Abstract
We have developed a new and effective method for bone marrow transplantation (BMT): bone marrow cells (BMCs) are injected directly into the bone marrow (BM) cavity of recipient mice. The intrabone marrow injection of BMCs (IBM-BMT) greatly facilitates the engraftment of donor-derived cells, and IBM-BMT can attenuate graft-versus-host reaction (GVHR), in contrast to conventional intravenous BMT (i.v.-BMT). Here, we examine the mechanisms underlying the inhibitory effects of IBM-BMT on GVHR using animal models where GVHR is elicited. Recipient mice (C57BL/6) were irradiated and splenic T cells (as donor lymphocyte infusion: DLI) from major histocompatibility complex-disparate donors (BALB/c) were injected directly into the BM cavity (IBM-DLI) or injected intravenously (i.v.-DLI) along with IBM-BMT. The BM stromal cells (BMSCs) from these recipients were collected and related cytokines were examined. The recipient mice that had been treated with IBM-BMT + i.v.-DLI showed severe graft-versus-host disease (GVHD), in contrast to those treated with IBM-BMT + IBM-DLI. The suppressive activity of BMSCs in this GVHD model was determined. The cultured BMSCs from the recipients treated with IBM-BMT + IBM-DLI suppressed the proliferation of responder T cells remarkably when compared with those from the recipients of IBM-BMT + i.v.-DLI in mixed leucocyte reaction. Furthermore, the level of transforming growth factor-β and hepatocyte growth factor in cultured BMSCs from IBM-BMT + IBM-DLI increased significantly when compared with those from the recipients of IBM-BMT + i.v.-DLI. Thus, the prevention of GVHD observed in the recipients of IBM-BMT + IBM-DLI was attributable to the increased production of immunosuppressive cytokines from BMSCs after interaction with host reactive T cells (in DLI).
Keywords: bone marrow stromal cells, donor lymphocyte infusion, graft-versus-host disease, intrabone marrow-bone marrow transplantation
Introduction
Allogeneic bone marrow transplantation (BMT) has been used as a potentially curative therapy for patients with a wide variety of diseases, including haematological disorders, congenital immunodeficiencies, metabolic disorders, autoimmune diseases and solid tumours [1–6]. However, there are several problems to be resolved in allogeneic BMT. One of the important issues is how to control graft-versus-host disease (GVHD), which remains a major cause of post-transplantation morbidity and mortality.
We have recently developed intrabone marrow (IBM)-BMT, in which bone marrow cells (BMCs) are injected directly into the bone marrow (BM) cavity [7]. We have found that IBM-BMT allows us not only to use low-dose irradiation as a preconditioning regimen [7,8] but also to suppress GVHD [9], as IBM-BMT can efficiently recruit donor-derived stromal cells [including mesenchymal stem cells (MSCs)] that can support donor-derived haemopoietic stem cells [1,9–12].
It is noted that IBM-BMT can be used to prevent GVHD, even when intensive donor lymphocyte infusion (DLI) is carried out [9]. We attempted to inject allogeneic T cells as DLI into the BM cavity (IBM-DLI) or intravenously (i.v.-DLI) with IBM-BMT. The prolongation of survival rate and reduction of GVHD were observed clearly in the recipients treated with IBM-BMT + IBM-DLI, but not in those with IBM-BMT + i.v.-DLI [13]. These findings prompted us to examine the regulatory function of BM stromal cells (BMSCs) after interaction with T cells that had been injected into the BM cavity. Evidence has been accumulated that BMSCs play a critical role in the regulation of haemopoiesis by promoting cell-to-cell interactions and constitutively secreting immunoregulatory soluble factors [14–23]. In fact, BMSCs suppress the proliferation of allogeneic T cells in a major histocompatibility complex (MHC)-independent manner [24–31].
In the present study, we examine the suppressive activity of BMSCs that had been in contact with T cells in vivo, and evaluate the effect of T cell polarization and several factors produced by BMSCs.
Materials and methods
Mice
C57BL/6 (B6, H-2b), BALB/c (H-2d) mice were purchased from Shimizu Laboratory Supplies (Kyoto, Japan). C57BL/6 mice at the age of 7–9 weeks were used as recipients, and BALB/c mice at the age of 7–9 weeks were used as donors. All mice were kept in our animal facilities under specific pathogen-free conditions. All animal procedures were performed in accordance with protocols approved by the Animal Experimentation Committee, Kansai Medical University.
Irradiation
C57BL/6 mice were irradiated at 8·5 Gy (1·0 Gy/min) from a 137Cs source (Gammacell 40 Exactor, Nordion, International Inc., Ottawa, Ontario, Canada) 1 day before the BMT.
Bone marrow transplantation and donor lymphocyte infusion
Bone marrow cells were flushed from the femoral and tibial bones of the BALB/c mice, and then suspended in RPMI-1640. The BMCs were then filtered through a 70-μm nylon mesh (Becton Dickinson Labware, Franklin Lakes, NJ, USA) to remove debris, washed and adjusted to 1·5 × 109 cells/ml in RPMI-1640. The BMCs, thus prepared, were injected directly into the BM cavity as described previously [7]. Briefly, the region from the inguen to the knee joint was shaved and a 5-mm incision was made on the thigh. The knee was flexed to 90 degrees and the proximal side of the tibia was drawn to the anterior. A 26-gauge needle was inserted into the joint surface of the tibia through the patellar tendon and then inserted into the BM cavity. Using a microsyringe (10 μl; Hamilton Co., Reno, NV, USA) containing the donor BMCs (1·5 × 109 cells/ml), the donor BMCs were injected from the said bone holes into the BM cavity of the left tibia (107 cells/7 μl/tibia) (IBM-BMT). In some groups, BMCs were injected intravenously.
T cells were purified from the spleens by positive selection by a MACS® system using CD4 and CD8α microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) after depletion of red blood cells, or by an EPICS ALTRA flow cytometer (Coulter, Hialeah, FL, USA) after staining with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD4/CD8 monoclonal antibodies (mAbs) (BD Pharmingen, San Diego, CA, USA).
Splenic T cells were injected into the BM cavity of the right tibia (107 cells/7 μl/tibia: intrabone marrow T cell injection as DLI; IBM-DLI) or injected intravenously (i.v.-DLI; 107 cells/0·5 ml) into the recipient mice along with the IBM-BMT. Recipients treated with IBM-BMT alone (without DLI) served as negative controls (termed NO-DLI) [13].
Preparation of freshly isolated BMSCs
Three days after the DLI, BMCs were flushed from the right tibial bones of the recipient mice, and non-haemopoietic MSC-enriched cells (defined as CD45–/CD106+ cells) were sorted immediately by an EPICS ALTRA flow cytometer (Coulter, Hialeah, FL, USA) after staining with FITC- or PE-conjugated anti-CD45/CD106 mAbs (BD Pharmingen, San Diego, CA, USA). Freshly isolated non-haemopoietic BMSCs-enriched populations, sorted as CD45–/CD106+ cells, were prepared from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI, or IBM-BMT alone (NO-DLI). Haemopoietic BMC-enriched populations, sorted as CD45+/CD106– cells, were also prepared from the recipients and used as controls.
Preparation of cultured BMSCs
Bone marrow cells from the right tibia, into which T cells had been injected as DLI, were collected from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI) 3 days after treatment, and cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS). Two days later, non-adherent cells were removed. Adherent cells were detached using trypsin-ethylenediamine tetraacetic acid, and passaged when 80% confluence was reached and then replated. After 2 weeks (short-term culture) or 3 months (long-term culture) the cultures were discontinued, and BMSCs were harvested and used for further experiments, including mixed leucocyte reaction (MLR) and real-time reverse transcription–polymerase chain reaction (RT–PCR) assay. The culture-expanded BMSCs from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI) were stained with FITC-anti-CD45 and PE-anti-CD106 mAbs and analysed by a fluorescence activated cell sorter (FACScan) (BD Pharmingen).
Mixed leucocyte reaction
Various numbers of freshly prepared (defined as CD45–/CD106+ BM cells) or cultured BMSCs from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.V-DLI or IBM-BMT alone (without DLI) were added to the culture of one-way MLR (4-day culture) where 2 × 105 responder CD4+ T cells from BALB/c mice were stimulated with 12 Gy irradiated stimulator spleen cells (2 × 105 cells) from B6 mice in a 96-well flat-bottomed plate in a total volume of 0·2 ml. CD45+/CD106– haemopoietic cells or whole BMCs served as controls for BMSCs added to the culture. The cultures were pulsed with 0·5 μCi of [3H]-TdR for the last 16 h of the culture period.
Activation of T cells with concanavalin A
Splenic T cells (2 × 106 cells) from BALB/c mice were cultured with 2·5 μg/ml of concanavalin A (ConA) for 4 days. Activated T cells, thus prepared, were used as a positive control in real-time RT–PCR assay and enzyme-linked immunosorbent assay (ELISA) to detect cytokines.
Flow cytometric analyses of intracellular cytokines
CD4-enriched T cells from BALB/c mice were cultured with irradiated stimulator spleen cells from B6 mice with cultured BMSCs from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI) in round-bottomed plates (RPMI-1640) with 10% FCS. Cells were harvested 6 days later and stained with biotin-conjugated anti-H-2Kd (visualized by streptavidin–peridinin chlorophyll-Cy5·5) and FITC-anti-CD4 mAb (BD Pharmingen) to detect responder (donor) CD4 T cells. The cells were next fixed and permeabilized with Cutofix/Cytoperm solution™ (BD Pharmingen). Intracellular cytokines were detected after the staining of cells with PE-anti-interleukin (IL)-2, -interferon (IFN)-γ or -IL-4 using an Intracellular Cytokine Staining Kit® (BD Pharmingen). Cells stained with isotype control cocktail (BD Pharmingen) served as a control. The stained cells were analysed by a FACScan® (Becton Dickinson Co., Mountain View, CA, USA).
Real-time RT–PCR assay
Cytokine messages of BMSCs were determined by real-time RT–PCR. We prepared some primers for transforming growth factor (TGF)-β (forward: TTTCGATTCAGCGCTCACTGCTCTTGTGAC, reverse: ATGTTGGACAACTGCT CCACCTTGGGCTTGC), hepatocyte growth factor (HGF) (forward: AAGAGTGGCATCAAGTGCCAG, reverse: CTGGATTGCTTGTGAAACACC), IL-2 (forward: TGGAGCAGCTGTTGATGGAC, reverse: CAATTCTGTGGCCTGCTTGG), IL-4 (forward: ACAGGAGAAGGGACGCCAT, reverse: GAAGCCCTACAGACGAGCTCA), IL-10 (forward: GGTTGCCAAGCCTTATCGGA, reverse: ACCTGCTCCACTGCCTTGCT) and IL-15 (forward: CATCCATCTCGTGCTACTTGTGTT, reverse: CATCTATCCAGTTGGCCTCTGTTT) (Nisshinbo, Chiba, Japan).
Real-time RT–PCR was conducted on a DNA engine Opticon2 System (MJ Japan Ltd, Tokyo, Japan) by using SYBR Green I as a double-stranded DNA-specific binding dye and continuous fluorescence monitoring. The cycling conditions consisted of a denaturation step for 10 min at 95°C, 40 cycles of denaturation (94°C for 15 s), annealing (60°C for 30 s) and extension (72°C for 30 s). After amplification, melting curve analysis was performed with denaturation at 95°C, then continuous fluorescence measurement from 65°C to 95°C at 0·1°C/s. All reactions were run at least in duplicate, and included control wells without cDNA.
Detection of cytokines in MSC culture supernatant
Mesenchymal stem cell culture supernatants were collected 2 weeks later, and the amounts of IL-2, IL-4, IFN-γ and TGF-β were determined by ELISA kits.
Statistical analyses
Non-parametric analyses (Mann–Whitney U-test and log-rank test) were performed using StatView software (Abacus Concepts, Berkley, CA, USA). Values of P < 0·05 were considered statistically significant.
Results
In vitro immunosuppressive effects of BMSCs on T cell proliferation
Three days after DLI, BMCs were collected from the recipients, and non-haemopoietic BMCs (defined as CD45–/CD106+ cells) were isolated immediately as shown in Fig. 1a. The average number of these sorted cells per mouse were as follows. CD45–/CD106+ cells from the recipients of IBM-BMT + IBM-DLI: 31 033 ± 2450 cells (four mice), CD45–/CD106+ cells from the recipients of IBM-BMT + i.v.-DLI: 29 850 ± 2728 cells (four mice), CD45–/CD106+ cells from the recipients of IBM-BMT alone (without DLI): 36 630 ± 5244 cells (four mice). There were no statistical differences among these groups regarding the yields of CD45–/CD106+ cells. The sorted CD45–/CD106+ cells from these recipients were added to the culture of one-way MLR. As shown in Fig. 2, all the CD45–/CD106+ cells isolated from the BM of IBM-DLI, i.v.-DLI and IBM-BMT alone (without DLI) suppressed MLR only slightly, but not significantly (not statistically significant among three groups). This is the case when haemopoietic CD45+/CD106– cells or whole BMCs were added to the culture. Thus, non-haemopoietic BMCs freshly isolated from the site of IBM-DLI could not significantly suppress T cell proliferation in MLR. This might be due to the heterogeneity of non-haemopoietic BMCs. Therefore, we next examined the inhibitory effect of cultured BMSCs after IBM-DLI.
Fig. 1.
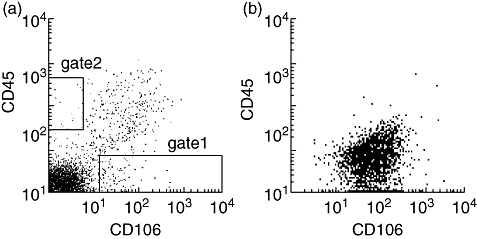
Flow cytometric profiles of freshly isolated and cultured bone marrow stromal cells (BMSCs). (a) Non-haemopoietic mesenchymal stem cell-enriched cells, defined as CD45–/CD106+ cells, were sorted immediately (gate 1) from the recipient of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI) after the staining of cells with fluorescein isothiocyanate (FITC)-anti-CD45 and phycoerythrin (PE)-anti-106 monoclonal antibodies (mAbs). The dot-plot profile of CD45–/CD106+ cells from the recipients of IBM-BMT + intravenous (i.v.)-DLI or IBM-BMT alone (without DLI) was similar to (a). Haemopoietic bone marrow cell-enriched populations, sorted as CD45+/CD106– cells (gate 2), were also prepared from the recipients, and used as controls. (b) Cultured BMSCs (for 2 weeks) obtained originally from the right tibia of the recipients of IBM-BMT + IBM-DLI were stained with FITC-anti-CD45 and PE-anti-106 mAbs, and analysed by a fluoresence activated cell sorter scan. The dot-plot profile of cultured BMSCs from the recipients of IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI) was similar to (b).
Fig. 2.
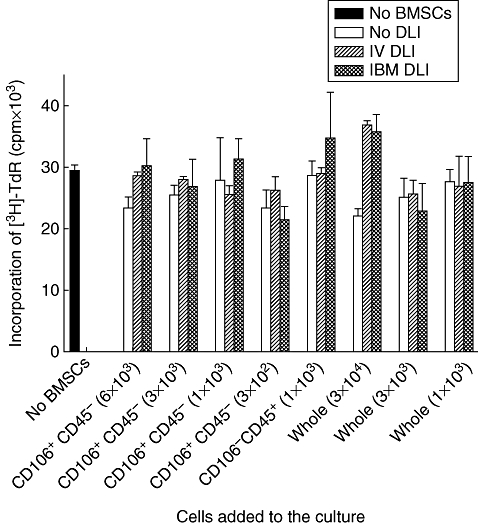
Effect of freshly isolated bone marrow stromal cells (BMSCs) on T cell proliferation. Non-haemopoietic mesenchymal stem cell-enriched cells, defined as CD45–/CD106+ cells, were sorted immediately (gate 1) from the recipient of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI), IBM-BMT + intravenous (i.v.)-DLI or IBM-BMT alone (without DLI) after the staining of cells with fluorescein isothiocyanate-anti-CD45 and phycoerythrin-anti-106 monoclonal antibodies (mAbs). Haemopoietic cells in the bone marrow (BM), defined as CD45+/CD106– cells, were also obtained by a cell sort (gate 2). Graded numbers of CD45–/CD106+ BMSCs (3 × 102−6 × 103), CD45+/CD106– haemopoietic cells (1 × 103) or whole BM cells (1 × 103−3 × 104) were added to the culture of one-way mixed leucocyte reaction where 2 × 105 responder CD4+T cells from BALB/c mice were stimulated with 12 Gy irradiated stimulator spleen cells (2 × 105 cells) from B6 mice in a 96-well flat-bottomed plate in a total volume of 0·2 ml and cultured for 96 h. The cultures were pulsed with 0·5 μCi of [3H]-TdR for the last 16 h of the culture period. This figure shows the representative result of three experiments. The data are expressed as mean counts per minute ± standard deviation of three mice (separately cultured BMSCs obtained from the recipient).
Three days after DLI, BMCs were collected from the recipients, and cultured in DMEM with 10% FCS for 2 weeks, as shown in Materials and methods. The phenotypes of BMSCs, thus prepared, were negative for CD45 and CD34, but positive for CD90 and CD106 (Fig. 1b). These BMSCs were added to the culture of MLR to examine their suppressive effects.
As shown in Fig. 3a and b, the BMSCs prepared from the recipients treated with IBM-BMT + IBM-DLI significantly suppressed MLR in a dose-dependent fashion when compared with those from the recipients treated with IBM-BMT + i.v.-DLI. It is surprising that the BMSCs from the recipients of IBM-BMT + IBM-DLI still showed a suppressive effect on T cell proliferation even after long-term culture (3 months) when compared with those prepared from the recipients of IBM-BMT + i.v.-DLI (Fig. 3c), suggesting that the suppressive effects of BMSCs on the BM (IBM-DLI) are long-lasting.
Fig. 3.
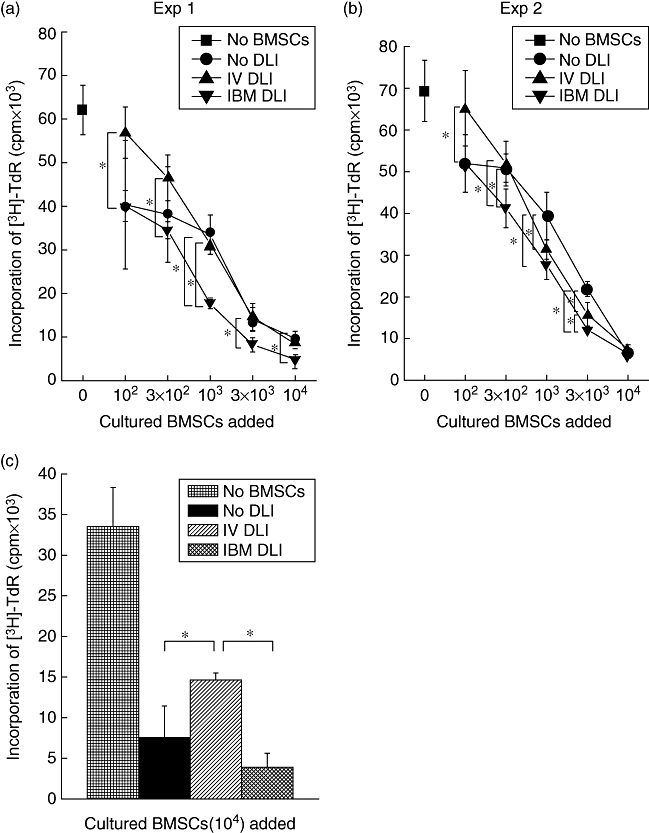
Inhibitory effect of cultured bone marrow stromal cells (BMSCs) on T cell proliferation. Bone marrow cells from the right tibia were collected from the recipients of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI), IBM-BMT + intravenous (i.v.)-DLI or IBM-BMT alone (without DLI) and cultured for 2 weeks. Graded numbers of cultured BMSCs (102−104 cells) were added to the culture of one-way mixed leucocyte reaction (MLR) (a, b). BMSCs were obtained after the long-term culture (cultured for 3 months), and were added to the culture of one-way MLR (c). The data in figures are expressed as mean counts per minute ± standard deviation of three mice (separately cultured BMSCs obtained from the recipient). Symbols in the boxes represent origins of cultured BMSCs. *Statistically significant when compared with MLRs performed in the groups (P < 0·05).
The frequency of IFN-γ- and IL-4-producing T cells after coculture with BMSCs
To examine the effects of BMSCs on T cell polarization, CD4-enriched T cells from donor BALB/c mice were cultured with irradiated stimulator spleen cells from B6 mice and BMSCs cultured from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI, or IBM-BMT alone (without DLI). The development of T helper 1 (Th1) or Th2 cells was defined by intracellular staining of IFN-γ or IL-4. The frequency of IL-4-producing cells was slightly but significantly higher in the culture with BMSCs from IBM-BMT + IBM-DLI than in that with BMSCs from IBM-BMT + i.v.-DLI (Fig. 4a and bversus4c and summarized in 4g). Conversely, the percentage of IFN-γ-producing cells was lower in the culture with BMSCs from IBM-BMT + IBM-DLI than in that with BMSCs from IBM-BMT + i.v.-DLI (Fig. 4d and eversus4f, and summarized in 4g). Furthermore, this is the case when intracellular IL-2 was examined (data not shown). Thus, the polarization of Th2 cells is facilitated strongly after co-culture with the BMSCs from the recipients of IBM-BMT + IBM-DLI, while Th1 cells are induced dominantly by co-culture with the BMSCs from the recipients of IBM-BMT + i.v.-DLI. These findings suggest strongly that T cells injected into the BM cavity can modulate the function of BMSCs after their interaction.
Fig. 4.
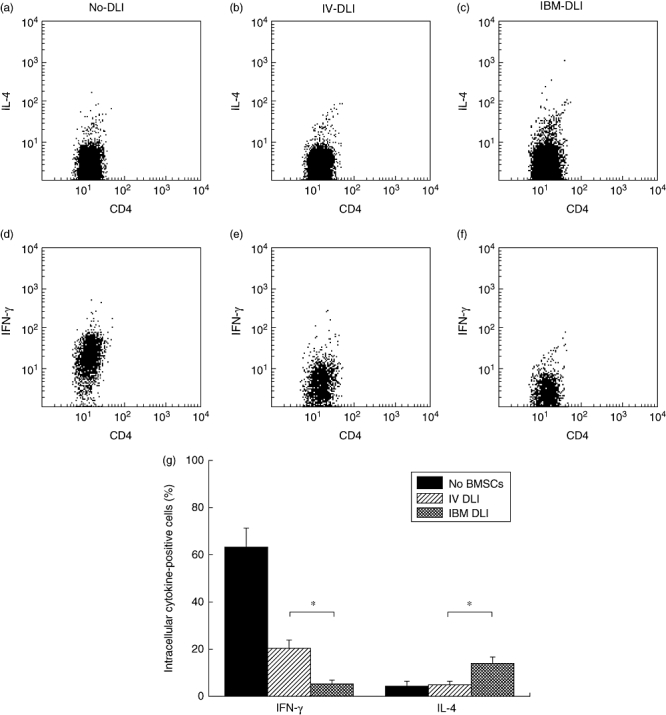
Interaction of bone marrow stromal cells (BMSCs) with T cells and induction of T helper 2 cells. CD4+ cell-enriched T cells from BALB/c mice were cultured with irradiated stimulator spleen cells from B6 and BMSCs cultured from the recipients of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI), IBM-BMT + intravenous (i.v.)-DLI or IBM-BMT alone (without DLI) in a round-bottomed plate (RPMI-1640) with 10% fetal calf serum. Cells were harvested 6 days later and stained with biotin-conjugated anti-H-2Kd (visualized by streptavidin–peridinin chlorophyll-Cy5·5) and fluorescein isothiocyanate-anti-CD4 monoclonal antibody (mAb) to detect responder (donor) CD4 T cells. The cells were next fixed and permeabilized and intracellular cytokines were detected after the staining of cells with phycoerythrin-anti-interleukin (IL)-4 and -interferon (IFN)-γ mAbs. Representative dot-plot profiles of CD4+/IL-4+ cells (a, b, c) or CD4+/IFN-γ+ cells (d, e, f) are shown, co-cultured with BMSCs from the recipients of IBM-BMT alone (without DLI) (a, d), IBM-BMT + i.v.-DLI (b, e), or IBM-BMT + IBM-DLI (c, f). Cells in dot-plot profiles were gated positively as H-2Kd+ responder cells. Cells stained with isotype control cocktail served as a control. (g) Representative result of three experiments. Columns represent mean percentage of IFN-γ or IL-4 bearing cells ± standard deviation of three mice (separately cultured BMSCs obtained from the recipient). Symbols in the boxes represent origins of cultured BMSCs. *Statistically significant when compared with intracellular cytokines performed in the groups (P < 0·05).
Bone marrow stromal cells produce immunoregulatory cytokines: TGF-β and HGF
Previous reports have shown that BMSCs can modify T cell functions by soluble factors [18,19]. Therefore, we attempted to identify molecules involved in the immune modulation by BMSCs. First, we determined the levels for IL-2, IL-10, IFN-γ and TGF-β in the culture supernatant of BMSCs using an ELISA. The culture supernatants of enriched T cells stimulated with ConA served as a control. As shown in Table 1, IL-2, IL-10 or IFN-γ were not detected in the culture supernatants of BMSCs from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI), while a significant amount of TGF-β was detected in the culture supernatants of BMSCs from the recipients of IBM-BMT + IBM-DLI, but not in those from the recipients of IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI). These results indicate that TGF-β secreted from the BMSCs obtained from the recipients of IBM-BMT + IBM-DLI might be one of the candidates for attenuation of GVHD in our model system.
Table 1.
Measurement of cytokines.
| No DLI† | i.v.-DLI | IBM-DLI | T cells with ConA‡ | |
|---|---|---|---|---|
| IL-2 (pg/ml) | 0 | 0 | 0 | 87·3 ± 15·5 |
| IFN-γ (pg/ml) | 0 | 0 | 0 | 1418·2 ± 369·4 |
| IL-10 (pg/ml) | 0 | 0 | 0 | 1114·6 ± 103·1 |
| TGF-β (ng/ml) | 0 | 0·27 ± 0·4 | 14·2 ± 2·4 | 0·6 ± 0·5 |
Bone marrow stromal cell (BMSC) culture supernatants from the recipients of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI), IBM-BMT + intravenous (i.v.)-DLI, or IBM-BMT alone (without DLI) were collected 2 weeks later. The cell supernatants were analysed for the amount of interleukin (IL)-2, IL-4, interferon (IFN)-γ and trandforming growth factor (TGF)-β by enzyme-linked immunosorbent assay.
Splenic T cells from BALB/c mice were activated with concanavalin A (ConA) and used as a positive control.
It has been reported that HGF also inhibits T cell proliferation or activation [18,19]. Therefore, we next determined in the culture supernatants of BMSCs whether the levels of HGF in BMSCs increased after IBM-BMT + IBM-DLI. We measured HGF (and also TGF-β) in the message level by a quantitative real-time RT–PCR because no ELISA kit is available to detect murine HGF. As shown in Fig. 5a (HGF) and 5b (TGF-β), the relative levels of both HGF and TGF-β were significantly higher in the BMSCs from the recipients of IBM-BMT + IBM-DLI than in those from the recipients of IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI). Furthermore, as summarized in Table 2, we did not detect substantial levels of IL-2, IL-4 or IL-15 mRNA in BMSCs from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI). However, it is noted that a slight but significant level of IL-10 message was detected only in the BMSCs from recipients of IBM-BMT + IBM-DLI, but not in those from recipients of IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI). Therefore, T cells injected directly into the BM cavity can induce the production of suppressive cytokines from BMSCs, and BMSCs might exert their inhibitory effect on T cell activation or proliferation via HGF and/or TGF-β.
Fig. 5.
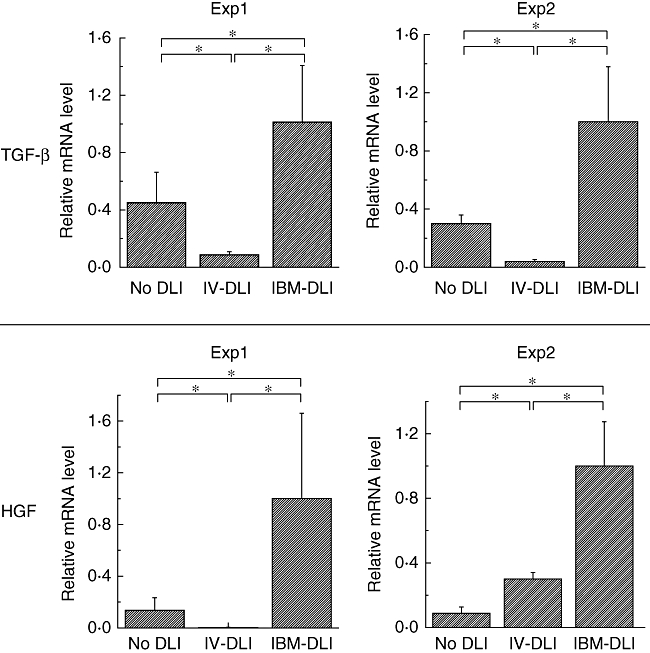
Production of transforming growth factor (TGF)-β and hepatocyte growth factor (HGF) in bone marrow stromal cells (BMSCs). Culture expanded BMSCs from the recipients of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI), IBM-BMT + intravenous (i.v.)-DLI or IBM-BMT alone (without DLI) were used for analysis of cytokine messages by real-time PCR. After DNase I treatment, cDNA was synthesized, amplified using HGF or TGF-β primer, and visualized with SYBR Green by real-time reverse transcription–polymerase chain reaction. Relative intensity of HGF or TGF-β mRNA was calculated on the basis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) intensity. Columns represent relative cytokine message levels of TGF-β and HGF. Each column shows mean ± standard deviation of three mice (separately cultured BMSCs obtained from the recipient), and we performed two separate experiments. Symbols in the boxes represent origins of cultured BMSCs. *Statistically significant when compared with cytokine message performed in the groups (P < 0·05).
Table 2.
Analyses of cytokine messages by real-time reverse transcription–polymerase chain reaction (RT–PCR).
| Cytokines examined | No DLI† | i.v.-DLI | IBM-DLI | T cells with ConA‡ |
|---|---|---|---|---|
| IL-2 | 0·47 ± 0·3§ | 0·31 ± 0·2 | 0·47 ± 0·3 | 8·51 ± 6·1 |
| IL-4 | 0 | 0 | 0·025 ± 0·03 | 1277·2 ± 357·4 |
| IL-10 | 0 | 0 | 2·7 ± 2·3 | 95 000 ± 16 000 |
| IL-15 | 0 | 0 | 0 | n.d. |
Culture expanded bone marrow stromal cells (BMSCs) from the recipients of intrabone marrow-bone marrow transplantation (IBM-BMT) + IBM-donor lymphocyte infusion (DLI), IBM-BMT + intravenous (i.v.)-DLI, or IBM-BMT alone (without DLI) were used for analysis of cytokine messages by real-time RT–PCR. After DNase I treatment, cDNA was synthesized, amplified using interleukin (IL)-2, IL-4, IL-10 or IL-15 primer, and visualized with SYBR Green by real-time RT–PCR.
Splenic T cells from BALB/c mice were activated with concanavalin A (ConA) and used as a positive control.
Relative intensities of soluble factors were calculated on the basis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Numbers in the table represent mean intensities of cytokines ± standard deviation of three mice (separately cultured BMSCs obtained from the recipient). We performed two separate experiments. n.d., not done.
Discussion
Transplantation biology has been one of the major advances in medicine during the last few decades. BMT, in particular, can cure a variety of malignancies by exploiting graft-versus-tumour effects exerted by the lymphocytes. In this procedure, one of the major problems to be solved is GVHD. We have developed recently a new protocol for BMT: IBM-BMT can induce persistent allogeneic donor-specific tolerance without the use of immunosuppressants after the treatment, even when the radiation doses are reduced to sublethal levels. Therefore, we have aimed to develop a new strategy for the successful engraftment of donor-derived haematolymphoid cells without developing GVHD even in the presence of T cells in the donor inoculum. We have found that GVHD could be alleviated when BMCs containing T cells were inoculated into the BM cavity [9]. We compared the severity of GVHD induced by the intravenous injection of T cells (i.v.-DLI) with that induced by the IBM injection of T cells (IBM-DLI). Acute GVHD was observed in recipients treated with IBM-BMT + i.v.-DLI, while reduced GVHD was seen in those treated with IBM-BMT + IBM-DLI. However, the mechanisms underlying this inhibition still remain unresolved and therefore we focused on the function of BMSCs, because T cells can interact with BMSCs in the BM cavity after the IBM-DLI. The ability of MSCs to interact with immune cells and to modulate their response has important implications in the transplantation biology. We have carried out experiments in which the sorted CD45–/CD106+ cells from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI or IBM-BMT alone (without DLI) were added to the culture of one-way MLR. The inhibitory ability of non-haemopoietic BMCs to activated T cells was insufficient (Fig. 2). However, cultured BMSCs from the recipients of IBM-BMT + IBM-DLI, IBM-BMT + i.v.-DLI and IBM-BMT alone (without DLI) showed an immunosuppressive effect in MLR in a dose-dependent fashion (Fig. 3). Furthermore, of interest and of importance is that the cultured BMSCs from the recipients of IBM-BMT + IBM-DLI suppressed MLR strongly even in small numbers (102−3 × 103) when compared with BMSCs from the recipients of IBM-BMT + i.v.-DLI.
Furthermore, the conversion of Th1 cells (defined by intracellular staining of IFN-γ) was clearly inhibited while the polarization of Th2 cells (defined by intracellular staining of IL-4) was facilitated by BMSCs from the recipients treated with IBM-DLI. In contrast to this, BMSCs from the recipients of i.v.-DLI prompted the polarization of Th1 cells (Fig. 4). These data suggest that BMSCs from the recipients of IBM-BMT + IBM-DLI interact with naive T cells to convert Th2 cells, which might be beneficial for GVHD management.
Several recent reports have described how BMSCs produce soluble factors, including TGF-β and HGF, which regulate T cell proliferation [18,21,22,32]. In our present study, BMSCs from the recipients of IBM-BMT + IBM-DLI produced significantly higher amounts of HGF and TGF-β than those from the recipients of IBM-BMT + i.v.-DLI and IBM-BMT alone (without DLI) (Fig. 5 and Table 1).
Collectively, our findings indicate clearly that BMSCs can interact with T cells that have been injected into the BM cavity as IBM-DLI, and that the function(s) of BMSCs might somehow be modulated by this interaction to produce inhibitory cytokines and to possess the ability to convert Th0 cells to Th2 cells, but not to Th1 cells. It should be noted that the modulated features of BMSCs were maintained for at least 6 weeks, thus leading to the reduction of GvH responses. We have shown, in our GVHD model, that IBM-DLI (in vivo injection of donor T cells into the BM cavity) (but not i.v.-DLI) can attenuate GVHD. Therefore, our present study provides the basic information that IBM-BMT is an excellent strategy to engraft donor cells efficiently along with attenuation of GVHD, even when some quantities of T cells are contaminated in BMC preparations. Thus, IBM-BMT can control GVHD easily.
T cells can recognize MHC determinants on BMSCs in vivo, and the BMSC recognized by T cells can modulate their functions. Therefore, we are now investigating subcellular processes after the T–BMSC interaction and identifying molecules, other than MHC, to be essential for this interaction.
Acknowledgments
Supported by a grant from Haiteku Research Center of the Ministry of Education, a grant from the Millennium programme of the Ministry of Education, Culture, Sports, Science and Technology, a grant from the Science Frontier programme of the Ministry of Education, Culture, Sports, Science and Technology, a grant from the 21st Century Center of Excellence programme of the Ministry of Education, Culture, Sports, Science and Technology, a grant-in-aid for scientific research (B) 11470062, grants-in-aid for scientific research on priority areas (A)10181225 and (A)11162221 and Health and Labour Sciences research grants (Research on Human Genome, Tissue Engineering, Food Biotechnology) and also grants from the Department of Transplantation for Regeneration Therapy (Sponsored by Otsuka Pharmaceutical Company Ltd), Molecular Medical Science Institute, Otsuka Pharmaceutical Co. Ltd and Japan Immunoresearch Laboratories Co., Ltd. We thank Ms Y. Tokuyama for her expert technical assistant, and Mr Hilary Eastwick-Field, Mr Brian O'Flaherty and Ms K. Ando for their help in the preparation of the manuscript.
References
- 1.Ikehara S. Bone marrow transplantation: a new strategy for intractable disease. Drugs Today. 2002;38:103–11. doi: 10.1358/dot.2002.38.2.820106. [DOI] [PubMed] [Google Scholar]
- 2.Ikehara S, Ohtsuki H, Good RA, et al. Prevention of type I diabetes in non-obese diabetic mice by allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 1985;22:7743–7. doi: 10.1073/pnas.82.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasumizu R, Sugiura K, Iwai H, et al. Treatment of type 1 diabetes mellitus in non-obese diabetic mice by transplantation of allogeneic bone marrow and pancreatic tissue. Proc Natl Acad Sci USA. 1987;84:6555–7. doi: 10.1073/pnas.84.18.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Than S, Ishida H, Inaba M, et al. Bone marrow transplantation as a strategy for treatment of non-insulin-dependent diabetes mellitus in KK-Ay mice. J Exp Med. 1992;176:1233–8. doi: 10.1084/jem.176.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida T, Inaba M, Hisha H, et al. Requirement of donor-derived stromal cells in the bone marrow for successful allogeneic bone marrow transplantation. Complete prevention of recurrence of autoimmune diseases in MRL/MP-lpr/lpr mice by transplantation of bone marrow plus bones (stromal cells) from the same donor. J Immunol. 1994;152:3119–27. [PubMed] [Google Scholar]
- 6.Nakagawa T, Nagata N, Hosaka N, Ogawa R, Nakamura K, Ikehara S. Prevention of autoimmune inflammatory polyarthritis in male New Zealand black/KN mice by transplantation of bone marrow cells plus bone (stromal cells) Arthritis Rheum. 1993;36:263–8. doi: 10.1002/art.1780360220. [DOI] [PubMed] [Google Scholar]
- 7.Kushida T, Inaba M, Hisha H, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–9. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 8.Taira M, Inaba M, Takata K, et al. Treatment of streptozotocin-induced diabetes mellitus in rats by transplantation of islet cells from two major histocompatibility complex disparate rats in combination with intra bone marrow injection of allogeneic bone marrow cells. Transplantation. 2005;79:680–7. doi: 10.1097/01.tp.0000155500.17348.94. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Inaba M, Sugiura K, et al. Enhancement of allogeneic hematopoietic stem cell engraftment and prevention of graft-versus-host diseases (GvHD) by intra-bone marrow-bone marrow transplantation plus donor lymphocyte infusion. Stem Cells. 2004;22:125–34. doi: 10.1634/stemcells.22-2-125. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto F, Sugiura K, Inoue K, Ikehara S. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood. 1997;89:49–54. [PubMed] [Google Scholar]
- 11.Sugiura K, Hisha H, Ishikawa J, et al. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vitro. Stem Cells. 2001;19:46–58. doi: 10.1634/stemcells.19-1-46. [DOI] [PubMed] [Google Scholar]
- 12.Ikehara S. A novel strategy for allogeneic stem cell transplantation: perfusion method plus intra-bone marrow injection of stem cells. Exp Hematol. 2003;31:1142–6. doi: 10.1016/j.exphem.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Fukui J, Inaba M, Ueda Y, et al. Prevention of graft-versus-host disease by intra-bone marrow injection of donor T cells. Stem Cells. 2007;25:1595–601. doi: 10.1634/stemcells.2006-0234. [DOI] [PubMed] [Google Scholar]
- 14.Golde DW, Gasson JC. Hormones that stimulate the growth of blood cells. Sci Am. 1988;259:62–71. doi: 10.1038/scientificamerican0788-62. [DOI] [PubMed] [Google Scholar]
- 15.Gordon MY. Extracellular matrix of the marrow microenvironment. Br J Haematol. 1988;70:1–4. doi: 10.1111/j.1365-2141.1988.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 16.Dexter TM. Regulation of hemopoietic cell growth and development: experimental and clinical studies. Leukemia. 1989;3:469–74. [PubMed] [Google Scholar]
- 17.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 18.Muriel S, Francoise N, Aurelie T, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 19.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 20.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 21.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into non-human primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–64. [PubMed] [Google Scholar]
- 23.Sudeepta A, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 24.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–9. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 27.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–34. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 28.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 29.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 30.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 31.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 32.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]


