Abstract
The male Wistar Bonn/Kobori (WBN/Kob) rat is known to be a unique animal model for chronic pancreatitis with widely distributed fibrosis and degeneration of parenchyma because of the infiltration of lymphocytes. In this report, we show that female (but not male) rats develop dacryoadenitis at 3 months of age, and that both male and female WBN/Kob rats develop sialoadenitis, thyroiditis, sclerotic cholangitis and tubulointerstitial nephritis over 18 months of age. The infiltration of CD8+ cells and the deposits of tissue-specific IgG2b were observed in the injured pancreas and lachrymal glands. Furthermore, the number of regulatory T cells (defined as CD4+ Forkhead box P3+ cells) decreased in the periphery of both male and female WBN/Kob rats, suggesting that the onset of these diseases is attributable, at least, to the failure in the maintenance of peripheral immune tolerance. These features show clearly that WBN/Kob rats are a useful animal model for autoimmune pancreatitis and Sjøgren-like syndrome or multi-focal fibrosclerosis in humans. We also show that these autoimmune diseases can be prevented by a newly devised strategy of bone marrow transplantation (BMT) in which bone marrow cells are injected directly into the bone marrow cavity: intrabone marrow–BMT.
Keywords: animal model, autoimmune extrapancreatic exocrinopathy, autoimmune pancreatitis, Forkhead box P3, intra bone marrow–bone marrow transplantation
Introduction
Recently, autoimmune pancreatitis (AIP) has been reported as a part of chronic pancreatitis with pancreatic duct stenosis. AIP is characterized by hypergammaglobulinaemia and responsiveness to corticosteroid therapy, suggesting the involvement of autoimmune mechanisms, particularly at the onset of this disease [1]. Patients with autoimmune diseases such as Sjøgren's syndrome and other autoimmune diseases in the liver, bile ducts, intestine and blood vessels often show AIP [2]. Lesions in the pancreas have been observed in acinar cells, ductal cells and pancreatic islet cells, indicating that the target cells or target molecules for autoimmune reactions are considered to be variable [3]. The precise mechanism underlying the onset of AIP and its progress is still unknown [4], although the diagnosis and therapy have been investigated extensively and progressed. In various cases of pancreatitis, it is difficult to prove whether autoimmune mechanisms are involved in the development of pancreatitis [5]. Therefore, long-term studies on histopathological evaluation before the onset of pancreatitis are clinically important [6]. Imaging studies of patients with AIP characteristically show the diffuse enlargement of the pancreas and irregular narrowing of the main pancreatic duct. Typical immunological abnormalities are the elevation of serum IgG4 and the presence of autoantibodies. The histopathological findings show lymphoplasmacytic sclerosing pancreatitis (LPSP) associated with fibrotic changes with dense infiltration of lymphocytes and IgG4-positive plasma cells [7]. However, the role of IgG4 in the pathogenesis of AIP still remains unclear. Therefore, the establishment of an animal model for AIP is critically important. To date, there have been reported various artificial AIP models, most of which are chemical or antigen-induced ones [8,9]. Even if there are some spontaneous animal models which partially resemble human AIP [10,11], there are many differences in terms of symptoms, gene functions and pathological findings.
It has been shown that the male Wistar Bonn/Kobori (WBN/Kob) rat is an animal model for spontaneous diabetes, osteopenia and systemic haemosiderin deposition [12]. Spontaneous hyperglycaemia, glycosuria, hypoinsulinaemia and glucose intolerance are observed after the age of 17 months. Marked fibrosis is observed around the pancreatic ducts and blood vessels at 3–6 months of age [13,14]. The fibrous tissue gradually invades extensive areas of the pancreas, and the islets are also affected by fibrotic degeneration, leading to an obvious decrease in islet number and size. Distinct infiltration of inflammatory cells has been observed around the islets and among adjacent acinar cells, and most inflammatory cells are apparently lymphocytes. Macrophages or granulocytes are not often observed in the lesions. These pathological findings are found in male but not female WBN/Kob rats, and have been discussed regarding their pathogenesis in relation to sex hormone [15], genetic factor [16] and immune disturbance [17]. WBN/Kob rats have some interesting characteristics: (i) only male WBN/Kob rats appear with pancreatitis; (ii) they are a unique diabetic animal model whose islets are withered by progress with inflammatory fibrosis dissimilar to the other diabetic models [18,19]; (iii) infiltrating cells are nearly all lymphocytes, rather than plasma cells and eosinophils [12]; and (iv) immune suppressive drugs such as tacrolimus or steroid hormones could prevent the onset of pancreatitis [17]. These characteristics resemble human AIP.
We have shown previously that conventional allogeneic bone marrow transplantation (BMT) can be used to treat autoimmune disease using various autoimmune-prone mice [20,21]. Recently, we have developed a new BMT method in which donor bone marrow cells (BMCs) are injected directly into the bone marrow cavity of recipients: intrabone marrow–BMT (IBM–BMT) [22,23]. This strategy allows us to reduce radiation doses to 5 Gy × 2 (fractionated irradiation), which is equivalent to 8 Gy (one shot). Therefore, we attempted to prevent abnormal pathological, serological and immunological findings in the WBN/Kob rats by IBM–BMT; in our preliminary experiments, conventional BMT was found to be unable to be used to prevent these abnormalities in the rats. In line with these studies, the model described here can be considered to be highly relevant not only for a better understanding of the pathogenesis of AIP but also for the preclinical testing of novel strategies for the treatment of AIP.
Materials and methods
Rats
Male and female WBN/Kob rats (RT1au), male Wistar rats and Fischer 344 (F344:RT1a1) rats were purchased from SLC (Shizuoka, Japan). They were maintained until use in our animal facilities under specific pathogen-free conditions. All experimental protocols were approved by the Animal Experimentation Committee (06-026), Kansai Medical University.
Histopathological analysis
Histopathological studies of the systemic exocrine organs (pancreas, liver, lachrymal and parotid glands, kidney and thyroid) in male and female WBN/Kob rats 4 weeks, 12 weeks and 18 months of age were performed. In brief, each organ was fixed in paraformaldehyde and infiltrated with Histo-Clear (National Diagnostics, Atlanta, GA, USA) [24]. They were dehydrated with ethanol and embedded in paraffin (5 μm), and stained with haematoxylin and eosin or Masson-Trichrome and Sirius red to detect fibrosis.
Immunohistochemical analyses
Thyroid, liver and kidney from 18-month-old WBN/Kob rats, and pancreas, spleen, lachrymal gland and parotid gland were obtained serially from the recipients 4, 8, 12 and 16 weeks after IBM–BMT. The sections were prepared with the masked antigen for using Histo-Clear, rehydrated through a graded series of ethanol/water solutions and incubated at 85°C in antigen retrieval buffer. The specimens were also stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or biotin-conjugated IgG, IgG1, IgG2a, IgG2b and IgG2c (FITC–streptavidin was used to visualize biotin-conjugated antibodies). Nuclei were stained with 4,6-diamidino-2-phenylindole (Nacalai Tesque, Inc., Kyoto, Japan). Immunohistochemical staining for CD4+, CD8+ cells, anti-major histocompatibility complex (MHC) I (OX-18) and anti-MHC II (OX-6) were also performed using frozen tissues from pancreas and lachrymal gland. The sections (4 μm) were incubated with appropriate primary antibodies at 4°C overnight. They were incubated with biotinylated secondary anti-rat IgG antibodies (Vector Laboratories, Burlingame, CA, USA) and incubated with avidin–biotin horseradish peroxidase complex (Vector Laboratories). Samples were incubated with 3,3′-diaminobenzidine in the presence of H2O2 to develop the chromatic reaction. The stained samples were analysed using an optical microscope (Nikon Eclipse E1000M, Digital Sight ACT-1 for L-1 software version 2·62; Nikon Co. Ltd, Tokyo, Japan) and confocal laser microscope (LSM 510 META; Carl Zeiss IMT Corporation, Oberkochen, Germany).
Biochemical analyses
Blood glucose was measured by a Dextor ZIIR (Bayer Medical Ltd, Tokyo, Japan). Total amylase and total protein were determined by a biochemistry automated analyser (AU5400; Olympus Corp., Tokyo, Japan). Immunoelectrophoresis was carried out in our laboratory using standard equipment. Interleukin (IL)-4, IL-10 and IL-12 were analysed by an enzyme-linked immunosorbent assay kit (R&D Systems, Inc., Minneapolis, MN, USA; BioSource, Camarillo, CA, USA).
IBM–BMT experiments
Fludarabine at 50 mg/kg was given as a single intravenous injection and irradiated in fractionated irradiations (5·0 Gy × 2 = 10 Gy; 4-h interval) 1 day before transplantation. BMCs were collected from the femurs and tibias of Fischer 344 rats. CD4+ cells were depleted from the BMCs by the combination of anti-CD4 antibody (Caltag Laboratories, Burlingame, CA, USA) and sheep anti-mouse IgG-coupled Dynabeads® (Dynal, Oslo, Norway). Resultant CD4+ T cell-depleted BMCs (2 × 108 BMCs) were injected directly into the bone marrow cavity of the recipient's tibia (IBM–BMT) to facilitate the early recovery of haematopoiesis and donor cell engraftment [22]. In brief, the region from the thigh to the knee joint was shaved of hair with a razor. The knee was flexed to 90°, and the proximal side of the tibia was drawn to the anterior. A 21-gauge needle was inserted into the joint surface of the tibia through the patellar tendon and then inserted into the bone marrow cavity. Using a microsyringe (50 μl; Hamilton Co., Reno, NV, USA), the donor BMCs (3 × 107/30 μl) were injected into the bone marrow cavity. These WBN/Kob rats are abbreviated as (F344→WBN/Kob).
Surface marker analyses
The spleen cells, peripheral blood mononuclear cells or BMCs were prepared from recipient rats, and the cells were then stained with FITC-anti-RT1Al monoclonal antibodies (mAb) (PharMingen, San Diego, CA, USA) to identify the donor-derived cells. Donor-derived cells bearing a lineage-specific phenotype were also analysed by FITC-anti-RT1Al mAb plus PE-conjugated mAb against CD45R (B220) (PharMingen), CD4, CD8 or CD11b (Caltag Laboratories). Furthermore, the cells were stained with anti-CD4 and anti-CD25 mAb (BD Pharmingen, Hamburg, Germany) or anti-CD4 and anti-Forkhead box P3 (FoxP3) mAb to detect regulatory T cells (Tregs). In the case of staining with anti-FoxP3 mAb, cells were stained with FITC-anti-CD4 mAb, and then fixed and permeabilized with Cutofix/Cytoperm solutionTM (BD Pharmingen). The cells thus treated were stained intracytoplasmically with PE-anti-FoxP3 mAb (eBioscience, Inc., San Diego, CA, USA). The stained cells were analysed by a fluorescence activated cell sorter (FACScan®; Becton Dickinson, Mountain View, CA, USA).
Statistical analysis
Survival data were analysed using the Kaplan–Meier method using StatMate software. Differences between groups were analysed using the log-rank test in the StatMate software. P < 0·05 was considered to be significant.
Results
Histopathological and immunological features of WBN/Kob rats
After 4 weeks of age male WBN/Kob rats showed chronic pancreatitis, while after 4 weeks of age female WBN/Kob rats showed Sjøgren-like dacryoadenitis. Histopathologically, significant oedema in the interstitium, the infiltration of lymphocytes and the destruction of acinar cells were seen in the pancreas of male WBN/Kob rats (Fig. 1a and b). At approximately 8 weeks of age fibrosis appeared in the lobules, and after 12 weeks of age the infiltration of inflammatory cells, oedema, haemorrhage and the deposit of haemosiderin were found in the interlobules or peri-pancreatic ducts, leading to the isolation of acini and pancreatic islets, followed by acceleration of fibrosis (Fig. 1c and d). On the other hand, in female WBN/Kob rats, the infiltration of inflammatory cells was found in the outer lachrymal glands after the age of 4 weeks, and the inflammation was aggravated dually. Significant infiltration of lymphocytes was noted in the periductal area, and the acceleration of fibrosis was found in the interlobules (Fig. 1g and h), although the lobular structure in the outer lachrymal glands was still maintained (Fig. 1e and f). Furthermore, many vacuoles and the degeneration of nucleus structure were detected in acinar cells. Thus, chronic dacryoadenitis (and partially parotiditis) resembling Sjøgren's syndrome was found in female WBN/Kob rats.
Fig. 1.
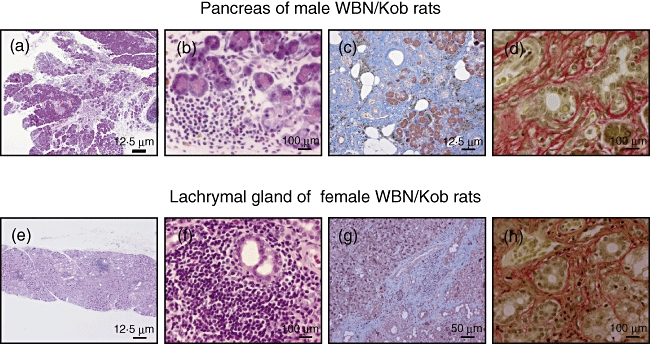
Pathological findings of pancreas and lachrymal grand of male and female Wistar Bonn/Kobori (WBN/Kob) rats. Paraffin sections of pancreas and lachrymal gland were prepared and stained with haematoxylin and eosin (H&E) (6 months of age). (a,b,e,f) Stained with H&E, (c) and (g); stained with Masson-Trichrome, (d) and (h); stained with Sirius red. Bars in the figure represent scales.
In aged male and female WBN/Kob rats (>18 months of age), sclerotic cholangitis, thyroiditis and even tubulointerstitial nephritis were observed along with pancreatitis and dacryoadenitis. The infiltration of inflammatory cells was found in the area of peripheral bile ducts, and hyperplasia of bile ducts and the fibrosis of peri-bile duct areas were also observed (Fig. 2a and b), resembling sclerotic cholangitis in humans. Colloids in the thyroid glands were found to be degenerated and partially destroyed. Infiltration of the inflammatory cells was detected in hyperplastic and fibrous interstitium (Fig. 2c and d). Furthermore, in male WBN/Kob rats, the hyperplasia of the mesangial cells and the demilune bodies in the uriniferous tubule were found clearly as evidence of diabetic nephritis associated with tubulointerstitial nephritis. Infiltration of the inflammatory cells was also detected in the interstitium as tubulointerstitial nephritis (Fig. 2e and f). On the other hand, infiltration of the inflammatory cells was detected only in the interstitium in female WBN/Kob rats where pancreatitis was not developed (Fig. 2g and h). Thus, it is noted that there are clear sex differences in pathological findings.
Fig. 2.
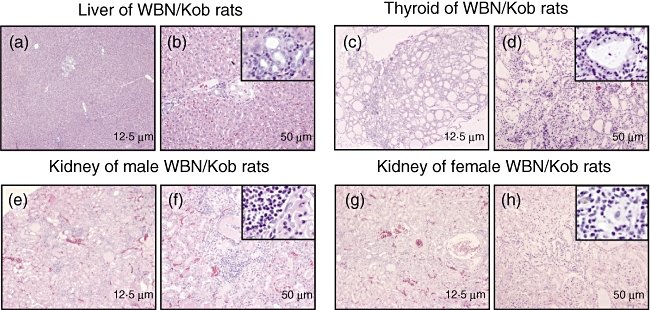
Pathological findings of liver, thyroid and kidney in the aged Wistar Bonn/Kobori (WBN/Kob) rats. Twenty months after birth, liver, thyroid and kidney were removed and paraffin sections were stained with haematoxylin and eosin. (a, b) Liver; (c,d) thyroid; (e,f) kidney of male WBN/Kob rat; (g,h) kidney of female WBN/Kob rat.
Immunohistochemical analyses revealed that a large number of CD8+ T cells were observed in the injured organs (Fig. 3b pancreas; Fig. 3f lachrymal gland), while a small number of CD4+ T cells were infiltrated into the pancreas (Fig. 3a) and lachrymal gland (Fig. 3e). The expression of both MHC class II (Fig. 3c pancreas; Fig. 3g lachrymal gland) and MHC class I (Fig. 3d pancreas; Fig. 3h lachrymal gland) was also found, although the former was less than the latter.
Fig. 3.
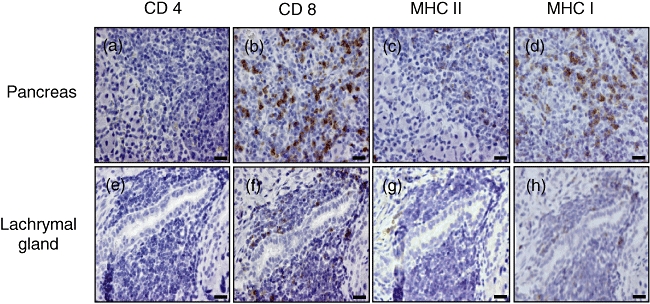
Immunohistological staining for CD4+, CD8+, major histocompatibility complex (MHC) I and anti-MHC II T cells. Pancreas and lachrymal gland were removed (4 months of age) and serial sections were stained with anti-CD4, anti-CD8 anti-MHC I and anti-MHC II monoclonal antibody. (a–d) Pancreas of male Wistar Bonn/Kobori (WBN/Kob) rat; (e–h) pancreas of female WBN/Kob rat.
Serum γ-globulin levels increased in both male and female WBN/Kob rats (Fig. 4), and the deposits of γ-globulin were also observed in the pancreas of male WBN/Kob rats and in the lachrymal gland of female rats. IgG, which was detected specifically in the injured region, was IgG2b subclass, but not any other subclass (Fig. 5) (IgG2c appeared with non-specific staining), and IgG2b was actually detected in the B cells which appeared in the injured region (Fig. 6i and j pancreas; Fig. 6l and m lachrymal gland) along with CD8+ T cells (Fig. 6c pancreas; Fig. 6h lachrymal gland). These findings indicate clearly that pancreatitis in male WBN/Kob rats and dacryoadenitis in female WBN/Kob rats develop as a result of immunological disorders, and can be categorized as AIP and autoimmune dacryoadenitis.
Fig. 4.
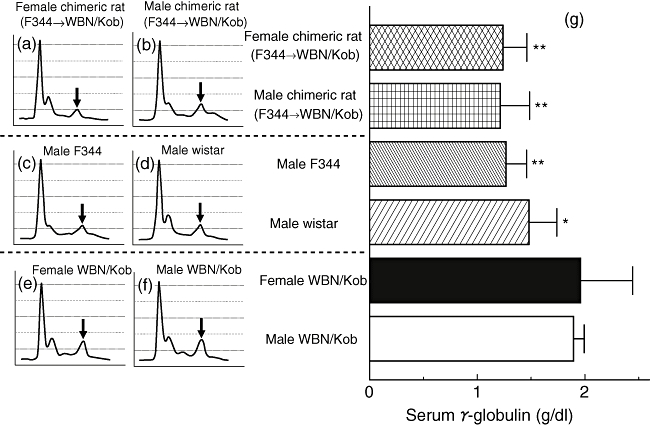
Analyses of serum γ-globulin. Sera were collected from male and female Wistar Bonn/Kobori (WBN/Kob) rat (4 months of age) and electrophoresis was carried out to examine the relative level of IgG. (a) Female WBN/Kob rat treated with intrabone marrow–bone marrow transplantation (IBM–BMT) from F344 (F344→Kob); (b) male WBN/Kob rat treated with IBM–BMT from F344 (F344→WBN/Kob); (c) male F344 rat as a normal control; (d) male Wistar rat as a normal control; (e) untreated female WBN/Kob rat; (f) untreated male WBN/Kob rat; and (g) amounts of γ-globulin in sera calculated from the results of electrophoresis and total amounts of serum protein. Columns and bars represent means ± standard deviations of seven rats. *P < 0·05; **<0·01.
Fig. 5.
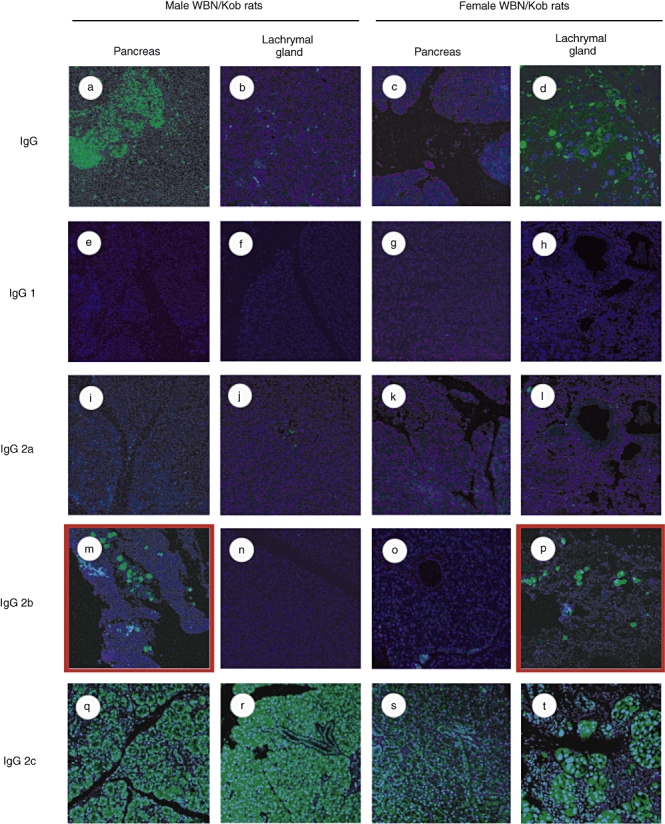
Detection of tissue-specific antibody. Paraffin sections of pancreas and lachrymal gland were prepared from male and female Wistar Bonn/Kobori (WBN/Kob) rats (4 months of age) and stained with biotinylated anti-IgG (a–d), anti-IgG1 (e–h), anti-IgG2a (i–l), anti-IgG2b (m–p) or IgG2c (q–t). They were visualized by additional staining with fluorescein isothiocyanate–streptavidin.
Fig. 6.
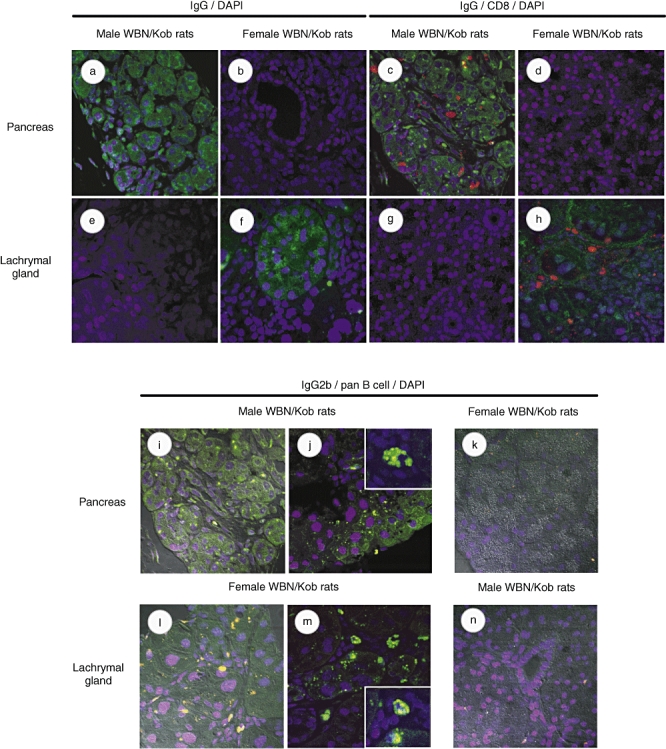
Detection of infiltrated IgG-producing cells and CD8+ T cells. Paraffin sections of pancreas and lachrymal gland were prepared from male and female Wistar Bonn/Kobori (WBN/Kob) rats (4 months of age) and stained with biotinylated anti-IgG monoclonal antibodies (mAb). (a,b) Pancreas; (e,f) lachrymal gland) followed by fluorescein isothiocyanate (FITC)–streptavidin. Nuclei were stained by 4,6-diamidino-2-phenylindole (DAPI). Paraffin sections were double-stained with anti-IgG mAb plus phycoerythrin (PE)-anti-CD8 mAb. (c,d) Pancreas; (g,h) lachrymal gland. Paraffin sections of the pancreas and lachrymal gland were stained with anti-IgG2b followed by FITC–streptavidin, PE-anti-pan B cell mAb and DAPI. (i–k) Pancreas; (l–n) lachrymal gland).
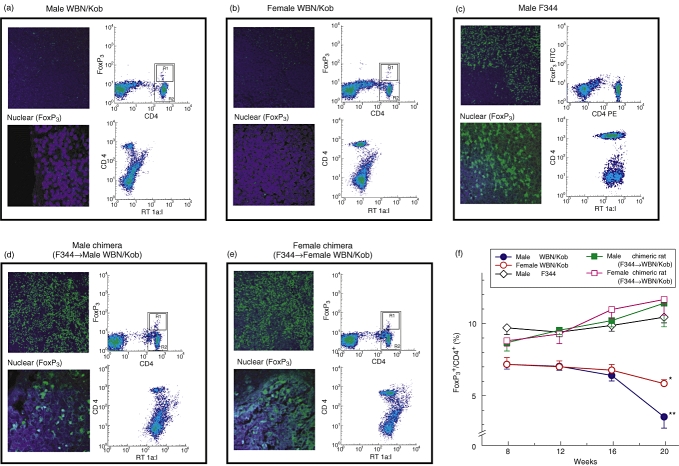
The involvement of immunological disorders in the onset of pancreatitis and dacryoadenitis was confirmed by the measurement of Tregs known to control negatively autoreactive T cells in the periphery. When compared with normal controls (F344), the number of Tregs (detected as CD4+ FoxP3+ cells in the spleen cells) was decreased significantly in WBN/Kob rats (∼7% in WBN/Kob rats at 8 weeks, ∼ 10% in untreated normal F344 rats). The frequency of Tregs decreased gradually to ∼ 4% in male WBN/Kob rats and to ∼ 6% in females at 20 weeks (Fig. 7f), and the representative fluorescence activated cell sorter profiles are shown in Fig. 6a–e.
Fig. 7.
Detection of Forkhead box P3 (FoxP3)+ cells in the spleen. Paraffin sections of the spleen were prepared (4 months of age) and stained with fluorescein isothiocyanate (FITC)-anti-FoxP3 monoclonal antibody (mAb) and 4,6-diamidino-2-phenylindole. Spleen cells were prepared and stained with FITC-anti-CD4 mAb followed by the intracytoplasmic staining with phycoerythrin (PE)-anti-FoxP3 mAb to detect T regulatory cells. The stained cells were analysed by a fluorescence activated cell sorter scan. (a) Male Wistar Bonn/Kobori (WBN/Kob) rat; (b) female WBN/Kob rat; (c) male F344 rat as a normal control; (d) male WBN/Kob rat treated with intrabone marrow–bone marrow transplantation (IBM–BMT) from F344; (e) female WBN/Kob rat treated with IBM–BMT from F344). Spleen cells were also stained with FITC-anti-RT1A1[donor type rat major histocompatibility complex (MHC)] and PE-anti-CD4 mAbs to detect donor-derived cells. (a) Male WBN/Kob rat; (b) female WBN/Kob rat; (c) male F344 rat as a normal control; (d) male WBN/Kob rat treated with IBM–BMT from F344; (e) female WBN/Kob rat treated with IBM–BMT from F344). The frequency of FoxP3+/CD4+ cells was measured kinetically and summarized in Fig. 7F. Symbols and bars represent means ± standard deviations of seven rats. *P < 0·05.
Effects of IBM–BMT on the development of pancreatitis and dacryoadenitis
We have found recently that IBM–BMT can facilitate the engraftment of not only donor-derived haemopoietic cells but also mesenchymal stem cells (bone marrow stromal cells), and thereby prevent or treat various intractable diseases [22,25,26]. We therefore applied this strategy to the prevention of pancreatitis and dacryoadenitis in WBN/Kob rats.
We first examined chimerism in the WBN/Kob rats that had received BMCs of donor F344 rats by IBM–BMT [(F344→WBN/Kob)]. Chimerism was analysed flow cytometrically using FITC-conjugated donor-specific anti-RT1Al mAb. As shown in Fig. 7[compare (a, b, c) with (d,e)], haematolymphoid cells were of donor origin and the donor chimerism remained stable until the experiments were finished (20 weeks of age). The tolerant state was confirmed by mixed lymphocyte reaction. Splenic T cells from (F344→WBN/Kob) rats were used as responders and stimulated with irradiated spleen cells from F344 and unrelated BN rats. T cells from (F344→Kob) rats responded significantly to the unrelated BN spleen cells, but not to the spleen cells of F344 or WBN/Kob rats (data not shown), indicating that the T cells of (F344→WBN/Kob) rats were tolerant of the donor and recipient MHCs, but could respond to the third-party MHC determinants.
After IBM–BMT, the male or female recipients [(F344→WBN/Kob)] did not develop pancreatitis (Fig. 8b and c) or dacryoadenitis (Fig. 8g and h) respectively, showing no hyperglycaemia for more than 16 months (Fig. 9b) after IBM–BMT in the male recipients [(F344→WBN/Kob)], whereas chronic pancreatitis (Fig. 8d and e) or dacryoadenitis (Fig. 8i and j) was observed in the untreated WBN/Kob rats with the destruction of pancreatic islets, and hyperglycaemia was observed at 1 month and thereafter elevated gradually more than 400 mg/dl at 20 weeks of age. Infiltration of CD8+ and CD4+ T cells in the pancreas or lachrymal glands was not detected in the recipients [(F344→WBN/Kob)] (data not shown), and the frequency of Tregs in the periphery and spleen was normalized in both male and female (F344→WBN/Kob) rats and maintained even 20 weeks after IBM–BMT (Fig. 7f).
Fig. 8.
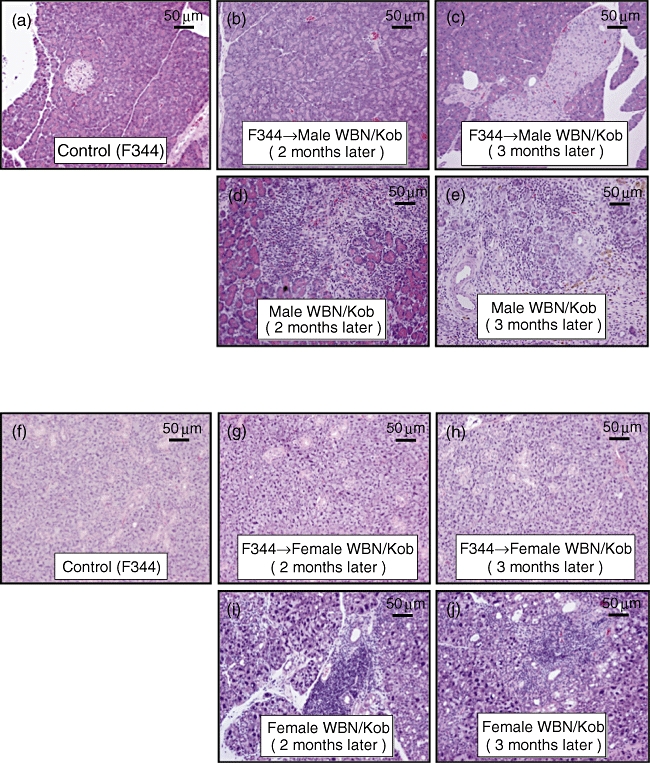
Pathological findings of pancreas and lachrymal gland after intrabone marrow–bone marrow transplantation (IBM–BMT). Male and female Wistar Bonn/Kobori (WBN/Kob) rat treated with IBM–BMT from F344 (F344→WBN/Kob) were examined histologically after staining with haematoxylin and eosin. Paraffin sections of pancreas and lachrymal grand were prepared. (a–e) Pancreas; (f–j) lachrymal gland. (a) Male F344 rat as a normal control; (b) male WBN/Kob rat treated with IBM–BMT from F344 (F344→WBN/Kob, 3 months of age, 2 months after IBM–BMT); (c) male F344→WBN/Kob, 4 months of age, 3 months after IBM–BMT; (d) male WBN/Kob rat 3 months of age; (e) male WBN/Kob rat 4 months of age; (f) female F344 rat as a normal control; (g) female F344→WBN/Kob, 3 months of age, 2 months after IBM–BMT; (h) female F344→WBN/Kob, 4 months of age, 3 months after IBM–BMT; (i) female WBN/Kob rat 3 months of age; (j) female WBN/Kob rat 4 months of age.
Fig. 9.
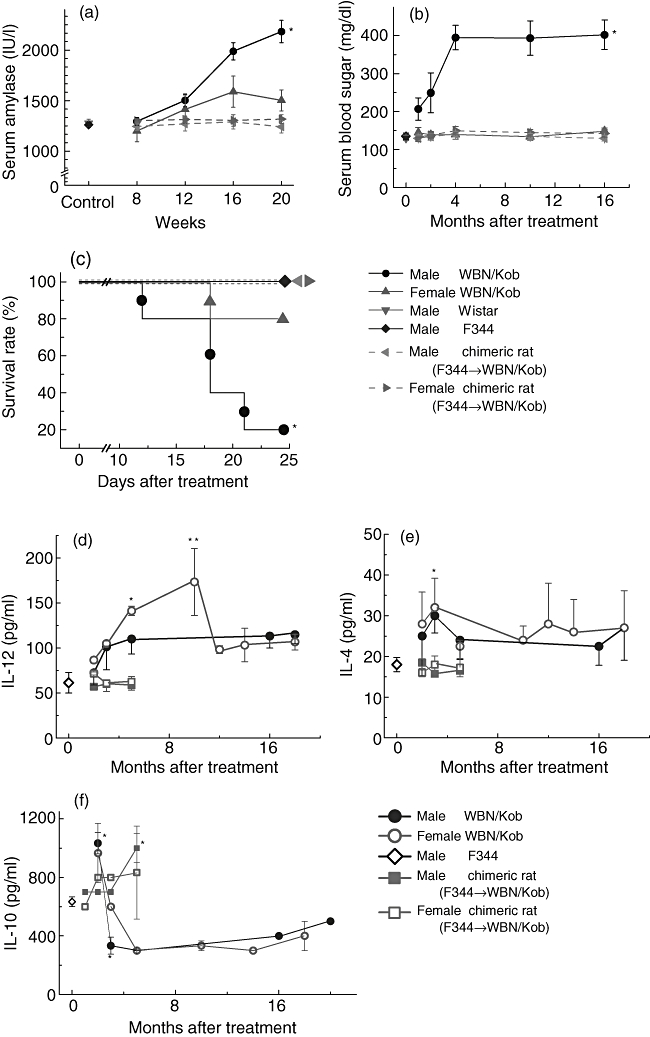
Measurement of serum amylase, glucose, survival rate and cytokine levels. Serum amylase (a), glucose (b), survival rate (c), interleukin (IL)-12 (d), IL-4 (e) and IL-10 (f) were measured kinetically. Symbols and bars represent means ± standard deviations of seven rats. *P < 0·05.
In accordance with the amelioration of pancreatitis or dacryoadenitis, serum amylase (Fig. 9a) and γ-globulin levels (Fig. 4) were normalized, and hyperglycaemia observed in the untreated male WBN/Kob rats was not detected (Fig. 9b).
Measurement of cytokines
To analyse the mechanism underlying the development of AIP and dacryoadenitis in the WBN/Kob rats, we next examined kinetically the cytokine levels of untreated WBN/Kob rats. The production of IL-10 in male and female WBN/Kob rats decreased at 3 months of age and remained unchanged to 20 months (Fig. 9f). In contrast, serum IL-12 levels in WBN/Kob rats increased at the age of 3 months, and the elevated levels of IL-12 were maintained until 20 months (Fig. 9d). The levels of IL-4 were slightly higher in male and female WBN/Kob rats than in the control male F344 rats, but there was statistically no significant difference between them (Fig. 9e).
Discussion
This study was carried out to clarify whether autoimmune mechanisms are involved in the development of pancreatitis in WBN/Kob rats, which allowed us to understand more clearly the pathogenic mechanism of AIP. Recently, AIP has been divided histologically into two distinctive types, LPSP and granulocyte epithelial lesion (GEL). In Japan and Korea, most patients with AIP show the LPSP type associated with systemic exocrinopathy in the aged male. By contrast, in Caucasians, younger patients with the GEL type of AIP associated with ulcerative colitis are often observed without gender difference as well as LPSP. The LPSP type of AIP has been thought to be a multi-focal fibrosclerosing disease with abundant infiltration of IgG4-positive plasmacytes, because extrapancreatic lesions, such as sclerosing cholangitis, sialoadenitis or dacryoadenitis (similar to Mikulicz's disease or Kuttner's tumour), retroperitoneal fibrosis, interstitial nephritis or thyroiditis, are often associated with AIP. It is noted that these lesions can be treated by administration of steroid hormone [27], suggesting that sclerosing cholangitis or sialoadenitis with AIP is different from primary sclerosing cholangitis or typical Sjøgren's syndrome respectively [28].
In the present study, we found that WBN/Kob rats showed different manifestations, depending on gender. In the pancreas of the male rats, many lymphocytes infiltrated around the pancreatic ducts, which seemed to destroy the pancreatic ducts and acinar cells. On the other hand, the lachrymal and salivary glands of the female rats showed massive infiltration of lymphocytes similar to the male pancreas (Fig. 1). Moreover, aged WBN/Kob rats (>18 months) showed thyroiditis, sclerotic cholangitis and even tubulointerstitial nephritis in both genders (Fig. 2). These findings suggest that WBN/Kob rats are an animal model for the LPSP type of AIP, showing extra-pancreatic sclerosing lesions without inflammatory bowel disease, but not the GEL type. Subsequently, analyses of the lymphocyte surface markers (Fig. 3), serum γ-globulin levels (Fig. 4), specific autoantibodies (Fig. 5) and the deposits of immune globulin (Fig. 6) showed that these lesions met the characteristics of autoimmune diseases defined by Witebsky and Mackay [29,30]. In addition to immunohistological findings, the prevention of pancreatitis and dacryoadenitis by the reconstitution of immune tolerance using BMT supports the correspondence as an autoimmune disease model (Fig. 7).
Human AIP involves both humoral immunity (autoantibody) and cell-mediated immunity (cytotoxic lymphocyte). We have reported previously that autoantibodies against CA-II and LF are identified frequently in patients with AIP, and that the prevalence of these two antibodies is independent [31]. Although the stage-dependent immunity in human AIP still remains unclear, T helper type 1/T helper type 2 (Th1/Th2) imbalance in the microenvironment is thought to reflect exacerbation progress. In fact, cytokine balance in the WBN/Kob rats suggested that Th2 cells are involved mainly in early development and Th1 cells are involved in progression, in comparison with the immunity of other AIP models that shifted into Th1 balance only because of artificial alloimmunization [18,19,32]. We also detected the specific autoantibodies of IgG2b type in WBN/Kob rats. Although details of the IgG subclass in rats remain unclear, rat IgG2b, a minor subclass of IgG, is separated in a similar position to human IgG4 by electrophoresis [33]. IgG2b subclass antibodies in the rats reflect the Th1/Th2 immune balance [34]. Up-regulation of the Th1 cytokine (interferon-γ) and down-regulation of IL-4 alter the Th1/Th2 balance to up-regulate IgG2b in the memory response, expand polyclonal activation and also expand the capacity of specific T helper cells. As reported previously, CD4+ T cells react to CA-II or LF, which allows them to escape from negative selection in the thymus and depletion of Tregs such as CD4+ CD25+ T cells in the periphery. Although we identified the specific antibody belonging to the IgG2b subclass in WBN/Kob rats in the present study, the details of other targeting antigens still remain unclear. Further studies on target antigens are necessary.
Recently, great attention has been paid to relations between various autoimmune diseases and Tregs. Tregs control immunological self-tolerance in the periphery [35,36]. The regulatory function is not mediated via CTLA-4 and cannot be blocked by antibodies to IL-4, IL-10 or transforming growth factor-β[6,13]. It has been clarified that their transcriptional factor, FoxP3, is the master gene for differentiation and function of Tregs[36]. In mice, neonatal thymectomy (NTx) induces the escape of T cells from negative selection in the thymus and depletes Tregs in the periphery, which results in the failure of both humoral and cellular immunity. Therefore, the NTx BALB/c mice, which had been immunized with CA-II or lactoferrin, nude mice (in which spleen cells of NTx mice had been transferred) developed immune-mediated pancreatitis and exocrinopathy [37]. Similarly, the WBN/Kob rats showed decreased numbers of peripheral CD4+ CD25+ T cells and FoxP3+ splenic cells as reported in other autoimmune disease patients or animal models [38,39]. Our results showed that reconstitution of immune tolerance using BMT recovered the numbers of peripheral CD4+ CD25+ T cells and FoxP3+ splenic cells, which resulted in the prevention of exocrinopathy in WBN/Kob rats. However, it still remains unclear as to whether or not all autoimmune diseases can originate from the abnormalities of Tregs. In WBN/Kob rats, cytokine alteration indicates that Th2 cells are involved mainly in early development and Th1 cells are involved in later development. Although it still remains unclear as to why organ-specific inflammation in human AIP and WBN/Kob rats depends on gender difference, factors such as sex hormones and genetic diathesis might be involved.
New immune therapies, such as suppressive, supportive and anti-cytokine therapies, can help to maintain and induce the remission of various autoimmune diseases [40]. However, as serious problems such as side effects of immune suppression often occur, these are radical treatments. Recently, haematopoietic stem cell transplantation has shown therapeutic efficacy in various kinds of autoimmune diseases [41]. In the WBN/Kob rats, we succeeded finally in preventing the development of inflammation by the achievement of complete chimerism; direct injection of the donor BMCs into the bone marrow cavity (by IBM–BMT) reduced the donor bone marrow trapping in the lung or liver [42]. The increased size of the CD4+CD25+ population and the up-regulation of FoxP3 after IBM–BMT as a treatment for ongoing pancreatitis and dacryoadenitis provide important evidence for the involvement of Tregs in the process of tolerance observed after induced relapses. The efficacy of IBM–BMT is not only a transient immunosuppression but, as we demonstrated, multiple interconnected mechanisms of immunomodulation. These findings suggest that IBM–BMT therapy may be a promising treatment of AIP in humans, although some complications such as rejection and infection should be carefully monitored. Again, taken together the WBN/Kob rats represent a novel model for the study of AIP with autoimmune extrapancreatic exocrinopathy.
Acknowledgments
Supported by grants from the Haiteku Research Center, the Millennium programme, the Science Frontier programme and the 21st Century Center of Excellence programme of the Ministry of Education, Culture, Sports, Science and Technology; a grant-in-aid for scientific research [(B) 11470062] and grants-in-aid for scientific research on priority areas [(A) 10181225 and (A) 11162221]; research grants from Health and Labor Sciences (Research on Human Genome, Tissue Engineering, Food Biotechnology), Department of Transplantation for Regeneration Therapy (sponsored by Otsuka Pharmaceutical Company Ltd), Molecular Medical Science Institute, Otsuka Pharmaceutical Company Ltd, Japan Immunoresearch Laboratories Co. Ltd, and the Ministry of Culture and Science of Japan (C18590755); and by a health and labour science research grant on intractable diseases of the pancreas from the Japanese Ministry of Health, Labor and Welfare. We also thank Mr Hilary Eastwick-Field, Mr Brian O'Flaherty and Ms K. Ando for their help in the preparation of the manuscript.
References
- 1.Kawaguchi K, Koike M, Tsuruta K, et al. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–95. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 2.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic development. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Tu Y, Egawa N, et al. Involvement of pancreatic and bile ducts in autoimmune pancreatitis. World J Gastroenterol. 2006;12:612–14. doi: 10.3748/wjg.v12.i4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelberg DL, Sahani D, Deshpande V, et al. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–6. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamboni G, Luttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–63. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Kim M, Song M, et al. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–16. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 8.Mufti R, Williamson R. Experimental models of pancreatitis. Ann Acad Med Singapore. 1999;28:133–40. [PubMed] [Google Scholar]
- 9.Sakaguchi Y, Kusafuka K, Inaba M, et al. Establishment of animal models for three types of pancreatitis and analyses of regeneration mechanisms. Pancreas. 2006;33:371–80. doi: 10.1097/01.mpa.0000236734.39241.99. [DOI] [PubMed] [Google Scholar]
- 10.Kanno H, Nose M, Itoh J, et al. Spontaneous development of pancreatitis in the MRL/Mp strain of mice in autoimmune mechanism. Clin Exp Immunol. 1992;89:68–73. doi: 10.1111/j.1365-2249.1992.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallance B, Hewlett B, Snider D, et al. T cell-mediated exocrine pancreatic damage in major histocompatibility complex class II-deficient mice. Gastroenterology. 1998;115:978–87. doi: 10.1016/s0016-5085(98)70270-7. [DOI] [PubMed] [Google Scholar]
- 12.Mori Y, Yokoyama J, Nishimura M, et al. Diabetic strain (WBN/Kob) of rat characterized by endocrine–exocrine pancreatic impairment due to distinct fibrosis. Pancreas. 1990;5:452–9. doi: 10.1097/00006676-199007000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi K, Kim J, Hara H, et al. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol. 1990;6:231–47. [PubMed] [Google Scholar]
- 14.Tago Y, Katsuta O, Tsuchitani M, et al. Glomerular lesions in spontaneously occurring diabetic WBN/Kob rats. J Comp Pathol. 1991;104:367–77. doi: 10.1016/s0021-9975(08)80147-8. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Yamada T, Hashimoto T, et al. Estradiol alleviates acinar cell apoptosis and chronic pancreatitis in male Wistar Bonn/Kobori rats. Pancreas. 2003;26:59–66. doi: 10.1097/00006676-200304000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji A, Nishikawa T, Mori M, et al. Quantitative trait locus analysis for chronic pancreatitis and diabetes mellitus in the WBN/Kob rat. Genomics. 2001;74:365–9. doi: 10.1006/geno.2001.6557. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Yamada T, Yokoi T, et al. Apoptosis of acinar cells is involved in chronic pancreatitis in WBN/Kob rats: role of glucocorticoids. Pancreas. 2000;21:296–304. doi: 10.1097/00006676-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Zipris D. Evidence that Th1 lymphocytes predominate in islet inflammation and thyroiditis in the BioBreeding (BB) rat. J Autoimmun. 1996;9:315–19. doi: 10.1006/jaut.1996.0043. [DOI] [PubMed] [Google Scholar]
- 19.Piccirillo CA, Tritt M, Sgouroudis E, et al. Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann NY Acad Sci. 2005;1051:72–87. doi: 10.1196/annals.1361.048. [DOI] [PubMed] [Google Scholar]
- 20.Ikehara S, Good RA, Nakamura T, et al. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc Natl Acad Sci USA. 1985;82:2483–7. doi: 10.1073/pnas.82.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikehara S. Treatment of autoimmune diseases by hematopoietic stem cell transplantation. Exp Hematol. 2001;29:661–9. doi: 10.1016/s0301-472x(01)00645-2. Review. [DOI] [PubMed] [Google Scholar]
- 22.Kushida T, Inaba M, Hisha H, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–9. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 23.Ikehara S. A novel strategy for allogeneic stem cell transplantation: perfusion method plus intra-bone marrow injection of stem cells. Exp Hematol. 2003;31:1142–6. doi: 10.1016/j.exphem.2003.08.020. Review. [DOI] [PubMed] [Google Scholar]
- 24.Masuya M, Drake C, Fleming P, et al. Hematopoietic origin of glomerular mesangial cells. Blood. 2003;101:2215–18. doi: 10.1182/blood-2002-04-1076. [DOI] [PubMed] [Google Scholar]
- 25.Takada K, Inaba M, Ichioka N, et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells. 2006;24:399–405. doi: 10.1634/stemcells.2005-0068. [DOI] [PubMed] [Google Scholar]
- 26.Adachi Y, Oyaizu H, Taketani S, et al. Treatment and transfer of emphysema by a new bone marrow transplantation method from normal mice to Tsk mice and vice versa. Stem Cells. 2006;24:2071–7. doi: 10.1634/stemcells.2005-0575. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Takahashi H, Ohara M, et al. A new conceptualization for Mikulicz's disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–40. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsubota K, Fujita H, Tsuzaka K, et al. Mikulicz's disease and Sjøgren's syndrome. Invest Ophthalmol Vis Sci. 2000;41:1666–73. [PubMed] [Google Scholar]
- 29.Witebsky E. Concept of autoimmune disease. Ann NY Acad Sci. 1966;26:443–50. doi: 10.1111/j.1749-6632.1966.tb45493.x. [DOI] [PubMed] [Google Scholar]
- 30.Mackay IR. Autoimmune disease. Med J Aust. 1969;29:696–9. doi: 10.5694/j.1326-5377.1969.tb105459.x. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–81. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 32.Davidson T, Longnecker D, Hickey W. An experimental model of autoimmune pancreatitis in the rat. Am J Pathol. 2005;166:729–36. doi: 10.1016/S0002-9440(10)62294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aithal P, Breslin P, Gumustop B, et al. High serum IgG4 concentrations in patients with aclerosing pancreatitis. N Engl J Med. 2001;345:147–8. doi: 10.1056/NEJM200107123450215. [DOI] [PubMed] [Google Scholar]
- 34.Bowman M, Holt G. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect Immun. 2001;69:3719–27. doi: 10.1128/IAI.69.6.3719-3727.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 36.Fontenot D, Gavin A, Rudensky Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K, Okazaki K, Nishi T, et al. Experimental immune-mediated pancreatitis in neonatally thymectomized mice immunized with carbonic anhydrase II and lactoferrin. Lab Invest. 2002;82:411–24. doi: 10.1038/labinvest.3780435. [DOI] [PubMed] [Google Scholar]
- 38.Viglietta V, Baecher-Allan C, Weiner H, et al. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Skurkovich S, Skurkovich B. Anticytokine therapy, especially anti-interferon-gamma, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann N Y Acad Sci. 2005;1051:684–700. doi: 10.1196/annals.1361.113. [DOI] [PubMed] [Google Scholar]
- 41.Tyndall A, Saccardi R. Haematopoietic stem cell transplantation in the treatment of severe autoimmune disease: results from phase I/II studies, prospective randomized trials and future directions. Clin Exp Immunol. 2005;141:1–9. doi: 10.1111/j.1365-2249.2005.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikehara S. Intra-bone marrow-bone marrow transplantation: a new strategy for treatment of stem cell disorders. Ann NY Acad Sci. 2005;1051:626–34. doi: 10.1196/annals.1361.107. [DOI] [PubMed] [Google Scholar]