Abstract
Non-immediate reactions to iodine contrast media (ICM) affect 2–5% of patients receiving these agents. We studied the immunological mechanisms involved in patients with a confirmed non-immediate reaction, maculopapular exanthema, after administration of ICM. The diagnosis was carried out by skin testing or drug provocation test. The immunological study was performed in sequential peripheral blood mononuclear cells taken from the onset of the reaction by flow cytometry and in skin biopsy by immunohistochemistry, with specific recognition by the lymphocyte transformation test (LTT) with different ICM. Flow cytometry showed an increase in the different activation markers [CD69, CD25 and human leucocyte antigen D-related (HLA-DR)] and the skin homing receptor [cutaneous lymphocyte-associated antigen (CLA)] in CD4 lymphocytes, whereas perforin was higher in the CD8 lymphocytes. The skin biopsy showed a perivascular mononuclear infiltrate composed of CD4 lymphocytes, expressing CD25, HLA-DR and CLA, with eosinophils. Intradermal skin tests and the LTT were positive to several ICM, including the culprit agent in four and three patients, respectively, with negative results in all 10 tolerant controls. We showed that a specific immunological mechanism was implicated in patients with non-immediate reactions to ICM. Moreover, the positive results in skin tests and lymphocyte proliferation tests indicated that an important cross-reactivity exists.
Keywords: allergy, iodine contrast media, maculopapular exanthema, monitoring, non-immediate reactions, T cells
Introduction
Adverse reactions after the administration of iodine contrast media (ICM) can be associated with the pharmacological actions of the drug (type A reactions) or be unpredictable, uncommon, and usually unrelated to its pharmacological actions (type B) [1,2]. This latter type includes hypersensitivity reactions, which can be immediate, occurring within 1 h of ICM administration, or non-immediate reactions (NIR), appearing later [3]. Immediate reactions are the most common type, with symptoms appearing within minutes of ICM administration in about 70% of cases [4]. These reactions can be anaphylactoid, mediated by a non-specific release of histamine or, more uncommonly, immunoglobulin E (IgE)-mediated reactions. Although probably underestimated, the frequency of NIR to ICM has been reported to be 2–5% of patients who receive ICM [5,6]. The majority of NIR are cutaneous, involving pruritus, maculopapular rash, urticaria and angio-oedema [7–9]. More severe reactions, even occasionally life-threatening, have also been reported, including erythema multiforme, fixed drug eruption, cutaneous vasculitis, Stevens–Johnson syndrome, toxic epidermal necrolysis and drug reaction with eosinophilia and systemic symptoms [8–12].
Iodine contrast media are highly concentrated solutions of tri-iodinated benzene derivatives, which are characterized further by their ionic or non-ionic side chains as well as by their monomeric or dimeric ring structure. Non-ionic dimers seem to be more implicated than non-ionic monomers in NIR [13], although a study in Sweden found no differences between non-ionic monomers and non-ionic dimers [14].
Although NIR to ICM have been reported to show a specific immunological mechanism mediated by T lymphocytes [4,7,8] few detailed studies have been undertaken, especially considering that the study of these reactions is hindered because of the absence of well-validated in vivo or in vitro tests. In order to demonstrate the implication of lymphocytes in NIR to ICM, we monitored the response during the acute phase and the resolution period of six patients who developed a cutaneous NIR to ICM. We evaluated the immunological activation and recognition in both peripheral blood and in skin.
Materials and methods
Patients and controls
The study included all subjects referred to our clinic with a NIR attributed to an ICM over a period of 2 years. From the onset of the reaction this was monitored by obtaining sequential blood samples and a skin biopsy. After obtaining the first sample, the patients received treatment with corticosteroids and anti-histamines. The final study included only those patients with a confirmed NIR induced by ICM after the allergological work-up, consisting of skin testing and, if negative, a drug provocation test (DPT).
A control group was composed of 10 age- and sex-matched patients who were given ICM for the same indications as the patients in the study group, but who had good tolerance.
The institutional review board approved the study, and informed consent for all the diagnostic procedures was obtained from all the patients and controls.
Skin testing
Intradermal tests were performed following the recommendations of the European Network for Drug Allergy Group [3]. Briefly, 0·01 ml of a fresh 1:10 dilution in 0·9% NaCl of an ionic ICM (sodium meglumide ioxaglate, Hexabrix®; Guerbet, Paris, France) and a series of non-ionic ICM (iomeprol, Iomeron® 300: Bracco, Milan, Italy; iodixanol, Visipaque®: Amersham Health, Buckinghamshire, UK; iopramide, Clarograf®: Shering AG, Mullerstrausse, Berlin, Germany; iobitridol, Xenetix®: Guerbet, Paris, France; and ioversol, Optiray®: Mallinchrodt, St Louis, MO, USA) were injected intradermally. Several readings were taken at 20 min and 8, 24 and 48 h after the injection. These concentrations of ICM proved to be non-irritant in a control group of healthy subjects with good tolerance to ICM.
Drug provocation test
To establish the diagnosis in the two cases where the intradermal testing with the battery of ICM was negative, DPT was carried out with the culprit ICM, as described previously [3]. Briefly, two increasing doses of ICM were given; the first was 1/10 of the amount administered during the procedure that caused the reaction. The patients were then monitored in the drug allergy unit for 8 h, after which they were monitored with a paging system. If good tolerance was observed the second dose, consisting of the full recommended amount of ICM, was administered 1 week later, after which the patient was monitored and followed-up as before.
Sample collection
During the acute phase of the reaction, sequential blood samples were obtained 3, 15 and 45 days after onset of the symptoms. In the control group two blood samples were obtained, one before the administration of iomeprol and another 24 h afterwards. Peripheral blood mononuclear cells (PBMC) were obtained from 6 ml of heparinized venous blood by Ficoll density gradient centrifugation (Nycomed, Oslo, Norway). A 4 mm punch skin biopsy was obtained during the acute phase of the reaction and if the patient had a positive intradermal skin test another biopsy was obtained and the specimens fixed in 10% formalin and embedded in paraffin.
Phenotype immunofluorescence analysis
Different lymphocyte subsets, activation and cytotoxic markers and the skin homing receptor were measured by flow cytometry using the following monoclonal antibodies: CD3-peridinin chlorophyll, CD4- and CD8-allophycocyanin, CD69-, CD25- and human leucocyte antigen D-related-phycoerythrin (HLA-DR-PE), cutaneous lymphocyte-associated antigen (CLA) and perforin-fluorescein isothiocyanate (Becton-Dickinson, San Jose, CA, USA), as described previously [15]. Briefly, 2 × 105 cells were stained sequentially with different monoclonal antibodies and fixed in 1% paraformaldehyde in phosphate-buffered saline until analysis on a fluorescence activated cell sorter (FacsCalibur) flow cytometer using CellQuest software. Negative isotype controls were used to verify the staining specificity.
Skin biopsy studies
Microtome sections (8 mm) of skin biopsies were processed as described [15] for haematoxylin and eosin and immunohistochemical staining with the following monoclonal antibodies: CD4, CD69, CD25, Granz-B and perforin (Novocastra Laboratory, Newcastle upon Tyne, UK), CD8 and HLA-DR (Dako, Ely, Cambridgeshire, UK), and CLA (BD Pharmingen, San Diego, CA, USA). The binding of these primary antibodies (mouse IgG) was detected using an anti-mouse IgG conjugated to peroxidase-labelled dextran polymer (Zymed Laboratory, San Francisco, CA, USA) and a 3-3′-diaminobenzidine substrate kit (Sigma, St Louis, MO, USA).
Lymphocyte transformation test
This was performed 2 months after the resolution of symptoms, as described previously [16]. Briefly, 1·5 × 105 PBMC/well were cultured in RPMI-1640 medium with 2 mM l-glutamine and 10% heat-inactivated autologous serum in triplicate in 96-well plates, in the presence of different ICM (iomeprol, iodixanol, iopramide, iobitridol, ioversol and sodium meglumide ioxaglato) at three different concentrations (1000, 100 and 10 μg/ml). Three-well cultures without antigen were used as controls to estimate the background proliferation. A purified protein derivative at a concentration of 10 IU/ml was used as a positive control. The cultures were incubated for 6 days at 37°C in 5% CO2, and 18 h before harvesting 1 μCi [3H]-thymidine was pulsed to each well. The cultures were harvested onto filters and the radionuclide incorporation was measured by scintillation counting. The stimulation index (SI) was calculated as the ratio between the mean values of counts per minute obtained in cultures with drug and those obtained in cultures without drug. A SI ≥ 2·0 was regarded as a positive response.
Statistical analysis
Comparisons for quantitative variables for related samples at different times were performed using the Wilcoxon test. All reported P-values represented two-tailed tests, with values of 0·05 or less considered statistically significant. Statistical analysis was performed using the spss program, version 11·5.
Results
Fourteen patients with a suspected NIR to ICM were monitored throughout the whole episode by taking sequential samples of peripheral blood and at least one skin biopsy. Two months after the episode had resolved, an allergological evaluation was undertaken with a skin test with a complete battery of ionic and non-ionic contrast media, including the culprit agent. Six of these 14 patients were diagnosed finally as having a NIR to ICM and therefore included in the study; all six had a maculopapular exanthema. Cases 1 and 5 developed a reaction with the same ICM on two occasions. Three were women and three men, with a mean age of 61·8 ± 7·44 years; in four cases the culprit agent was iomeprol and in two iodixanol. Four cases were diagnosed by skin testing and two by DPT with the culprit drug. In all cases the skin test positive results were obtained after 48 h with the culprit ICM, with a high cross-reactivity with both ionic and non-ionic ICM. In the two patients diagnosed by DPT, the reaction occurred at the ICM concentration of 1/10 (2·5 g) in case 3 and the full dose (25 g) in case 4. Table 1 shows the clinical characteristics (sex, age, symptoms), as well as the results of the allergological work-up (skin test and DPT) of the patients.
Table 1.
Clinical characteristics and results of the allergological work-up of the patients monitored.
| Patient | Sex | Age | Drug | Reaction | Skin test | DPT |
|---|---|---|---|---|---|---|
| 1 | F | 51 | IOM | MPE | +SMIOX, IOM, IOD, IOV, | Not done |
| 2 | M | 66 | IOD | MPE | +IOM, IOD, IOP, IOV | Not done |
| 3 | M | 65 | IOM | MPE | Negative | +IOM |
| 4 | F | 55 | IOM | MPE | Negative | +IOM |
| 5 | F | 63 | IOD | MPE | +IOM, IOD, IOV | Not done |
| 6 | M | 71 | IOM | MPE | +SMIOX, IOM, IOD, IOP, IOV | Not done |
IOB, iobitridol; IOD, iodixanol; IOM, iomeprol; IOP, iopramide; IOV, ioversol; MPE, maculopapular exanthema; SMIOX, sodium meglumide ioxaglate.
Phenotype immunofluorescence analysis
Figure 1 shows the results obtained by phenotype immunofluorescence analysis with the different markers in sequential samples. The expression of the activation makers (CD69, CD25 and HLA-DR) in the T cell subpopulation (CD4 and CD8) (Fig. 1a–c) showed that the early activation marker, CD69, was increased significantly in the first sample, corresponding to the third day of the reaction, and fell to normal values on day 45 (P = 0·028) (Fig. 1a). A significant increase in the percentage of CD25-expressing cells (late activation marker) was observed in the CD4 cells, with a maximum on day 15, falling to control values on day 45 (P = 0·027). These CD25 values were unchanged in the CD8 population (Fig. 1b).
Fig. 1.
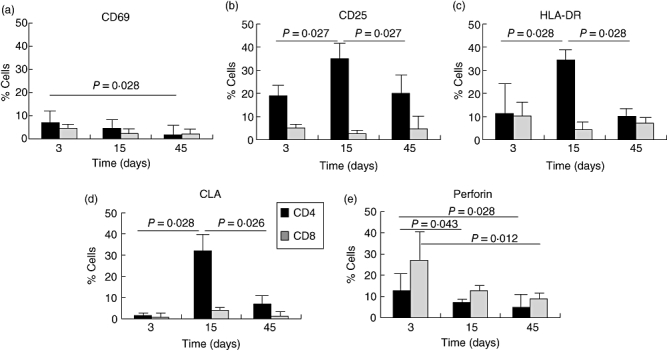
Mean and standard deviation of sequential measurement of activation markers CD69, CD25 and human leucocyte antigen D-related (HLA-DR) (a–c), the skin homing receptor [cutaneous lymphocyte-associated antigen (CLA)] (d) and perforin (e) in two T lymphocyte subpopulations, CD4 and CD8 from the six patients studied. Statistical differences are considered significant with a P < 0·05.
Figure 1c shows a large increase in HLA-DR expression in CD4 T cells on day 15, followed by a decrease until reaching normal values on day 45 (P = 0·028). The levels of this marker paralleled those of CLA (Fig. 1d) (P = 0·026). No increase was detected in CD8 CLA.
The percentage of cells containing perforin (Fig. 1e) was increased earlier on day 3 in both CD4 and CD8, slightly higher in the latter (P = 0·028 and 0·012 respectively).
During the skin test in patient 1, as well as a positive result at 48 h, a mild exanthematic reaction also appeared. At this time peripheral blood lymphocytes were obtained and the phenotype analysis showed a mild increase in perforin in CD4/CD8 cells, as occurred during the acute episode (data not shown in figures). No significant changes were observed in HLA-DR or CLA.
Skin biopsy studies
Different cell markers were detected in the biopsy obtained during the acute phase of the reaction. The immunohistochemical studies showed a perivascular mononuclear cell infiltrate, mainly in the dermis, with higher levels of CD4 lymphocytes than CD8 T lymphocytes, with moderate expression of CD25 and a higher expression of HLA-DR and CLA. There was a high presence of eosinophils, as well as the existence of foci of vacuoles containing lymphocytes. A skin biopsy was obtained at the site of the positive intradermal test to the culprit ICM and immunohistochemistry showed similar results to those seen in the initial acute phase biopsy, with higher expression of CD69 in lymphocytes. Figure 2 shows the immunohistochemical results of different markers in skin biopsies obtained during the initial acute phase of the reaction (Fig. 2a) and from the site of the positive skin test (Fig. 2b) in patient 1.
Fig. 2.
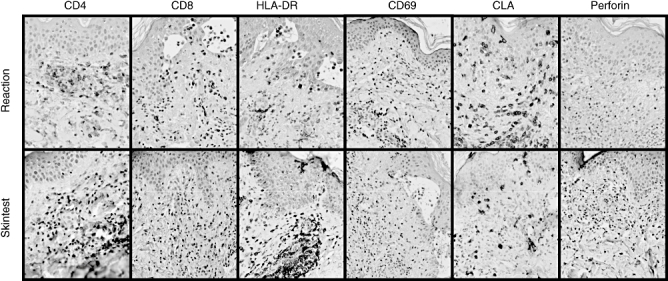
Immunohistochemical analysis of different markers, CD4, CD8, CD69, human leucocyte antigen D-related, CLA and perforin, in skin biopsies obtained from the acute reaction (a) and the positive skin test (b), and in patient 1. HLA-DR, human leucocyte antigen D-related; CLA, cutaneous lympho-cyte-associated antigen.
Lymphocyte transformation test
The lymphocyte transformation test (LTT) results showed a positive proliferation with a SI > 2 to the ICM responsible for the NIR in three patients (patients 1, 2 and 6) (Fig. 3), with different degrees of cross-reactivity with other ICM, especially to sodium meglumide ioxaglate and ioversol. None of the patients' PBMCs proliferated in the presence of iobitridol and only one to iopramide. With the different assay concentrations, in most cases 10 μg/ml gave positive results, with the response decreasing at higher concentrations, probably because of toxic effects. This test was negative to all the ICM tested in the control subjects.
Fig. 3.
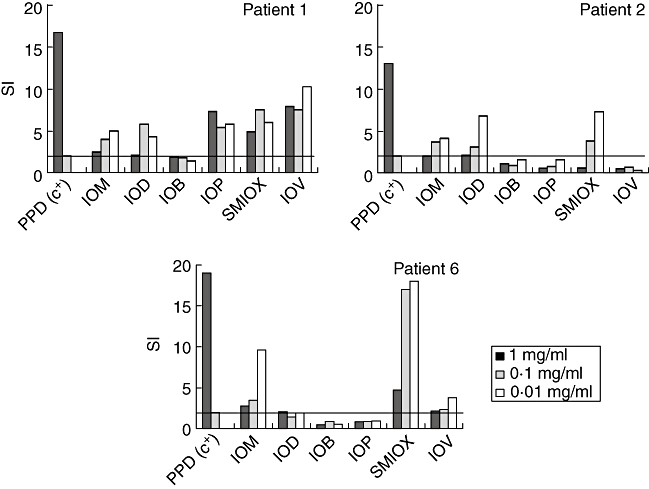
Lymphocyte transformation test results from patients 1, 2 and 6 who gave a positive proliferation [stimulation index (SI) > 2] to different iodine contrast media: IOM, iomeprol; IOD, iodixanol; IOB, iobitridol; IOP, iopramide; SMIOX, sodium meglumide ioxaglate; IOV, ioversol. PPD, purified protein derivative.
Discussion
Different studies have emphasized the relevance of NIR in allergy to ICM [17–22]. However, unlike immediate reactions, prophylaxis with steroids and anti-histamines does not guarantee tolerance. If a NIR is not properly identified, the patient may have another reaction on subsequent exposure. In fact, patients 1 and 5 in our series had a reaction on two occasions. This fact, plus a positive intradermal test in four cases and positive LTT in three, suggests that an immunological mechanism was involved. Additionally, we found an increase in the activation of T cells expressing the skin homing receptor, CLA, mainly in CD4 lymphocytes, in both peripheral blood and skin biopsies that paralleled the reaction. The implication of a specific T cell subpopulation and the cell marker expression in parallel with the development of the reaction are in agreement with studies carried out with other drugs, including betalactams and anti-convulsants [15,23,24]. We also detected an earlier increase in perforin, mainly in CD8 T lymphocytes, even in a reaction induced with a quantity of ICM as low as the one used in intradermal testing, indicating that cytotoxic lymphocytes were involved. These data have also been observed with other drugs [25].
Of note during the monitoring of the reaction was the fact that the late activation marker (CD25) decreased later in comparison with the very late activation marker, HLA-DR. The expression of this latter marker paralleled very closely both the percentage of CD4 cells and the cells expressing CLA. The delay in the decrease in CD25 expression in CD4 lymphocytes could be explained by the presence of regulatory T lymphocytes that show this phenotype. Importantly, during the reaction the patients were treated with corticosteroids, which have been reported to induce proliferation of regulatory T cells (CD4+ CD25+) [26]. The skin test biopsy showed the same pattern as that observed in the reaction, but with a higher expression of CD69, attributed to the fact that the biopsy sample was obtained earlier in the skin tests than in the reaction.
The specific recognition of ICM by T lymphocytes was demonstrated by their increased proliferation in the presence of the culprit drug in the LTT. This confirms the involvement of this drug in the reaction and that this was T cell-mediated. The usefulness of this test has been demonstrated previously with penicillins [16] although, unfortunately, few reports exist regarding other drugs or contrast media. We also observed high cross-reactivity between the different ionic and non-ionic ICM assayed, although the patients had never been exposed to the other ICM, as has been found by others [8,27].
With respect to the skin response to ICM, skin testing may show different results depending on the type of the reaction, and is more useful in immediate reactions to ICM [9]. However, we obtained delayed positive intradermal tests in 66% of the patients finally diagnosed. Similar results were described by Vernassiere, who found 50% positivity with intradermal tests with ICM and just 13% positivity with patch testing [28].
Regarding cross-reactivity, we observed a high rate with a good correlation between skin (detected by skin testing) and blood in those cases where the LTT was positive. Several reports have shown this cross-reactivity both in vivo and in vitro[18,20,27], although selective responses have also been found [17,19,21,22]. The cross-reactivity may be due to the common nuclear chemical structure shared by all ICM.
To our knowledge, this is the first report of patients with NIR to ICM in whom detailed immunological in vitro monitoring (peripheral blood and skin) has been undertaken, the results of which support an effector T cell mechanism.
Acknowledgments
We thank Ian Johnstone for help with the final English language version of this manuscript. This study was supported by the Spanish Ministry of Health (Fondo Investigacion Sanitaria) FIS grants PIO20640 and PI031165 and the FIS Network RIRAAF (RD07/0064).
References
- 1.Rodriguez RM, Gueant JL, Aimone-Gastin I, et al. The increased histamine release in ischaemic heart disease patients undergoing coronaroangiography is not mediated by specific IgE. Allergy. 2002;57:61–6. doi: 10.1034/j.1398-9995.57.s72.17.x. [DOI] [PubMed] [Google Scholar]
- 2.Böhm I, Speck U, Schild H. Cytokine profiles after nonionic dimeric contrast: medium injection investigative. Radiology. 2003;38:776–83. doi: 10.1097/01.rli.0000091650.57015.5a. [DOI] [PubMed] [Google Scholar]
- 3.Brockow K, Christiansen C, Kanny G, et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2005;60:150–8. doi: 10.1111/j.1398-9995.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Webb JA, Stacul F, Thomsen H, Morcos SK. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol. 2003;13:181–4. doi: 10.1007/s00330-002-1650-5. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda R, Munechika H. Delayed adverse reactions to non-ionic monomeric contrast-enhanced media. Invest Radiol. 1998;33:1–5. doi: 10.1097/00004424-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Christiansen C. X-ray contrast media. An overview. Toxicology. 2005;209:185–7. doi: 10.1016/j.tox.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Romano A, Artesani MC, Andriolo M, Viola M, Pettinato R, Vecchioli-Scaldazza A. Effective prophylactic protocol in delayed hypersensitivity to contrast media: report of a case involving lymphocyte transformation studies with different compounds. Radiology. 2002;225:466–70. doi: 10.1148/radiol.2251011654. [DOI] [PubMed] [Google Scholar]
- 8.Kanny G, Pichler W, Morisset M, et al. T cell-mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol. 2005;115:1791–85. doi: 10.1016/j.jaci.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Kvedariene V, Martins P, Rouanet L, Demoly P. Diagnosis of iodinated contrast media hypersensitivity: results of a 6-year period. Clin Exp Allergy. 2006;36:1072–7. doi: 10.1111/j.1365-2222.2006.02532.x. [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123–9. doi: 10.1016/j.tox.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Laroche D. Immediate reactions to contrast media: mediator release and value of diagnostic testing. Toxicology. 2005;209:193–4. doi: 10.1016/j.tox.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Morcos SK. Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol. 2005;78:686–93. doi: 10.1259/bjr/26301414. [DOI] [PubMed] [Google Scholar]
- 13.Morcos SK, Thomsen HS. Adverse reactions to iodinated contrast media. Eur Radiol. 2001;11:1267–75. doi: 10.1007/s003300000729. [DOI] [PubMed] [Google Scholar]
- 14.Rydber JC, Aspelin P. Frequency of late allergy-like adverse reactions following intravascular injection of non-ionic contrast media. A retrospective study comparing a non-ionic contrast monomeric contrast medium with non-ionic dimeric contrast medium. Act Radiol. 1998;39:219–22. doi: 10.1080/02841859809172183. [DOI] [PubMed] [Google Scholar]
- 15.Torres MJ, Mayorga C, Fernandez TD, et al. T cell assessment in allergic drug reactions during the acute phase according to the time of occurrence. Int J Immunopathol Pharmacol. 2006;19:119–30. [PubMed] [Google Scholar]
- 16.Luque I, Leyva L, Torres MJ, et al. In vitro T lymphocyte responses to betalactam drugs in immediate and nonimmediate allergic reactions. Allergy. 2001;56:611–18. doi: 10.1034/j.1398-9995.2001.000115.x. [DOI] [PubMed] [Google Scholar]
- 17.Kanzaki T, Sakagami H. Late phase reaction to a CT contrast medium (iotrolan) J Dermatol. 1991;18:528–31. doi: 10.1111/j.1346-8138.1991.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 18.Courvoisier S, Bircher AJ. Delayed-type hypersensitivity to a nonionic, radiopaque contrast medium. Allergy. 1998;53:1221–4. doi: 10.1111/j.1398-9995.1998.tb03846.x. [DOI] [PubMed] [Google Scholar]
- 19.Brockow K, Becker EW, Worret WI, Ring J. Late skin test reactions to radiocontrast medium. J Allergy Clin Immunol. 1999;104:1107–8. doi: 10.1016/s0091-6749(99)70096-5. [DOI] [PubMed] [Google Scholar]
- 20.Gall H, Pillekamp H, Peter RU. Late-type allergy to the X-ray contrast medium Solutrast (iopamidol) Contact Dermatitis. 1999;40:248–50. doi: 10.1111/j.1600-0536.1999.tb06058.x. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, Sueki H, Nakada T, Akiyama M, Lijima M. Multiple fixed drug eruption caused by iomeprol (Iomeron), a non-ionic contrast medium. Dermatology. 1999;198:291–4. doi: 10.1159/000018133. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi R, Morita A, Tsuji T. Fixed drug eruption caused by iopamidol, a contrast medium. J Dermatol. 1997;24:243–5. doi: 10.1111/j.1346-8138.1997.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 23.Cornejo-Garcia JA, Fernandez TD, Torres MJ, et al. Differential cytokine and transcription factor expression in patients with allergic reactions to drugs. Allergy. 2007;62:1429–38. doi: 10.1111/j.1398-9995.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 24.Leyva L, Torres MJ, Posadas S, et al. Anticonvulsant-induced toxic epidermal necrolysis: monitoring the immunologic response. J Allergy Clin Immunol. 2000;105:157–65. doi: 10.1016/s0091-6749(00)90191-x. [DOI] [PubMed] [Google Scholar]
- 25.Posadas SJ, Padial A, Torres MJ, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol. 2002;109:155–61. doi: 10.1067/mai.2002.120563. [DOI] [PubMed] [Google Scholar]
- 26.Chung Y, Fang Dong H, Zhang X, et al. Effects of IL-7 and dexamethasone: induction of CD25, the high affinity IL-2 receptor, on human CD4+ cells. Cell Immunol. 2004;232:57–63. doi: 10.1016/j.cellimm.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Lerch M, Keller M, Britschgi M, et al. Cross-reactivity patterns of T cells specific for iodinated contrast media. J Allergy Clin Immunol. 2007;119:1529–36. doi: 10.1016/j.jaci.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Vernassiere C, Trechot P, Commun N, Schmutz JL, Barbaud A. Low negative predictive value of skin tests in investigating delayed reactions to radio-contrast media. Contact Dermatitis. 2004;50:359–66. doi: 10.1111/j.0105-1873.2004.00367.x. [DOI] [PubMed] [Google Scholar]


