cell subsets towards CD8 predominance and CD4 reduction (Fig. 6). Furthermore, the data from ex vivo experiments showing that plasminogen activators stimulate the proliferation of lymphocytes in general, and increase the absolute numbers of CD8, support the assertion that the plasminogen activation system is involved in regulating the immune response to injury. As our ex vivo lymphocyte experiments and the in vivo scuPA treatment lacked a direct fibrotic stimulation and response, they remain a limitation of this study.
On the other hand, the link between the decrease in liver fibrosis and attenuation in the immune response is supported by our previous study [20], where the induction of liver fibrosis was associated with increased total CD8+ T cells, IL-4 secretion and an increased CD8 : CD4 ratio. The present data show that induction of fibrosis in WT mice increased the pro-fibrotic CD8 and IL-4, but decreased CD4 cells (Figs 3 and 4). Naive knock-out mice, however, exhibited decreased CD8, CD4 and IL-4 levels, which did not change after carbon tetrachloride treatment.
As well as the failure to increase levels of pro-fibrotic CD8 and IL-4 in knock-out mice, we found no increase in the anti-fibrotic factors IL-10 or IFN-γ. These findings suggest that the decrease in liver fibrosis is due primarily to attenuation of immune-mediated pro-fibrotic signals rather than an increase of inhibitory signals. Our data, combined with the finding that IL-10 transgenic mice [20] are protected from hepatic fibrosis, suggest that the increase in IL-10 during liver fibrosis in WT mice may be a response to the increased fibrogenic process.
The differences in phenotype between uPA−/− and tPA−/− in liver size and in the capacity to remove necrotic tissue suggest strongly that uPA has more than one role in the liver. Combining our results with those of Bezzera et al. [3–7], who demonstrated that the effect on necrotic tissue removal in uPA−/− mice depends on plasminogen activation, we postulate that uPA is the principal plasminogen activator during liver recovery, in addition to its effect on lymphocyte activation and cytokine expression that is shared with tPA.
The complementary experiments show that injection of scuPA to WT non-fibrotic mice induces changes in T cells confined primarily to the intrahepatic lymphocytes. This finding explains the different impact of the absence of plasminogen activators on responses seen in diverse organs, e.g. the increase in pulmonary fibrosis in the same knock-out mice, and points to the importance of yet-to-be-discovered organ-specific regulatory pathways.
Different inflammatory cells, including Küpffer cells and lymphocytes, play a role in the convergence of pro- and anti-fibrotic stimuli. Therefore, B cells, macrophages, dendritic cells and the activity of Küpffer cells and HSC in livers of uPA−/− mice merit evaluation in future studies.
Our findings extend the known roles of the plasminogen activator system in liver fibrosis and eventual cirrhosis emerging from other studies. For example, plasminogen activator inhibitor-1 expression in the liver is higher in obese patients with diabetes mellitus than in non-obese patients [44,45] and is inhibited by thiazolidinediones and statins [45]. Its increase blocks the conversion of scuPA to the active uPA [46]. Thus, scuPA levels are expected to increase and may augment the lymphocyte activation in non-alcoholic steatohepatitis, an issue under investigation. Immune modulation of fibrosis induction by plasminogen activators is therefore among the multiple factors engaged in this complex response, and might reveal additional targets for therapeutic intervention.
Acknowledgments
The technical assistance of Sa'ed Akkawi and Sarit Doron is gratefully acknowledged. This work was supported by grants from the National Institutes of Health (DK56621), the Feld Foundation and the US–Israel Binational Science Foundation. This work was supported by grants from ‘Return to Hadassah’, ‘Compensatory’ and ‘Horovetz’ Awards (Grant No. 8033601, 8033603, 8033604), the Israel Science Foundation (Grant No. 930/04) and Grants HL06831, HL67381 and DK56621 (SLF) from the National Institutes of Health.
References
- 1.Safadi R, Friedman SL. Hepatic fibrosis-role of hepatic stellate cell activation. Medgenmed. 2002;15:27. [PubMed] [Google Scholar]
- 2.Iredale JP. Hepatic stellate cell behaviour during resolution of liver injury. Semin Liver Dis. 2001;21:427–36. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 3.Bezerra JA, Bugge TH, Melin-Aldana H, et al. Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver. Proc Natl Acad Sci USA. 1999;96:15143–8. doi: 10.1073/pnas.96.26.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezerra JA, Currier AR, Melin-Aldana H, et al. Plasminogen activators direct reorganization of the liver lobule after acute injury. Am J Pathol. 2001;158:921–9. doi: 10.1016/S0002-9440(10)64039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pohl JF, Melin-Aldana H, Sabla G, et al. Plasminogen deficiency leads to impaired lobular reorganization and matrix accumulation after chronic liver injury. Am J Pathol. 2001;159:2179–86. doi: 10.1016/S0002-9440(10)63069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng VL, Sabla GE, Melin-Aldana H, et al. Plasminogen deficiency results in poor clearance of non-fibrin matrix and persistent activation of hepatic stellate cells after an acute injury. J Hepatol. 2001;35:781–9. doi: 10.1016/s0168-8278(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 7.Currier AR, Sabla G, Locaputo S, et al. Plasminogen directs the pleiotropic effects of uPA in liver injury and repair. Am J Physiol Gastrointest Liver Physiol. 2003;284:G508–15. doi: 10.1152/ajpgi.00336.2002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LP, Takahara T, Yata Y, et al. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. J Hepatol. 1999;31:703–11. doi: 10.1016/s0168-8278(99)80351-1. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Schoonjans L, Kieckens L, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;369:419–24. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 10.Nassar T, Akkawi S, Shina A, et al. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 11.Kaur J, Zhao Z, Klein GM, et al. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–63. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Moons L, Dewerchin M, et al. Receptor-independent role of urokinase type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice. J Cell Biol. 1998;140:233–45. doi: 10.1083/jcb.140.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Moons L, Ploplis V, et al. Impaired arterial neointimal formation in mice with disruption of the plasminogen gene. J Clin Invest. 1997;99:200–8. doi: 10.1172/JCI119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guitierrez LS, Schulman A, Brito-Robinson R, et al. Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res. 2000;60:5839–47. [PubMed] [Google Scholar]
- 15.Ploplis VA, Castellino FJ. Gene targeting of components of the fibrinolytic system. Thromb Haemost. 2002;87:22–31. [PubMed] [Google Scholar]
- 16.May AE, Kanse SM, Lund LR, et al. Urokinase receptor (CD87) regulates leukocyte recruitment via b2 integrins in vivo. J Exp Med. 1998;188:1029–93. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ploplis VA, Wilberding J, McLennan L, et al. A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice. Am J Pathol. 2000;157:703–8. doi: 10.1016/S0002-9440(10)64582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005;36:2265–9. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- 19.Lebeurrier N, Liot G, Lopez-Atalaya JP, et al. The brain-specific tissue-type plasminogen activator inhibitor, neuroserpin, protects neurons against excitotoxicity both in vitro and in vivo. Mol Cell Neurosci. 2005;30:552–8. doi: 10.1016/j.mcn.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Safadi R, Ohta M, Alvarez CE, et al. Immune stimulation of hepatic fibrogenesis by CD8 lymphocytes and its attenuation by transgenic interleukin 10 from hepatocytes. Gastroenterology. 2004;127:870–82. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 22.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci USA. 1997;94:10663–8. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZE, Reiner SL, Zheng S, et al. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzel FP, Sadick MD, Holaday BJ, et al. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bdeir K, Murciano JC, Tomaszewski J, et al. Urokinase mediates fibrinolysis in the pulmonary microvasculature. Blood. 2000;96:1820–6. [PubMed] [Google Scholar]
- 26.Miranda SR, Erlich S, Visser JW, et al. Bone marrow transplantation in acid sphingomyelinase-deficient mice: engraftment and cell migration into the brain as a function of radiation, age, and phenotype. Blood. 1997;90:444–52. [PubMed] [Google Scholar]
- 27.Lyons C, Dowling V, Tedengren M, et al. Variability of heat shock proteins and glutathione S-transferase in gill and digestive gland of blue mussel, Mytilus edulis. Mar Environ Res. 2003;56:585–97. doi: 10.1016/S0141-1136(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 28.Nassar T, Haj-Yehia A, Akkawi S, et al. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem. 2002;277:40499–504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- 29.Laso FJ, Iglesias-Osma C, Ciudad J, et al. Alcoholic liver cirrhosis is associated with a decreased expression of the CD28 costimulatory molecule, a lower ability of T cells to bind exogenous IL-2, and increased soluble CD8 levels. Cytometry. 2000;42:290–5. [PubMed] [Google Scholar]
- 30.Lombardo L, Capaldi A, Poccardi G, et al. Peripheral blood CD3 and CD4 T-lymphocyte reduction correlates with severity of liver cirrhosis. Int J Clin Lab Res. 1995;25:153–6. doi: 10.1007/BF02592558. [DOI] [PubMed] [Google Scholar]
- 31.Luikart S, Masri M, Wahl D, et al. Urokinase is required for the formation of mactinin, an alpha-actinin fragment that promotes monocyte/macrophage maturation. Biochim Biophys Acta. 2002;1591:99–107. doi: 10.1016/s0167-4889(02)00255-0. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Maurer F, Medcalf RL. Plasminogen activator inhibitor type 2: a regulator of monocyte proliferation and differentiation. Blood. 2002;99:2810–18. doi: 10.1182/blood.v99.8.2810. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida E, Tsuchiya K, Sugiki M, et al. Modulation of the receptor for urokinase-type plasminogen activator in macrophage-like U937 cells by inflammatory mediators. Inflammation. 1996;20:319–26. doi: 10.1007/BF01488206. [DOI] [PubMed] [Google Scholar]
- 34.Sitrin RG, Todd RF, Mizukami IF, et al. Cytokine-specific regulation of urokinase receptor (CD87) expression by U937 mononuclear phagocytes. Blood. 1994;84:1268–75. [PubMed] [Google Scholar]
- 35.de Larranaga GF, Petroni A, Deluchi G, et al. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinol. 2003;14:15–18. doi: 10.1097/00001721-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Speth C, Pichler I, Stockl G, et al. Urokinase plasminogen activator receptor (uPAR; CD87) expression on monocytic cells and T cells is modulated by HIV-1 infection. Immunobiology. 1998;199:152–62. doi: 10.1016/S0171-2985(98)80071-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang SC, Tsukamoto H, Rippe RA, et al. Expression of interleukin-10 by in vitro and in vivo activated hepatic stellate cells. J Biol Chem. 1998;273:302–8. doi: 10.1074/jbc.273.1.302. [DOI] [PubMed] [Google Scholar]
- 38.Mathurin P, Xiong S, Kharbanda KK, et al. IL-10 receptor and coreceptor expression in quiescent and activated hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G981–90. doi: 10.1152/ajpgi.00293.2001. [DOI] [PubMed] [Google Scholar]
- 39.Napoli J, Bishop GA, McGuinness PH, et al. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–65. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 40.Louis H, Van Laethem JL, Wu W, et al. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607–15. doi: 10.1002/hep.510280621. [DOI] [PubMed] [Google Scholar]
- 41.Arai T, Hiromatsu K, Kobayashi N, et al. IL-10 is involved in the protective effect of dibutyryl cyclic adenosine monophosphate on endotoxin-induced inflammatory liver injury. J Immunol. 1995;155:5743–9. [PubMed] [Google Scholar]
- 42.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 43.Swaisgood CM, French EL, Noga C, et al. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol. 2000;157:177–87. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alessi MC, Bastelica D, Mavri A, et al. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 2003;23:1262–8. doi: 10.1161/01.ATV.0000077401.36885.BB. [DOI] [PubMed] [Google Scholar]
- 45.Takeshita Y, Takamura T, Hamaguchi E, et al. Tumor necrosis factor-α-induced production of plasminogen activator inhibitor 1 and its regulation by pioglitazone and cerivastatin in a nonmalignant human hepatocyte cell line. Met Clin Exp. 2006;55:1464–72. doi: 10.1016/j.metabol.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Higazi AA, Mazar A, Wang J, et al. Single-chain urokinase-type plasminogen activator bound to its receptor is relatively resistant to plasminogen activator inhibitor type 1. Blood. 1996;87:3545–9. [PubMed] [Google Scholar]


