Abstract
Secretory immunoglobulin A (SIgA), although generated at mucosal surfaces, is also found in low concentrations in the circulation. Recently, SIgA was demonstrated in mesangial deposits of patients with immunoglobulin A nephropathy (IgAN), suggesting a role in the pathogenesis. This finding is in line with the belief that high molecular weight (HMW) immunoglobulin A (IgA) is deposited in the kidney. However, there is little information on the size distribution of antigen-specific IgA in circulation upon mucosal challenge. In this study we measured antigen-specific IgA, including SIgA, in serum following challenge of IgAN patients and controls via intranasal vaccination with a neoantigen, cholera toxin subunit B (CTB). We size-fractionated serum and nasal washes to study the size distribution of total IgA, SIgA and CTB-specific IgA. Finally, we compared the size distribution of antigen-specific IgA after mucosal immunization with the distribution upon systemic immunization. A significant induction of antigen-specific SIgA was detectable in serum of both patients with IgAN and controls after mucosal immunization with CTB. Independent of the route of immunization, in both groups the antigen-specific IgA response was predominantly in the polymeric IgA fractions. This is in contrast to total IgA levels in serum that are predominantly monomeric. We conclude that mucosal challenge results in antigen-specific SIgA in the circulation, and that the antigen-specific IgA response in both IgAN patients and in controls is of predominantly HMW in nature. No differences between IgAN patients and controls were detected, suggesting that the size distribution of antigen-specific IgA in the circulation is not disturbed specifically in IgAN patients.
Keywords: antigen-specific SIgA, IgA nephropathy, immunization, secretory IgA, size distribution
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide, leading to renal insufficiency in 30–40% of patients [1–3]. The disease is characterized by deposits of polymeric immunoglobulin A1 (IgA1) in the mesangium of glomeruli [4,5]. The pathogenesis of IgAN is still not clear. Plasma IgA levels are elevated in about 50% of IgAN patients, but how this higher concentration of plasma IgA contributes to mesangial deposition remains unclear [4,6]. Qualitative changes of plasma IgA1, such as the glycosylation pattern, are probably involved in the pathogenesis of IgAN [7,8]. IgA isolated from IgAN patients shows more terminal N-acetyl galactosamine (Tn antigen), which could contribute to deposition of IgA in the mesangium. Interestingly, after renal transplantation IgA depositions may appear in the renal allograft suggesting, next to renal factors, a pathogenic role for serum IgA [9].
The mesangial IgA deposition in IgAN consists of IgA1, together with complement factor C3 and sometimes IgG. Most of the deposited IgA consists of high molecular weight (HMW) IgA [10–12]. The composition of HMW IgA is diverse and may contain dimeric IgA, secretory IgA (SIgA) and complexes of IgA and the Fcα-receptor (CD89) and fibronectin [2,13]. Recently we showed that in approximately 25% of cases, mannose binding lectin (MBL) is present in renal biopsies of IgAN patients. The presence of MBL was associated with more histological damage and more proteinuria [14]. In about 15% of IgAN cases mesangial SIgA is present [15,16]. The presence of SIgA in renal biopsies showed a strong correlation with the presence of MBL and the complement degradation fragment C4d. Moreover, a strong co-localization of SIgA, MBL and C4d was observed [15]. A potential pathogenic role of SIgA is supported further by higher serum levels of SIgA in IgAN patients compared with controls and the observation that higher levels of SIgA are correlated with more haematuria [12].
Several immunization studies have shown different results with regard to the specific serum and mucosal IgA responses in IgAN patients. Both hypo and hyper IgA responses have been described, dependent on the type of antigen and the route of administration of the antigen [17–22]. How these differences in immune responses are related to the pathogenesis of IgAN is unknown. No data are available about antigen-specific SIgA in circulation after mucosal immunization. In addition, very little is known about the size distribution of IgA during primary immune responses in IgAN patients.
Therefore, we have investigated the presence of antigen-specific SIgA in the circulation after mucosal immunization. In addition we size-fractionated sera and nasal washes collected from an earlier vaccination study in IgAN patients and controls, who underwent nasal mucosal and subcutaneous immunization with two different neoantigens. In the fractions we determined, in addition to total IgA and SIgA, the titres of antigen-specific IgA. As every individual was immunized with two different neoantigens via two different routes we were able to study whether the route of vaccination influences the size distribution of antigen-specific IgA. We conclude that after mucosal immunization antigen-specific SIgA appears in the circulation in both the patient and control group, and that antigen-specific IgA in serum is predominantly polymeric in both patients and controls, independent of the route of immunization. The amount of antigen-specific SIgA in IgAN patients and in controls is similar, suggesting that additional factors are involved in the pathogenesis of IgAN.
Subjects and methods
Human subjects and immunization protocol
In an earlier vaccination study we investigated the primary immune response after simultaneous mucosal and systemic vaccination of IgAN patients and controls in a quantitative manner [17]. Participants were immunized with 0·33 mg of cholera toxin subunit B (CTB) intranasally by spray, and 250 μg of keyhole limpet haemocyanin (KLH) subcutaneously, repeated by two administrations of identical doses after 2 and 4 weeks, as described earlier [17]. None of the patients had clinical or laboratory evidence of Henoch–Schoenlein purpura, kidney function was normal or mildly impaired (creatinine clearance >80 ml/min) and none of the patients used corticosteroids or any other immunosuppressive drug at the time of the study or at least 3 months before. Mean age of the IgAN patients was 40 years (range 30–47 years). Healthy volunteers were recruited as controls with a mean age of 29 years (range 24–37 years). The study was approved by the Ethical Committee of the Leiden University Medical Centre. All individuals gave informed consent.
From 20 controls and 11 IgAN patients, pre- and post-immunization samples, taken 2 weeks after the second booster immunization, i.e. 42 days from the start of the immunization protocol, were available for the assessment of total SIgA and antigen-specific SIgA. From six IgAN patients, all men with biopsy proven IgAN and six controls (five men) sufficient amounts of material were present to perform a size separation of serum.
Quantification of IgA and antigen-specific IgA
Total IgA, SIgA and antigen-specific (S)IgA levels were determined by specific sandwich enzyme-linked immunosorbent assay (ELISA). Polystyrene 96-well plates (Greiner, Alphen a/d Rijn, the Netherlands) were coated with 100 μl/well of the capturing antibody, diluted appropriately in phosphate-buffered saline (PBS). Total IgA was detected by heavy chain-specific, affinity purified goat F(ab')2 fragments against IgA (Jackson, West Grove, PA, USA). SIgA was detected using a monoclonal antibody specific for secretory component (NI194-4; 3F8) as capturing antibody in a concentration of 2 μg/ml.
In the antigen-specific ELISA, plates were coated with 100 μl of CTB (2·5 μg/ml) (Sigma, St Louis, MO, USA) or KLH (10 μg/ml) (Calbiochem, La Jolla, CA, USA). Subsequently the plates were washed with PBS/0·05% Tween (PBST). Plates were incubated with appropriate dilutions of samples from IgAN patients and controls in PBS/1% bovine serum albumin/0·05% Tween for 2 h. Bound IgA was detected using mouse anti-human IgA (4E8) conjugated to biotin, followed by incubation with streptavidin conjugated to horseradish peroxidase (Zymed, Sanbio BV, Uden, the Netherlands). CTB-specific SIgA was detected by polyclonal sheep anti-human secretory component (5 μg/ml) (Nordic, Tilburg, the Netherlands), followed by rabbit anti-sheep conjugated to horseradish peroxidase (10 μg/ml) (Nordic). Enzyme activity of horseradish peroxidase was developed using 3-ethylbenzthiazoline-6-sulphonic acid (Sigma). Between each step the wells were washed three times with PBST.
Size fractionation of IgA
Serum and nasal washes, containing antigen-specific IgA, were size-separated with a HiLoadTM 16/60 HR200 Superdex prep grade gel filtration column (120 ml; Amersham Pharmacia, Roosendaal, the Netherlands), run in Veronal-buffered saline containing 2 mM ethylenediamine tetraacetic acid. Fractions were assessed for the presence of IgA, SIgA, antigen-specific IgA and total protein.
Percentages of mIgA and polymeric IgA (pIgA) in serum for each individual were determined as follows: fractions containing pIgA (44–51 ml) or mIgA (52–60 ml) were pooled and assessed for total IgA and for antigen-specificity towards CTB and KLH. The percentages of pIgA and mIgA were calculated by dividing the amount of pIgA by the sum of pIgA and mIgA together.
Statistical analysis
Statistical analysis was performed by Student's t-test. Differences were considered significant when P-values were less than 0·05.
Results
Antigen-specific SIgA in serum
Using specific ELISA we tested pre- and post-immunization serum fractions of controls and IgAN patients for the presence of total SIgA. The mean ± standard error of the mean of total SIgA concentrations in serum of the control group was 18 ± 5·6 μg/ml before and 22·0 ± 6·2 μg/ml after immunization [not significant (n.s.)] (Fig. 1a). In the IgAN group preimmunization SIgA concentration was 23·3 ± 8·6 μg/ml and post-immunization was 20·7 ± 6·2 μg/ml (n.s.). Comparison of the SIgA concentration of the controls and patients revealed no significant difference, neither before nor after immunization. For each individual there was a very high correlation between the total SIgA concentration before and after immunization (control group r = 0·92, P < 0·0001, IgAN group r = 0·99, P < 0·0001), suggesting that SIgA levels are stable over time.
Fig. 1.
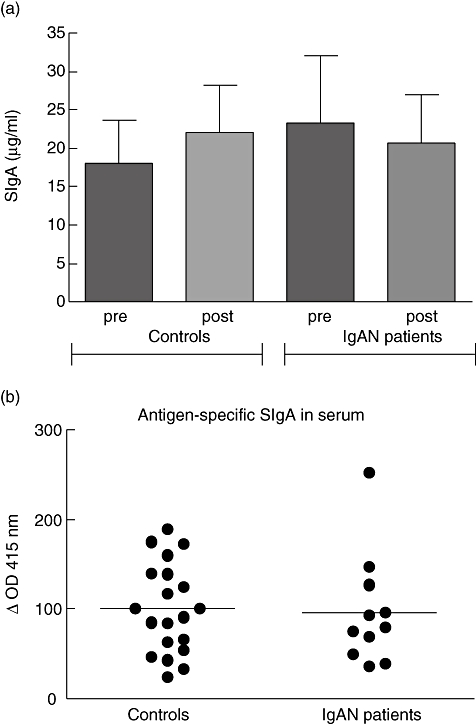
Total secretory immunoglobulin A (SIgA) and cholera toxin subunit B (CTB)-specific SIgA in serum of controls and immunoglobulin A nephropathy (IgAN) patients. Pre- and post-immunization sera of controls and IgAN patients were tested for total SIgA by enzyme-linked immunosorbent assay (a). CTB-specific SIgA was measured before and after immunization, and expressed by increase in optical density (OD) × 1000. Shown are individual increases in OD values of 20 controls and 11 IgAN patients (b).
Next, we measured SIgA specific for CTB in serum before and after immunization. In both the control group and the patient group we were able to detect a small but significant increase in SIgA anti CTB after the second booster (P < 0·001). The amount of CTB-specific SIgA was not significantly different between controls and IgAN patients (Fig. 1b). In these samples we were not able to detect secretory IgM (data not shown). As a control, sera of non-immunized individuals were tested for the presence of antigen-specific SIgA. These sera were negative for antigen-specific SIgA (data not shown).
Secretory immunoglobulin A in nasal washes and serum
As the antigen-specific total IgA response in serum was strong [17] and there was only a slight increase in serum for antigen-specific SIgA, we were interested in the size distribution of the antigen-specific IgA. We size-fractionated nasal washes and sera to test for total IgA and SIgA concentrations. In nasal wash only one peak of total IgA was found, exactly overlapping the SIgA peak, suggesting that most, if not all, IgA in nasal washes is SIgA (Fig. 2a). In serum the profile of total IgA showed two distinct peaks, corresponding with pIgA (44–51 ml) and monomeric IgA (mIgA) (52–60 ml) (Fig. 2b). A high percentage of total IgA in circulation was monomeric, in accordance with previous observations. SIgA was found in the pIgA fractions.
Fig. 2.
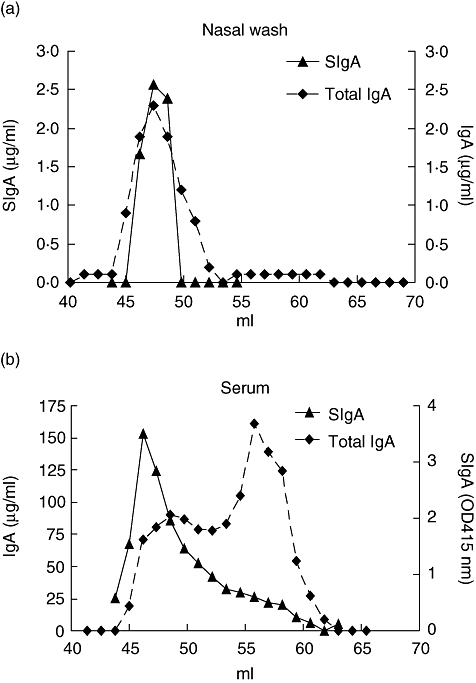
Secretory immunoglobulin A (SIgA) is present mainly in the high molecular weight fractions of immunoglobulin A (IgA). IgA of nasal wash and serum was size-fractionated with a HiLoadTM 16/60 HR200 Superdex prep grade gelfiltration column. Total IgA and SIgA was determined by specific enzyme-linked immunosorbent assay. In nasal wash the total IgA peak and SIgA peak overlap (a). In serum total IgA exists mainly of monomeric IgA and to a lesser extent of polymeric IgA. SIgA is present in the high molecular weight fractions of IgA (b). Shown are representative profiles of an IgA nephropathy patient.
Antigen-specific IgA in serum after mucosal immunization consists mainly of pIgA
To determine the size of antigen-specific IgA, sera of 12 immunized individuals (six controls and six IgAN patients) were size-fractionated as described in the Methods section. Fractions were measured for total IgA, antigen-specific IgA and total protein. CTB-specific IgA concentrations were determined in the fractions, relative to an internal standard and expressed as arbitrary units. IgA anti-CTB was present in both pIgA and mIgA fractions, with higher levels in the pIgA fractions (Fig. 3a). The percentage of pIgA anti-CTB was 57 ± 21 in the control group and 63 ± 24 in the IgAN patients (n.s.) (Fig. 3b). For total IgA the percentage of pIgA was lower than antigen-specific IgA in both the patient and the control groups. The mean percentage pIgA of total IgA, was 33 ± 2·9 in the control group and 32 ± 3·6 in the IgAN group (n.s) (Fig. 3c).
Fig. 3.
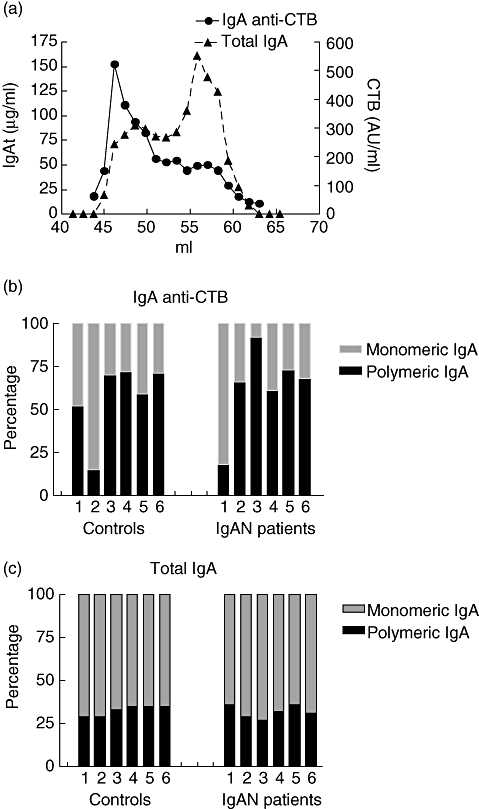
Percentage of polymeric immunoglobulin A (pIgA) and monomeric immunoglobulin A (mIgA) total IgA and cholera toxin subunit B (CTB)-specific IgA. IgA of serum was size-fractionated with a HiLoadTM 16/60 HR200 Superdex prep grade gelfiltration column. Total IgA and CTB-specific IgA were determined by enzyme-linked immunosorbent assay (ELISA). (a) Serum profile of total IgA and CTB-specific IgA of a representative nephropathy (IgAN) patient. Concentrations of high molecular weight IgA (pIgA) and of low molecular weight IgA (mIgA) in serum were determined by ELISA for total and CTB-specific IgA. (b) Percentages of CTB-specific mIgA and pIgA of six controls and six IgAN patients. In (c) percentages total mIgA and pIgA are depicted of six controls and six IgAN patients.
Size distribution of antigen-specific IgA in serum after systemic immunization
To compare the size distribution of antigen-specific IgA after a mucosal challenge with a systemic immunization, fractions were also analysed for the presence of IgA anti-KLH antibodies. IgA anti-KLH was detectable in all samples (six IgAN patients and six controls). IgA anti-KLH is present almost exclusively in the polymeric fractions (Fig. 4a). The percentage of pIgA anti-KLH was 83 ± 5·7 in the controls and 88 ± 4·9 in the IgAN patients (n.s.) (Fig. 4b). In both groups the percentage pIgA was significantly higher after systemic vaccination compared with mucosal vaccination.
Fig. 4.
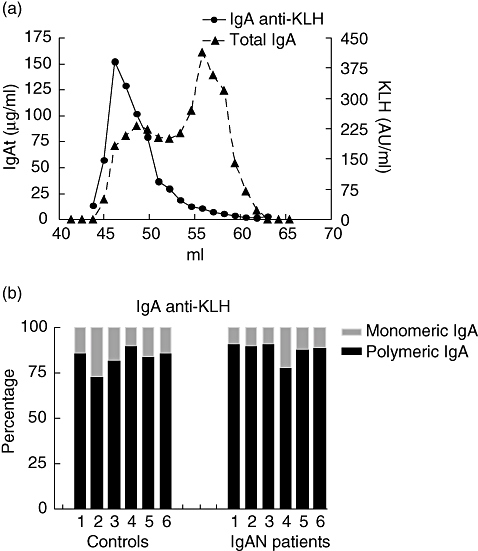
Antigen-specific immunoglobulin A (IgA) after systemic immunization consists almost exclusively of polymeric IgA (pIgA). IgA of serum was size-fractionated with a HiLoadTM 16/60 HR200 Superdex prep grade gelfiltration column. Total IgA and keyhole limpet haemocyanin (KLH)-specific IgA were determined by enzyme-linked immunosorbent assay (ELISA). (a) Serum profile of an IgA nephropathy (IgAN) patient for KLH-specific IgA (black line) and for total IgA (dotted line). Concentrations of high molecular weight IgA (pIgA) and of low molecular weight IgA [monomeric IgA (mIgA)] in serum were determined by ELISA for total and KLH-specific IgA. (b) Percentages of KLH-specific mIgA and pIgA of six controls and six IgAN patients.
Discussion
This is the first study showing induction of antigen-specific SIgA in serum, upon mucosal immunization. This is also the first study that compares the size distribution of antigen-specific IgA in serum following different routes of immunization. We demonstrate that the antigen-specific IgA response is mainly polymeric and independent of the route of immunization. The size distribution of antigen-specific IgA in patients with IgAN is not different from controls.
Secretory immunoglobulin A might play an important role in the pathogenesis of IgAN. This view is supported by the fact that higher concentrations of SIgA are present in serum of IgAN patients and by an association of higher SIgA concentrations with more pronounced haematuria [12]. Another argument for the involvement of SIgA in the pathogenesis of IgAN comes from the observation that in about 15% of cases SIgA can be detected in renal biopsies of IgAN patients [15,16,23]. The presence of SIgA is correlated with deposition of MBL and C4d. It has been described that renal injury is worse in patients with MBL deposition [14]. Recently we showed that high concentrations of SIgA were present in IgA eluted from a removed allograft of an IgAN patient [15,16]. An additional argument, suggesting a pathogenic role of SIgA in IgAN, is that about 40% of patients show a sudden increase in haematuria [24] within 2 days after an upper respiratory tract infection. It is tempting to speculate that this so-called synpharyngitic haematuria is mediated by SIgA produced during a mucosal infection.
In the present study we were able to show antigen-specific SIgA in plasma. As SIgA in serum is present in low concentrations we expected to find only very low concentrations of antigen-specific SIgA. The increase in antigen-specific SIgA was small but highly significant in both IgAN patients and controls. SIgA is present in sera of humans in low concentration. The mechanism by which SIgA, produced at mucosal surfaces, is transported to the circulation is not clear. This could be by leakage of SIgA or by active transport through the epithelial layer [25]. Whether SIgA in serum has a role in the immunological response is also a matter of debate. In our study all individuals had low concentrations of antigen-specific SIgA in their serum. We have shown recently that SIgA is present in renal biopsies of a small group of IgAN patients [15]. It would be interesting to correlate the SIgA immune response with the absence or presence of SIgA in the biopsy. Unfortunately, this material is not available at present. Similarly, it has been shown that the glycosylation pattern of SIgA differs from serum IgA in several ways [26]. Whether the glycosylation patterns of (antigen-specific) SIgA differ between IgAN patients and controls and whether this influences deposition is not known at present and would require the design of a new vaccination study.
It has long been recognized that deposited IgA in IgAN consists mainly of HMW IgA1[10,11] Recently it has been demonstrated that HMW IgA has specific effector activities including MBL pathway activation of complement [14]. Therefore we have analysed the size distribution of antigen-specific IgA upon simultaneous vaccination with two different neoantigens and show that the antigen-specific IgA response in both patients and controls is predominantly in the HMW fractions. Several immunization studies with different antigens and various routes of administration showed that IgAN patients have aberrant immune responses compared with controls [18,19,22]. However, there is only limited information about the size distribution of these specific IgA responses. After intramuscular vaccination with inactivated influenza virus, no differences in the size distribution of antigen-specific IgA were found between IgAN patients and controls [19]. In the present study it appeared that mucosally administered CTB induced a clear mucosal and systemic immune response, as described previously [17]. Measuring the antigen-specific IgA response revealed that mucosally administered CTB induced an antigen-specific pIgA response and also, to a lesser extent, an antigen-specific mIgA response in serum. The antigen-specific IgA anti-KLH consists almost exclusively of pIgA and a smaller fraction of mIgA. Both IgAN patients and controls showed a similar capacity to induce these HMW responses.
In several immunization studies it appeared that most of the antigen-specific IgA was of the IgA1 subclass [17–19]. Although two studies showed higher IgA1 : IgA2 ratios in IgAN patients [18,19] this finding was not shown in other studies [17,22]. It is important to realize that both the place of antigen presentation as well as the antigens used are of importance with respect of the subclass distribution. Overall, there is a tendency of higher IgA1 : IgA2 ratio in IgAN patients after immunization. In the current study we were not able to differentiate between antigen-specific SIgA1 and SIgA2.
Here we describe for the first time the size distribution of antigen-specific IgA after simultaneously performed mucosal and systemic immunization with two different neoantigens. With regard to IgAN patients, the size distribution of IgA after systemic recall immunizations has been described. Intramuscular vaccination with influenza virus showed higher mIgA titres than that of pIgA [19]. Systemic immunization with tetanus toxoid also showed predominantly mIgA, but higher levels of pIgA in IgAN patients than in controls [21]. Whether these differences in size of antigen-specific IgA are determined by the type of antigen used in the different studies, or by differences between primary or recall immune responses, is not clear.
In conclusion, in the present study we have investigated the size distribution of antigen-specific IgA responses upon mucosal and systemic immunization with a neoantigen. We observed that antigen-specific IgA responses were predominantly present in HMW IgA fractions, including antigen-specific SIgA. In view of the proposed pathogenic role of HMW IgA in IgAN, a more detailed analysis of antigen-specific IgA might be required to characterize the altered IgA response in patients with IgA nephropathy.
References
- 1.Berger J, Hinglais N. [Intercapillary deposits of IgA-IgG] J Urol Nephrol (Paris) 1968;74:694–5. [PubMed] [Google Scholar]
- 2.Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217. doi: 10.1016/j.semnephrol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 4.Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- 5.Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–13. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 6.Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171–9. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Allen AC, Feehally J. IgA1 glycosylation and the pathogenesis of IgA nephropathy. Am J Kidney Dis. 2000;35:551–6. doi: 10.1016/s0272-6386(00)70214-9. [DOI] [PubMed] [Google Scholar]
- 8.Hiki Y, Kokubo T, Iwase H, et al. Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J Am Soc Nephrol. 1999;10:760–9. doi: 10.1681/ASN.V104760. [DOI] [PubMed] [Google Scholar]
- 9.van der Boog PJ, de Fijter JW, Bruijn JA, van Es LA. Recurrence of IgA nephropathy after renal transplantation. Ann Med (Paris) 1999;150:137–42. [PubMed] [Google Scholar]
- 10.Feehally J, Allen AC. Structural features of IgA molecules which contribute to IgA nephropathy. J Nephrol. 1999;12:59–65. [PubMed] [Google Scholar]
- 11.Tomino Y, Sakai H, Miura M, Endoh M, Nomoto Y. Detection of polymeric IgA in glomeruli from patients with IgA nephropathy. Clin Exp Immunol. 1982;49:419–25. [PMC free article] [PubMed] [Google Scholar]
- 12.Oortwijn BD, van der Boog PJ, Roos A, et al. A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int. 2006;69:1131–8. doi: 10.1038/sj.ki.5000074. [DOI] [PubMed] [Google Scholar]
- 13.van der Boog PJ, Van Kooten C, de Fijter JW, Daha MR. Role of macromolecular IgA in IgA nephropathy. Kidney Int. 2005;67:813–21. doi: 10.1111/j.1523-1755.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 14.Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–34. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 15.Oortwijn BD, Rastaldi MP, Roos A, Mattinzoli D, Daha MR, Van Kooten C. Demonstration of secretory IgA in kidneys of patients with IgA nephropathy. Nephrol Dial Transplant. 2007;22:3191–5. doi: 10.1093/ndt/gfm346. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Kobayashi H, Sato H, Arakawa M. Immunohistochemical characterization of glomerular IgA deposits in IgA nephropathy. Clin Nephrol. 1990;33:66–71. [PubMed] [Google Scholar]
- 17.de Fijter JW, Eijgenraam JW, Braam CA, et al. Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int. 1996;50:952–61. doi: 10.1038/ki.1996.396. [DOI] [PubMed] [Google Scholar]
- 18.Layward L, Finnemore AM, Allen AC, Harper SJ, Feehally J. Systemic and mucosal IgA responses to systemic antigen challenge in IgA nephropathy. Clin Immunol Immunopathol. 1993;69:306–13. doi: 10.1006/clin.1993.1185. [DOI] [PubMed] [Google Scholar]
- 19.van den Wall Bake AW, Beyer WE, Evers-Schouten JH, et al. Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest. 1989;84:1070–5. doi: 10.1172/JCI114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldo FB. Systemic immune response after mucosal immunization in patients with IgA nephropathy. J Clin Immunol. 1992;12:21–6. doi: 10.1007/BF00918269. [DOI] [PubMed] [Google Scholar]
- 21.Layward L, Allen AC, Harper SJ, Hattersley JM, Feehally J. Increased and prolonged production of specific polymeric IgA after systemic immunization with tetanus toxoid in IgA nephropathy. Clin Exp Immunol. 1992;88:394–8. doi: 10.1111/j.1365-2249.1992.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roodnat JI, de Fijter JW, Van Kooten C, Daha MR, van Es LA. Decreased IgA1 response after primary oral immunization with live typhoid vaccine in primary IgA nephropathy. Nephrol Dial Transplant. 1999;14:353–9. doi: 10.1093/ndt/14.2.353. [DOI] [PubMed] [Google Scholar]
- 23.Obara W, Iida A, Suzuki Y, et al. Association of single-nucleotide polymorphisms in the polymeric immunoglobulin receptor gene with immunoglobulin A nephropathy (IgAN) in Japanese patients. J Hum Genet. 2003;48:293–9. doi: 10.1007/s10038-003-0027-1. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls KM, Fairley KF, Dowling JP, Kincaid-Smith P. The clinical course of mesangial IgA associated nephropathy in adults. Q J Med. 1984;53:227–50. [PubMed] [Google Scholar]
- 25.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Royle L, Roos A, Harvey DJ, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–53. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]


