Abstract
Haematopoietic stem cell transplantation is often complicated by the life-threatening graft-versus-host disease (GVHD) which consists of an allogeneic reaction of the graft cells against the host organs. The aim of this study was to investigate the putative involvement of soluble human leucocyte antigen (sHLA) class I molecules, and particularly sHLA-G molecules, in the occurrence and/or prevention of acute GVHD (aGVHD) in allogeneic peripheral blood stem cell (PSC) transplantation. Whole sHLA class I molecules seem to be involved in aGVHD pathogenesis because detection of a high concentration of these molecules in the first month post allograft is correlated with aGVHD occurrence. Conversely, a high level of sHLA-G molecules before and after allograft could indicate good prognosis in PSC allograft transplantation. sHLA-G molecules seem to be involved in aGVHD prevention, not only because they are enriched in plasma of patients without aGVHD, but also because: (i) a positive correlation has been found between sHLA-G level and CD4+ CD25+ CD152+ natural regulatory T cell (Treg) frequency in the blood of transplanted patients; and (ii) the presence of CD4+ CD25+ CD152+ natural Treg is correlated with increased sHLA-G expression in in vitro mixed leucocyte reaction cultures. Altogether, these results support the immunomodulatory function of sHLA-G molecules that might create a regulatory network together with the natural Treg to foster the induction of a tolerogenic environment and improve PSC transplantation favourable outcome.
Keywords: graft-versus-host disease, immunomodulation, natural regulatory T cell, peripheral blood stem cell transplantation, sHLA-G
Introduction
Allogeneic transplantation of haematopoietic stem cells (HSC) is a curative therapy for several malignancy and non-malignancy haematological disorders, which aims at the reconstitution of a normal haematopoietic system in patients. Sources of HSC are usually bone marrow stem cells [1] or peripheral blood stem cells (PSC) recruited by recombinant human granulocyte–colony-stimulating factor [2]. However, an important life-threatening complication of HSC allograft is acute graft-versus-host disease (aGVHD) that occurs in the 100 days following transplantation. Induction of peripheral tolerance is a crucial event to prevent GVHD. Actors of immunomodulation such as human leucocyte antigen-G (HLA-G) molecules and natural regulatory T cells (Treg) might potentially be involved.
Whole soluble HLA (sHLA) class I molecules have been reported to display immunosuppressive properties, as in the blood transfusion context [3], but studies on immunotolerance have focused upon HLA-G molecules. HLA-G is a non-classical class I molecule with low polymorphism [4]. The alternative splicing of its primary transcript results in at least seven HLA-G isoforms, including three soluble proteins (HLA-G5–HLA-G7) [5]. Alternative soluble forms can also be produced by metalloproteolytic cleavage of membrane-bound isoforms, such as HLA-G1 shedding form (sHLA-G1) [6]. HLA-G was first reported to play a role in semi-allogeneic fetus tolerance [7]. Furthermore, HLA-G expression was also described in the context of allotransplantation [8], and in several models it was correlated with better graft acceptance [9–11]. The tolerogenic role of HLA-G is highly supported by its in vitro properties: (i) inhibition of T CD4+ alloproliferative response [12]; (ii) inhibition of natural killer (NK) [13] and CD8+ T cells [14] cytotoxicity; (iii) apoptosis induction of CD8+ T and NK cells that is a property shared with sHLA class I molecules [15]; (iv) inhibition of antigen-presenting cell function [16]; and (v) induction [17]/definition [18] of immunosuppressive T cells subtype.
Immunosuppressive cells are also involved in peripheral tolerance such as Treg[19]. These cells were defined by the co-expression of CD4 and a high intensity of CD25 (interleukin-2Rα chain), even if other specific markers are now emerging, such as forkhead box P3 [20] or cytotoxic T lymphocyte antigen-4 (CTLA-4) [21]. They are in vitro anergic cells able to suppress proliferation of both CD4 and CD8 T cells in a contact-dependent manner [22].
The aim of the study was to examine the evolution of sHLA class I molecule expression and especially sHLA-G during the follow-up of PSC transplantation. We showed that sHLA class I molecules seem to be involved in aGVHD pathogenesis. On contrast, a high sHLA-G plasma level seems to be representative of a good prognostic factor considering aGVHD prevention. Consistent with those data, a positive correlation in vivo and in vitro was observed between sHLA-G level and CD4+ CD25+ CD152+ natural Treg.
Materials and methods
Patients
A cohort of 20 PSC-transplanted patients was studied between 2004 and 2007 at the Etablissement Français du Sang Bretagne, France. Patients were treated in the department of clinical haematology in Rennes Hospital for lymphoproliferative (n = 12) and myeloproliferative disorders (n = 1) or myelodysplasia (preleukaemia disease) and acute leukaemia (n = 7). Sixteen patients received an attenuated regimen (non-myeloablative treatment) and four, a standard regimen (myeloablative treatment). Among them, eight patients developed aGVHD, only one receiving an HLA-mismatch allograft together with a standard regimen. Ethylenediamine tetraacetic acid-anti-coagulated blood samples were drawn before pre-allograft conditioning regimen administration and at different time-points between PSC transplantation and day 90. Blood samples were not available for all patients at each time. Plasmas were obtained by a 10-min 1000 g centrifugation at room temperature and then cryopreserved at −80°C. Data obtained with plasmas of aGVHD patients after the occurrence of aGVHD have not been taken into account for this study.
Antibodies and fluorescence activated cell sorter analysis
Anti-CD4-fluorescein isothiocyanate, anti-CD25-phycoerythrin and anti-CD152-phycoerythrin-cyanin 5 (PC5) antibodies used for flow cytometry analysis were purchased from BD Pharmingen (Le Pont de Claix, France). Detection of cell surface molecules was performed using conjugated monoclonal antibodies incubated with cells at 4°C for 15 min. Intracellular CD152 staining was performed according to the manufacturer's recommendations (Fixation/Permeabilization Solution Kit; BD Pharmingen).
Isolation of Treg cells
Peripheral blood mononuclear cells (PBMC) were obtained from healthy patients by leucopheresis and cryopreserved at − 196°C. CD4+ CD25+ CD152+ T cells were purified using pre-enrichment with StemSepTM CD4+ T cell enrichment cocktail (StemCell, Grenoble, France) and anti-CD25 magnetic beads (Miltenyi Biotec, Paris, France), followed by cell sorting with BD FacsARIA (Le Pont de Claix, France), as described previously [21]. Cell purity, assessed by flow cytometry using intracellular staining with anti-CD152-PC5 antibody, was more than 90%.
Mixed leucocyte reaction assays
Cell cultures were performed in RPMI-1640 medium (Gibco, Cergy-Pontoise, France) supplemented with 10% pooled human AB serum, 100 U/ml penicillin/streptomycin, 2 mm L-glutamine and 1 mm sodium pyruvate (Gibco). Responder PBMC (5·104 cells/well) were stimulated with 2500 rad-irradiated allogeneic PBMC (5 × 104 cells/well) in the presence or absence of CD4+ CD25+ CD152+ T cells (2·5 × 104 cells/well) at 37°C, 5% CO2. Supernatants were harvested at 48 h. Cell proliferation was assessed at day 7 by incorporation of 1 μCi/well [3H]-thymidine for 18 h and analysed on a beta counter (Packard, Canberra, Australia).
Specific sHLA-G sandwich enzyme-linked immunosorbent assay
Soluble HLA-G concentration (ng/ml) was measured in plasmas or culture supernatants by a specific enzyme-linked immunosorbent assay (ELISA), as described previously [23]. Briefly, MEMG/9 (Exbio, Praha, Czech Republic) and anti-β2-microglobulin horseradish peroxidase (Dako, Trappes, France) were used, respectively, as capture and secondary antibodies. This ELISA was validated by the workshop for quantification of sHLA-G in Essen, 2004 [24] and allows specific estimation of sHLA-G1 and HLA-G5 levels.
Specific soluble human HLA class I sandwich ELISA
Soluble HLA class I molecules (HLA-A, -B, -C, -E, -F and -G) concentration was quantified in plasmas by a specific ELISA using W6/32 (Harlan Sera-Laboratory Ltd, Leicester, UK) as primary antibody, with the same methodology as described for sHLA-G ELISA.
Statistical analysis
GraphPad Prism 4 software was used for all statistics. Data are represented as means ± standard deviation. Comparison of sHLA-G plasma levels between groups of patients was performed by the Mann–Whitney U-test. Variations of sHLA-G concentration between time-points for without (w/o) aGVHD patients and between mixed leucocyte reaction (MLR) culture conditions were analysed using Wilcoxon's matched-pairs rank test. P < 0·05 was considered significant. Correlation analysis was achieved using Spearman's non-parametric test.
Results
Soluble HLA class I in PSC-transplanted patients
Because of their known role in immunotolerance, sHLA class I plasma level was assessed with specific ELISA in the context of PSC allograft. We first examined sHLA class I level in plasmas of PSC-transplanted patients w/o aGVHD. A significant increase of sHLA class I, from 1·4- to 2·1-fold, was observed between days 60 and 90 compared with the first month post allograft (Fig. 1; P < 0·05). We then analysed the potential involvement of sHLA class I in the occurrence of aGVHD. As shown in Fig. 2, a higher level of sHLA class I was observed during the first month post allograft in plasmas of patients who developed aGVHD later, compared with w/o aGVHD patients (2392 ± 226 versus 1493 ± 623 ng/ml; P < 0·01). Therefore, global quantification of sHLA class I implies a potential involvement of these molecules in aGVHD.
Fig. 1.
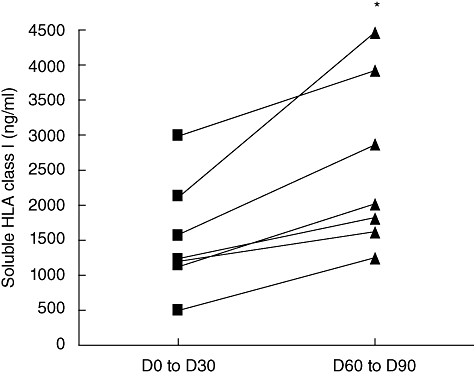
Comparison of soluble human leucocyte antigen (sHLA) class I level in without acute graft-versus-host disease patients (n = 7) between the first month post-allograft (days 0–30) and the third month post transplantation (days 60–90). sHLA class I level is increased significantly in the third month post allograft [Wilcoxon's matched-pairs test: P < 0·05 (*)].
Fig. 2.
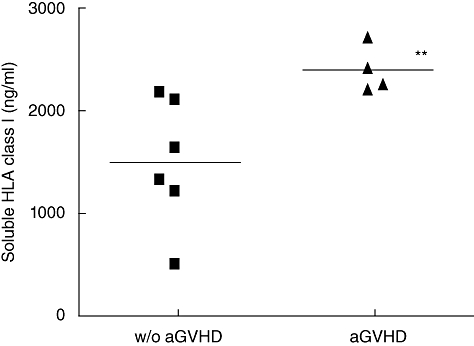
Comparison of soluble human leucocyte antigen (sHLA) class I between without (w/o) acute graft-versus-host disease (aGVHD) (n = 6) and aGVHD patients (n = 4) in the first month post allograft (day 30). sHLA class I level is significantly higher in the aGVHD group compared with the w/o aGVHD one in the first month post allograft [Mann–Whitney U-test: P < 0·01 (**)].
Soluble HLA-G in PSC-transplanted patients
We then focused on sHLA-G molecules, known widely to display important immunosuppressive properties, to determine whether they could be involved in the protection against aGVHD. Therefore, sHLA-G plasma level was assessed using specific ELISA. In the w/o aGVHD group, a significant increase of sHLA-G, from 1·1- to 3·2-fold, was observed between days 60 and 90 compared with the first month post allograft (Fig. 3; P < 0·05). Stratification of patients according to aGVHD occurrence showed asignificantly higher level of sHLA-G before transplantation in plasmas of w/o aGVHD patients (117·6 ± 38·14 ng/ml) compared with aGVHD patients (50·07 ± 31·86 ng/ml) (Fig. 4; P < 0·05). Moreover, when this quantification was conducted in the post-allograft period (Fig. 5), a significantly higher level of sHLA-G molecules was detected in plasmas of w/o aGVHD patients compared with aGVHD patients (65·85 ± 15·66 versus 40·02 ± 11·70 ng/ml; P < 0·01). Unlike whole sHLA class I molecules, sHLA-G molecules could be involved in aGVHD prevention.
Fig. 3.
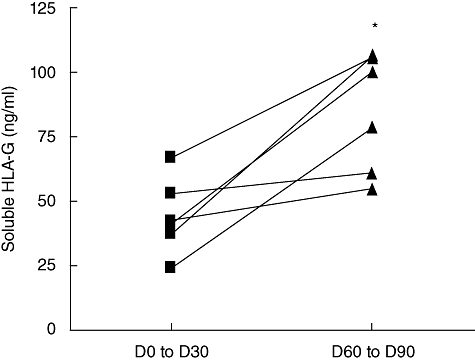
Comparison of soluble human leucocyte antigen-G (sHLA-G) plasma level between the first month (days 0–30) and the third month post allograft (days 60–90) in patients without acute graft-versus-host disease (n = 6). A significant increase of sHLA-G level is found in the third month post allograft [Wilcoxon's matched-pairs test: P < 0·05 (*)].
Fig. 4.
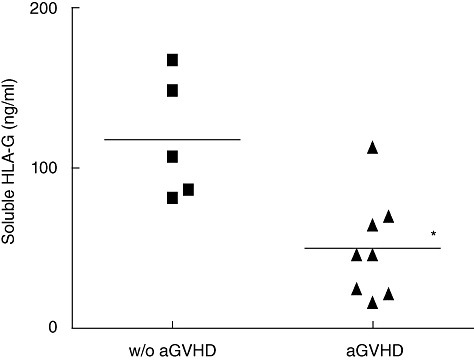
Comparison of soluble human leucocyte antigen-G (sHLA-G) molecule level before transplantation in the plasmas of without acute graft-versus-host disease (aGVHD) patients (n = 5) and aGVHD patients (n = 8). A low pre-allograft sHLA-G plasma level is associated with the occurrence of post-transplantation aGVHD [Mann–Whitney U-test: P < 0·05 (*)].
Fig. 5.
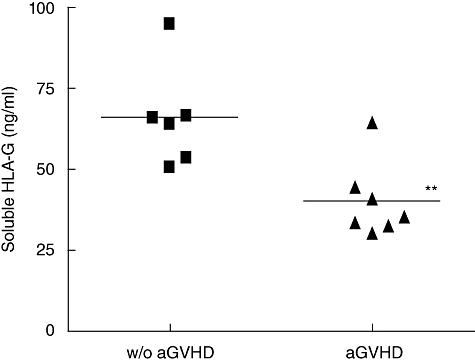
Comparison of soluble human leucocyte antigen-G (sHLA-G) molecule level in the plasmas of without acute graft-versus-host disease (aGVHD) patients (n = 6, day 60) and aGVHD patients (n = 7, patients tested before aGVHD occurrence). A correlation is observed between a low sHLA-G level post graft and the occurrence of aGVHD [Mann–Whitney U-test: P < 0·01 (**)].
A high sHLA-G plasma level is correlated with a high frequency of CD4+ CD25+ CD152+ T cells in allografted patients' blood samples
As we have demonstrated that sHLA-G plasma level is increased in the third month post allograft in patients w/o aGVHD, we wondered which cell type was responsible for sHLA-G secretion. We searched for an association, in day 90 blood samples, between sHLA-G level in plasmas and the percentage of one of the following blood cell subpopulations: CD3+, CD4+ and CD8+ T lymphocytes, CD19+ B lymphocytes, CD14+ monocytes, CD56+ NK cells and CD4+ CD25+ CD152+ Treg cells. Interestingly, a positive correlation between sHLA-G level and the percentage of CD4+ CD25+ CD152+ T cells among the CD3+ lymphocyte population was found at day 90 in w/o aGVHD patients (Fig. 6; P < 0·05). These data suggest the induction of a tolerogenic environment in patients w/o aGVHD, evidenced by high levels of both sHLA-G and CD4+ CD25+ CD152+ T cells in the periphery.
Fig. 6.
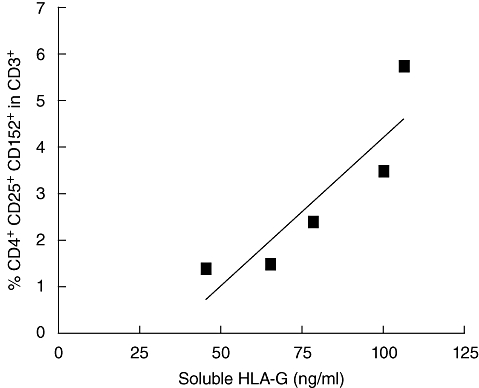
Correlation between soluble human leucocyte antigen-G (sHLA-G) level and percentage of CD4+ CD25+ CD152+ regulatory T cells (Treg) in the CD3+ lymphocyte population at day 90 post allograft in peripheral blood of without acute graft-versus-host disease patients (n = 5). A high level of sHLA-G is associated with a high percentage of CD4+ CD25+ CD152+ Treg cells in CD3+ lymphocyte population (Spearman's test: r = 0·89; P < 0·05).
Soluble HLA-G level is linked to natural Treg cells in MLR assays
In order to investigate whether CD4+ CD25+ CD152+ Treg cells could produce sHLA-G, we set up MLR assays, which are the in vitro equivalent of allogeneic transplantation. CD4+ CD25+ CD152+ population-defining Treg cells were added to MLR at a Treg : responder PBMC ratio of 1:2 (Fig. 7a). The average level of inhibition of alloproliferation by Treg cells was 72 ± 17% (range 45–92%; P < 0·01). In six independent experiments, an increase of sHLA-G level was reported in culture supernatants (Fig. 7b) in the presence of Treg cells in MLR compared with control MLR w/o addition of Treg (19·72 ± 5·52 versus 10·53 ± 4·80 ng/ml; P < 0·05). Therefore, natural Treg cells seem to be involved in sHLA-G secretion in the context of in vitro suppression of allogeneic response.
Fig. 7.
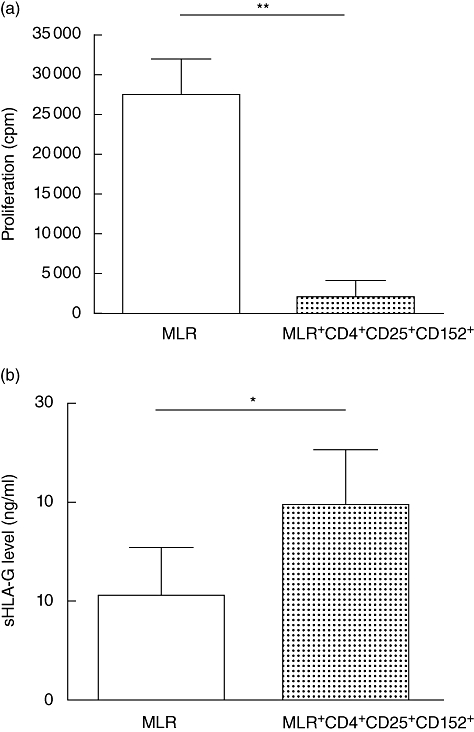
(a) Representative experiment of mixed leucocyte reaction (MLR) performed in the presence or absence of CD4+ CD25+ CD152+ regulatory T cells (Treg). Allogeneic proliferation is inhibited significantly by CD4+ CD25+ CD152+ Treg cells [Wilcoxon's matched-pairs test: P < 0·01(**)]. (b) Comparison of soluble human leucocyte antigen-G (sHLA-G) level in supernatants of MLR performed in the presence or absence of CD4+ CD25+ CD152+ Treg cells (n = 6 for each condition). sHLA-G level is significantly higher in the supernatants of MLR in the presence of Treg compared with MLR without Treg[Wilcoxon's match-pairs test: P < 0·05(*)].
Discussion
In the present study, we analysed the potential involvement of sHLA class I molecules including sHLA-G molecules in the occurrence of aGVHD in the context of PSC transplantation. In the first month after allotransplantation, a high sHLA class I level was found associated with aGVHD occurrence. This result is consistent with Puppo et al.'s study [25] showing the same correlation between a high sHLA class I level and aGVHD occurrence in bone marrow allotransplantation. This higher level of sHLA class I could be due to the release of sHLA class I molecules by damaged cells during aGVHD. Furthermore, interferon-γ, a cytokine highly involved in aGVHD pathogenesis [26], is known to increase sHLA class I release [27]. sHLA class I level is increased with time in w/o aGVHD patients and could be associated with protection against aGVHD at a later phase of the PSC allograft period. However, 43% of those w/o aGVHD patients developed a chronic GVHD later (data not shown). Liem et al. have already reported an increase of sHLA class I levels in bone marrow transplanted patients correlated with episodes of both acute and chronic GVHD [28].
Soluble HLA-G molecules are also involved in immune suppression and play a role in allograft acceptance. Indeed, rejection was associated with a low HLA-G expression in endomyocard biopsies and sera of heart-transplanted patients [10]. Recently, a correlation between low-level sHLA-G and rejection of kidney-allograft was also reported [11]. Here, a low sHLA-G (sHLA-G1 and HLA-G5) level before grafting could be representative of a poor prognostic factor concerning aGVHD in PSC allotransplantation. This result is strengthened by the fact that sHLA-G level is generally increased significantly in the plasmas of patients suffering from lymphoproliferative disorders compared with healthy subjects [23]. Also, in our study aGVHD and w/o aGVHD groups are similar in terms of patients cured for those pathologies (37·5% versus 40% of patients). GVHD incidence is also known to be associated with immunosuppressive treatments and HLA-mismatch. In the present study, those parameters were comparable in both groups of patients. Concerning post-allograft study, a high sHLA-G level seems to be a good marker of aGVHD prevention. As sHLA-G plasma level is increased significantly with time in w/o aGVHD patients, aGVHD might occur in patients that do not increase their sHLA-G plasma level over the time following transplantation. This indicates contradictory mechanisms mediated by sHLA class I and sHLAG molecules. The role of sHLA-G in preventing aGVHD is supported by several immunomodulatory capacities, and especially inhibition of dendritic cells (DC) [16], which play a key role in aGVHD occurrence [26]. Moreover, a recent report demonstrated the functional role of sHLA-G molecules from sera of liver-transplanted patients in the induction of Treg cells [29], and another study underlines this link between HLA-G, DC, Treg cells and allograft survival in a transgenic murine model [30]. Indeed, HLA-G1, via its ligation to immunoglobulin-like transcript 4 receptor, induces a tolerogenic phenotype in myeloid DC which results in the induction of Treg cells.
Therefore, we wondered whether sHLA-G increase in w/o aGVHD patients is associated with a particular cell subpopulation. We used CTLA-4 molecule (CD152) as a third co-marker of natural CD4+ CD25+ Treg cells, as we demonstrated higher immunosuppressive properties in CTLA-4+ Treg cells [21]. In the patients studied, sHLA-G plasma level was correlated positively with CD4+ CD25+ CD152+ T cell frequency in the w/o aGVHD group. A negative association has already been reported in humans between the number of Treg and the occurrence of aGVHD [31]. According to the link between Treg cells and sHLA-G expression ex vivo, a significantly higher level of sHLA-G was observed in culture supernatants when CD4+ CD25+ CD152+ Treg cells were added to MLR in vitro. It has already been reported that sHLA-G molecules, produced by CD4+ T cells in MLR assays, are able to suppress alloproliferation [32]. As no increase of sHLA-G level was observed in the supernatants of polyclonal activated Treg cells compared with other immune cell types (data not shown), sHLA-G expression might be dependent on allogeneic stimulation microenvironment and/or other cell types might be induced to produce sHLA-G molecules [33,34]. However, it remains to understand the mechanism by which sHLA-G expression could be induced in the presence of CD4+ CD25+ CD152+ Treg cells. A hypothesis might be an induction of sHLA-G expression by DC via action of indolamine-2,3-deoxygenase (IDO) [35], which catalyses tryptophan degradation in metabolites such as kynurenine. IDO is itself induced by interaction of CTLA-4 (CD152) with B-7 receptor interaction at the DC surface [36].
In conclusion, sHLA class I molecules seem to be involved in aGVHD pathogenesis, whereas sHLA-G molecules are involved specifically in aGVHD prevention. The potential link between sHLA-G molecules and natural Treg cells provides keys to the comprehension of aGVHD mechanism and opens up new monitoring strategies of PSC-transplanted patients.
Acknowledgments
A. L. M. is recipient of a fellowship of the Ministère de l'Education (France). This work was supported by the EFS and by grants from the Ligue contre le Cancer Ille et Vilaine. Thanks to C. Eyi Mba and D. Ollivier for their help in ELISA assays. Special thanks are due to Y. Sebti and J. Le Priol for their help in manuscript correction, to M. Alizadeh (EFS Bretagne) and E. Genin (Inserm U535) for their help in statistical analysis.
References
- 1.Thomas ED, Storb R. Technique for human marrow grafting. Blood. 1970;36:507–15. [PubMed] [Google Scholar]
- 2.Lane TA, Ho AD, Bashey A, Peterson S, Young D, Law P. Mobilization of blood-derived stem and progenitor cells in normal subjects by granulocyte–macrophage- and granulocyte–colony-stimulating factors. Transfusion. 1999;39:39–47. doi: 10.1046/j.1537-2995.1999.39199116893.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghio M, Contini P, Mazzei C, et al. In vitro immunosuppressive activity of soluble HLA class I and Fas ligand molecules: do they play a role in autologous blood transfusion? Transfusion. 2001;41:988–96. doi: 10.1046/j.1537-2995.2001.41080988.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirszenbaum M, Djoulah S, Hors J, et al. HLA-G gene polymorphism segregation within CEPH reference families. Hum Immunol. 1997;53:140–7. doi: 10.1016/S0198-8859(97)00038-4. [DOI] [PubMed] [Google Scholar]
- 5.Paul P, Cabestre FA, Ibrahim EC, et al. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5-G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000;61:1138–49. doi: 10.1016/s0198-8859(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y, Lieskovska J, Kedrin D, Porcelli S, Mandelboim O, Bushkin Y. Soluble nonclassical HLA generated by the metalloproteinase pathway. Hum Immunol. 2003;64:802–10. doi: 10.1016/s0198-8859(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 7.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 8.LeMaoult J, Rouas-Freiss N, Carosella ED. Immuno-tolerogenic functions of HLA-G: relevance in transplantation and oncology. Autoimmun Rev. 2005;4:503–9. doi: 10.1016/j.autrev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Creput C, Le Friec G, Bahri R, et al. Detection of HLA-G in serum and graft biopsy associated with fewer acute rejections following combined liver–kidney transplantation: possible implications for monitoring patients. Hum Immunol. 2003;64:1033–8. doi: 10.1016/j.humimm.2003.08.356. [DOI] [PubMed] [Google Scholar]
- 10.Lila N, Amrein C, Guillemain R, et al. Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation. 2002;105:1949–54. doi: 10.1161/01.cir.0000015075.89984.46. [DOI] [PubMed] [Google Scholar]
- 11.Qiu J, Terasaki PI, Miller J, Mizutani K, Cai J, Carosella ED. Soluble HLA-G expression and renal graft acceptance. Am J Transplant. 2006;6:2152–6. doi: 10.1111/j.1600-6143.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 12.Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48:17–26. doi: 10.1016/s0165-0378(00)00070-x. [DOI] [PubMed] [Google Scholar]
- 13.Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED. The alpha1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc Natl Acad Sci USA. 1997;94:5249–54. doi: 10.1073/pnas.94.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiendl H, Mitsdoerffer M, Hofmeister V, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–80. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 15.Contini P, Ghio M, Poggi A, et al. Soluble HLA-A, -B, -C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33:125–34. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 16.Liang S, Baibakov B, Horuzsko A. HLA-G inhibits the functions of murine dendritic cells via the PIR-B immune inhibitory receptor. Eur J Immunol. 2002;32:2418–26. doi: 10.1002/1521-4141(200209)32:9<2418::AID-IMMU2418>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA. 2004;101:7064–9. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feger U, Tolosa E, Huang YH, et al. HLA-G expression defines a novel regulatory T cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568–77. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 20.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 21.Birebent B, Lorho R, Lechartier H, et al. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–96. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 22.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+) CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebti Y, Le Friec G, Pangault C, et al. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Hum Immunol. 2003;64:1093–101. doi: 10.1016/j.humimm.2003.08.345. [DOI] [PubMed] [Google Scholar]
- 24.Rebmann V, Lemaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Report of the Wet Workshop for Quantification of Soluble HLA-G in Essen, 2004. Hum Immunol. 2005;66:853–63. doi: 10.1016/j.humimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Puppo F, Brenci S, Ghio M, et al. Serum HLA class I antigen levels in allogeneic bone marrow transplantation: a possible marker of acute GVHD. Bone Marrow Transplant. 1996;17:753–8. [PubMed] [Google Scholar]
- 26.Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–94. doi: 10.1016/s0268-960x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 27.Haynes LD, Bushkin Y, Love RB, Burlingham WJ. Interferon-gamma drives the metalloproteinase-dependent cleavage of HLA class I soluble forms from primary human bronchial epithelial cells. Hum Immunol. 2002;63:893–901. doi: 10.1016/s0198-8859(02)00461-5. [DOI] [PubMed] [Google Scholar]
- 28.Liem LM, Koelman CA, Doxiadis II, van Houwelingen JC, Goulmy E, Claas FH. Elevated serum HLA class I levels coincide with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 1997;20:227–34. doi: 10.1038/sj.bmt.1700877. [DOI] [PubMed] [Google Scholar]
- 29.Le Rond S, Azema C, Krawice-Radanne I, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol. 2006;176:3266–76. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- 30.Ristich V, Zhang W, Liang S, Horuzsko A. Mechanisms of prolongation of allograft survival by HLA-G/ILT4-modified dendritic cells. Hum Immunol. 2007;68:264–71. doi: 10.1016/j.humimm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–7. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci USA. 2001;98:12150–5. doi: 10.1073/pnas.201407398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Rond S, Le Maoult J, Creput C, et al. Alloreactive CD4+ and CD8+ T cells express the immunotolerant HLA-G molecule in mixed lymphocyte reactions: in vivo implications in transplanted patients. Eur J Immunol. 2004;34:649–60. doi: 10.1002/eji.200324266. [DOI] [PubMed] [Google Scholar]
- 34.Rebmann V, Busemann A, Lindemann M, Grosse-Wilde H. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64:1017–24. doi: 10.1016/j.humimm.2003.08.354. [DOI] [PubMed] [Google Scholar]
- 35.Lopez AS, Alegre E, LeMaoult J, Carosella E, Gonzalez A. Regulatory role of tryptophan degradation pathway in HLA-G expression by human monocyte-derived dendritic cells. Mol Immunol. 2006;43:2151–60. doi: 10.1016/j.molimm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–8. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]


