Abstract
Acute rejection remains a poor predictor of graft outcome. In this study, we measured serum levels of interferon (IFN)-γ and neopterin by enzyme-linked immunosorbent assay and a single nucleotide polymorphism (SNP) within the 3′ untranslated region of the interleukin (IL)-12 B gene (1188 A/C) to determine whether either of these factors could predict acute rejection in renal transplantation. Significantly higher early post-transplant neopterin levels (days 5–7; 35·7 versus 19·9 nmol/l) were observed in recipients who subsequently rejected their grafts. Post-transplant neopterin levels showed a strong positive correlation with 1-month creatinine levels (Spearman's correlation 0·62, P < 0·001), suggesting macrophage activation early after transplantation. Pretransplant neopterin and IFN-γ levels and the IL-12B gene SNP did not predict acute rejection in this small retrospective study. The ability to predict acute rejection non-invasively early after transplantation could lead to individual tailoring of immunosuppressive regimens and perhaps lead eventually to longer graft survival.
Keywords: acute renal allograft rejection, IFN-γ, IL-12 genetic polymorphisms, neopterin
Introduction
Transplantation provides the best survival benefits to patients with end-stage kidney disease compared with other forms of renal replacement therapy. Although the incidence of acute allograft rejection (AR) has fallen significantly with advances in immunosuppression, AR remains a strong predictor of poor graft outcome [1]. A clear correlation between subclinical acute cellular rejection and chronic graft dysfunction has been demonstrated in the study by Nankivell et al. [2], indicating that immunological rejection is a factor for progression to graft failure. AR is a complicated process involving both immune and non-immune mediated factors. A long-standing objective of transplantation immunology has been to identify reliable immunological markers for the prediction of AR and thus to enable tailoring of immunosuppressive regimens to individual patients.
Cytokines are soluble glycoproteins with proinflammatory and/or anti-inflammatory properties. Proinflammatory cytokines such as interleukin (IL)-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α modulate T cell activity during the immune response and are powerful mediators in AR.
We have demonstrated previously significant interindividual differences in cytokine production in vitro in both normal individuals [3] and in patients [4] prior to renal transplantation. Our group also showed that patients who secrete higher pretransplant levels of IFN-γin vitro are more likely to undergo AR [4,5] and have poorer long-term graft function [6] than patients who secrete lower levels. In contrast to the work of Pravica et al. [7], we were unable to show a correlation between IFN-γ gene polymorphisms and in vitro production of IFN-γ[8]. We found subsequently that although measuring in vitro production of IFN-γ was predictive of rejection in human leucocyte antigen D-related (HLA-DR) mismatched recipient–donor pairs, this was of limited value in beneficially matched patients because of the HLA-DR match not eliciting a significant mixed-lymphocyte reaction [9].
A number of cytokines orchestrate the production of IFN-γ, including IL-12, the key cytokine of T helper 1 (Th1)-mediated immunity in humans. IFN-γ, in turn, is a major stimulus to neopterin production by monocyte-derived macrophages. In vivo, there is a strong correlation between neopterin levels in the urine or serum and the severity, progression and outcome of many infectious and inflammatory diseases [10]. Although the cellular infiltrate of AR is composed primarily of T cells, a number of studies have shown that macrophages are prominent and are effectors of tissue damage [11,12]. In this current study, we set out to evaluate the role of pre- and post-renal transplant serum neopterin levels, and a single nucleotide polymorphism (1188 A/C) of the IL-12B gene on the outcome of AR in renal transplantation. Additionally, we analysed the in vivo pretransplant IFN-γ levels to assess whether these correlated with neopterin levels and/or acute rejection within our cohort of patients.
Materials and methods
Patients
Sixty-five renal transplant recipients (RTR) who received a kidney transplant between October 2003 and September 2005 from a single centre at Derriford Hospital were included in the study. All recipients received standard triple immunosuppression: cyclosporin (8 mg/kg/day with Ciclosporin 2-h post-dose level monitoring), Azathioprine (2 mg/kg/day) and prednisolone (20 mg/day). This regimen of anti-rejection therapy was used commonly within the United Kingdom at the time of the study period. The study was granted full approval by Plymouth Hospitals National Health Service Trust Research and Development Committee and the South-west Devon Research Ethics Committee.
Diagnosis of acute rejection
Acute rejection was defined by histological changes in renal allograft biopsy occurring within the first 3 months of transplantation using the Banff Classification 1997. Transplant biopsies were undertaken if there was clinical suspicion of rejection based on rising serum creatinine. Other clinical parameters recorded include the number of HLA mismatches between donors and recipients, panel reactive antibodies (PRA) pretransplantation, warm and cold ischaemic time, cytomegalovirus (CMV) infection, presence of delayed graft function and serum creatinine at 1 and 3 months post-transplant. CMV infection is defined by a viral load greater than 100 000 copies per millilitre as measured by polymerase chain reaction (PCR). Pre-emptive treatment with Valganciclovir was used in patients with CMV infection. Delayed graft function is defined as the need to initiate dialysis post-transplant.
Measurement of serum neopterin and IFN-γ
Serum was collected pretransplant on day 0 and between days 5 and 7 post-transplant and stored at −20°C. Pre-/post-transplant serum neopterin and pretransplant IFN-γ levels were measured retrospectively by commercial enzyme-linked immunosorbent assay (Brahms, Germany and Bender MedSystems, Austria respectively) in all 65 RTR.
Polymerase chain reaction amplification
Genomic DNA samples were extracted from peripheral blood in ethylenediamine tetraacetic acid or prepared lymphocytes from donor spleen cells, using a ‘salting-out’ method [13]. The polymorphic-3′untranslated region (UTR) end of the IL-12B gene at position 1188 A/C was investigated in donors and their corresponding recipients. In brief, PCR amplification of the region of interest was carried out with forward primer (5′-CTG ATC CAG GAT GAA AAT TTG G-3′), reverse primer (5′-CCC ATG GCA ACT TGA GAG CTG G-3′), 2 mmol/l 2′-deoxynucleosides 5′-triphosphate, 10× buffer solution, 25 mmol/l MgCl2 and Taq polymerase. PCR conditions consisted of an initial denaturation at 96°C for 1 min, followed by 10 cycles at 96°C for 15 s, at 55°C for 50 s, at 72°C for 40 s and then 20 cycles at 96°C for 10 s, at 60°C for 50 s and finally at 72°C for 40 s. The PCR product was digested with Taq1 (Fermentas Life Sciences, St. Leon-Rot, Germany) restriction enzyme. The product was then loaded onto a 3% agarose gel and run at 120 V for 30 min, and visualized under ultraviolet light staining with ethidium bromide. A 50 base pairs (bp) DNA ladder (Fermentas Life Sciences) was used to assess DNA size. With the rarer C allele, Taq1 would generate two products of 156 and 71 bp. The cytokine genetic analysis was found to be in Hardy–Weinberg equilibrium.
Statistical analyses
The numbers of patients in the study are sufficient to give a power of 80% for detecting a difference of 36% or more (e.g. 20% and 56%) in the percentages of IL-12 AC for the rejectors and non-rejectors, based on a χ2 test at the 0·05 significance level. Data are expressed as median (minimum–maximum) and numbers (%). Mann–Whitney U, Kruskal–Wallis, χ2, Fisher's exact tests and Spearman's correlation were used as appropriate. Multiple logistic regression analysis was used to determine variables associated with AR. Variables with P-value of < 0·10 in the univariate tests were included in the regression models. Findings were considered statistically significant if the corrected P-value was less than 0·05.
Results
During the study period, 65 patients (37 male, 28 female, median age 45 years) received renal transplants from 58 donors (median age 46 years). Three patients were re-transplanted, one of whom was transplanted for the third time. None of these three patients rejected their kidneys. Fifty patients received kidneys from deceased heart-beating donors, five from live donors and 10 from controlled non-heart-beating donors. Twenty patients (31%) were diagnosed with AR, most of whom (n = 15) experienced one episode of AR, while five patients had two episodes of AR. Patients with AR had significantly higher serum creatinine at 1 month, although it was no longer significant by 3 months (Table 1).
Table 1.
Comparison of quantitative and categorical variables between rejectors and non-rejectors.
| Variable | Rejectors | Non-rejectors | P-value |
|---|---|---|---|
| 1 month creatinine (μmol/l) | 202 (118–906) | 135 (71–519) | < 0·001 |
| 3 months creatinine (μmol/l) | 152 (89–802) | 130 (74–483) | 0·094 |
| Cold ischaemic time (min) | 1139 (138–2203) | 1046 (120–2020) | 0·31 |
| Warm ischaemic time (min) | 30·5 (15–75) | 30·5 (16–70) | 0·74 |
| Delayed graft function | 7 (35·0%) | 8 (17·8%) | 0·13 |
| HLA-A antigen mismatch | |||
| One-antigen mismatch | 13 (65·0%) | 32 (71·1%) | 0·18 |
| Two-antigen mismatch | 1 (5·0%) | 7 (15·6%) | |
| HLA-B antigen mismatch | |||
| One-antigen mismatch | 13 (65·0%) | 31 (68·9%) | 0·41 |
| Two-antigen mismatch | 3 (15·0%) | 10 (22·2%) | |
| HLA-DR antigen mismatch | |||
| One-antigen mismatch | 2 (10·0%) | 9(20·0%) | 0·24 |
| Two-antigen mismatch | 3 (15·0%) | 2 (4·4%) | |
| PRA (> 0%) | 1 (5·0%) | 6 (13·3%) | 0·42* |
| IL-12B AC genotype (R) | 4/19 (21·1%) | 14/40 (35·0%) | 0·28 |
| IL-12B genotype mismatch | |||
| One-locus mismatch | 8 (40·0%) | 15 (33·3%) | 0·87 |
| Two-loci mismatch | 2 (10·0%) | 5 (11·1%) | |
| Pretransplant neopterin (nmol/l) | 76·0 (40·1–154·0) | 86·3 (26·8–370·6) | 0·104 |
| Post-transplant neopterin (nmol/l) | 35·7 (8·7–100·0) | 19·9 (2·8–93·5) | 0·006 |
| Pretransplant IFN-γ (ng/ml) | 1·8 (0·3–47·8) | 1·8 (0–162) | 0·57 |
Fisher's exact test. Quantitative variables are expressed as median (range) and categorical variables as number (%). PRA, panel reactive antibodies; R, recipients; HLA, human leucocyte antigen; IL, interleukin; IFN, interferon.
Pre- and post-transplant serum neopterin levels were measured in all 65 patients. There was no difference in pretransplant levels between those with and without AR. However, post-transplant neopterin was significantly higher among rejectors (Table 1, Fig. 1) and remained significant in multivariate analysis (Table 2). There was a strong positive correlation between post-transplant neopterin levels and creatinine at 1 month (Spearman's correlation 0·62, P < 0·001). The correlation with creatinine at 3 months, albeit weaker, remained significant (Spearman's correlation 0·36, P = 0·007, data not shown). Multiple linear regression looking at the relationship with creatinine at 1 month determined that post-transplant neopterin level was the only independently significant factor (adjusted R2 = 0·40, P < 0·001).
Fig. 1.
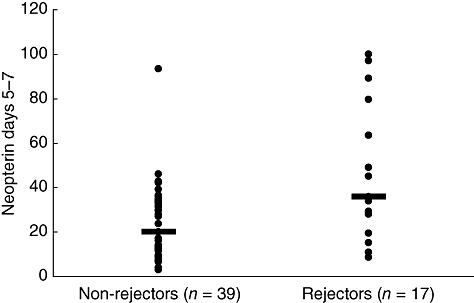
Early (days 5–7) post-transplant neopterin levels in non-rejectors versus rejectors. Significant differences in median neopterin levels were demonstrated in patients with acute rejection (35·7 nmol/l) (range 8·7–100·0) than those without rejection (19·9 nmol/l) (range 2·8–93·5) (Mann–Whitney U-test: P = 0·006).
Table 2.
Multiple logistic regression analysis demonstrating that post-transplant neopterin levels and cytomegalovirus (CMV) infection are both independent predictors of acute rejection.
| Variable | P-value | Odds ratio | 95% confidence interval |
|---|---|---|---|
| Neopterin d5–7 | 0·003 | 1·049 | 1·017–1·083 |
| CMV infection | 0·026 | 6·16 | 1·24–30·52 |
Pretransplant serum IFN-γ was also measured in all 65 patients. There was no correlation between levels of IFN-γ and acute rejection (data not shown).
Cytomegalovirus infection was found to be a risk factor for acute rejection in the logistic regression model, with an odds ratio of 6·16 (P = 0·026, 95% confidence interval 1·24–30·52).
A total of 59 renal recipients and 61 kidney donors were analysed for the IL-12B 1188 A/C polymorphism in the 3′ UTR of the IL-12 B gene (Table 3). Previous in vitro studies have suggested that IL-12 production varies according to the 1188 A/C genotype and therefore we speculated that it may have an effect on rejection by affecting the IL-12 levels within the recipient or donor kidney. In this study, the IL-12B polymorphism (1188 A/C) was not predictive of acute rejection in the RTR.
Table 3.
The effect of interleukin (IL)-12B 1188AC single nucleotide polymorphism (SNP) on rejection.
| IL-12 cytokine polymorphism | Rejectors | Non-rejectors | P-value |
|---|---|---|---|
| IL-12B 1188AC SNP recipient | 4/19 (21·1%) | 14/40 (35·0%) | 0·28 |
| IL-12B 1188AC SNP donor | 5/18 (27·8%) | 19/43 (44·2%) | 0·23 |
Discussion
Neopterin is a pteridine, its biosynthesis beginning with guanosine triphosphate (GTP). GTP is cleaved by GTP cyclohydrolase I to synthesize 7,8-dihydroneotperin triphosphate. Because of the relatively inefficient enzyme activity of 6-pyrovoyltetrahydropterin synthase in human monocytes/macrophages, this results in an accumulation of 7,8-dihydroneopterin triphosphate that is then cleaved by phosphatase to form neopterin [10,14]. Macrophages and dendritic cells in humans constitute the major source of production of neopterin. IFN-γ is a powerful stimulator of GTP cyclohydrolase I and hence of neopterin production. Production of IFN-γ is, in turn, governed by IL-12, a heterodimeric cytokine composed of p35 and p40 [15]. IL-12p70 production varies widely between individuals, and several polymorphisms in the gene encoding IL-12p40 (IL-12B) have been identified that influence susceptibility and severity of infectious, autoimmune and neoplastic disease. IL-12B promoter homozygotes have been shown to be associated with enhanced IL-12p70 production in vitro, while A/C heterozygotes are thought to be associated with decreased production [16–18]. However, the data seem to be affected by the in vitro stimuli used. In addition, IL-12p production has been also shown to be reduced by IL-10 polymorphisms [16]. This cascade of IL-12/IFN-γ–neopterin interaction represents the Th1 immune response. Current evidence suggests that IFN-γ is a powerful cytokine mediating AR. We therefore set out to examine the up-stream and down-stream factors governing IFN-γ production, i.e. the effects of IL-12B polymorphisms and pre- and post-transplant serum neopterin levels in acute renal AR.
In our earlier studies we have demonstrated a predictive effect of pretransplant IFN-γ levels measured in vitro on acute rejection [3,4]. However, the pretransplant serum levels of IFN-γ measured in vivo in this study were not predictive of rejection. This is in accordance with the work from Ghafari et al. [19], but in contrast to the work from Sadeghi et al. [20], which demonstrated an association with pretransplant IFN-γ levels and rejection.
To our knowledge, this is the only study in the published literature examining the effect of IL-12B gene polymorphism on acute rejection in renal transplantation. Despite the published correlation between IL-12B genetic polymorphisms and IL-12 production, we were unable to find an association between the IL-12B 1188 A/C polymorphism within the recipient or donor kidney DNA and the incidence of AR. Although the numbers of patients in our study were low there was sufficient power in the study to rule out a strong association, which was our objective. Studies of cytokine gene polymorphisms have attracted much attention in recent years but have yielded mixed results. While acute renal AR has been observed with increased frequency in patients with high IFN-γ and TNF-α producer genotypes [21], this was not demonstrated in patients on tacrolimus-based therapy [22], raising a question-mark over the significance of cytokine polymorphism in the modern era of immunosuppression. The role of IL-12 in the field of transplantation is not well studied. Although IL-12 is known to stimulate the differentiation of naive T cells into Th1, thereby initiating acute rejection, IL-12p70 was found to delay AR in several skin and heart allograft models [23]. Furthermore, while pretransplant serum IL-12 levels were found to be higher in adult patients who subsequently rejected their kidney grafts [24], this was not demonstrated in paediatric transplant recipients [25]. Another important determinant of cytokine gene polymorphism distribution may be ethnicity [26]. Recently, two other related cytokines, IL-23 and IL-27, have been described, both of which are related to the IL-12 heterodimer family [27]. IL-23 promotes IL-17 production by Th17 subsets, with evidence suggesting the proinflammatory nature of IL-17. Cross-regulation exists between IL-12/IFN-γ and IL-23/IL-17 pathways, with IFN-γ a suppressor of IL-23 and IL-17 synthesis. Future in-depth studies are necessary to dissect further their role in transplantation, but the evidence from the studies above suggest that IL-12 is not a good predictor of graft outcome.
Serum neopterin levels are a good marker of Th1 activation, and hence may provide a useful non-invasive adjunct tool to monitor acute rejection [28]. Our study demonstrated that although pretransplantation serum neopterin levels were not predictive of subsequent AR, in the early post-transplant period high neopterin levels were associated with a higher risk of acute rejection episodes. It also remained a significant factor in predicting AR in a logistic regression model. This is in accordance with findings from earlier studies [29–32]. In a retrospective analysis of 172 RTR plasma neopterin was found to be useful in the early detection of AR, with an overall sensitivity of around 90% [30]. It could be argued that raised neopterin levels represent activated macrophages within the kidney early after transplantation; however, without biopsies taken at the same time (days 5–7) this cannot be proved. Two more recent studies have also demonstrated significant increases in post-transplant neopterin levels in those with rejection [33,34]. Excretion of neopterin requires normal renal function; therefore elevated neopterin levels can be seen in those with delayed graft function [30,32]. Within this study, post-transplant neopterin levels were independently significant to delayed graft function by multiple linear regression looking at the relationship with creatinine at 1 month. Raised serum neopterin levels have been shown to be associated significantly with impaired 2-year graft function [33]. This effect was also observed in our cohort of patients, as creatinine at 1 and 3 months were correlated positively with post-transplant neopterin levels, although we do not have creatinine data beyond the 3-month period.
Higher neopterin levels seen in those who rejected their grafts might be explained by inadequate immunosuppression leading to ongoing Th1 activation and therefore raised serum levels. It is clear that AR can occur even when levels of immunosuppressive drugs are within recommended targets, implying the need to tailor immunosuppression individually. Measurement of neopterin levels post-transplantation might provide useful information under such circumstances. In situations where rising neopterin levels are encountered, vigilance and closer monitoring of renal function and immunosuppressive drug levels could be performed to ensure that levels are in the higher range of the recommended targets. Similarly, dose reduction could be considered in those with stable neopterin levels, in order to avoid over-immunosuppression.
Cytomegalovirus infection might provide another potential mechanism for raised neopterin levels in those who underwent AR. The relationship between CMV infection and AR has long been recognized. Logistic regression analysis in our study determined that CMV infection was an independent factor for AR. The immunomodulatory effect of CMV could possibly be an explanation for this effect. Weimer et al. suggested recently that CMV disease might induce long-term macrophage activation resulting in increased risk of AR and graft survival [33]. Macrophage activation could translate to increased neopterin production. This is supported by the observation that increased serum and urine neopterin correlated with the appearance of symptoms or signs of CMV infection, preceding CMV-specific immunoglobulin M response by a median of 9 days [35].
Weaknesses in our study include the relatively small numbers of patients, the retrospective nature of the study and the relatively short follow-up period, although our intention was to assess the incidence of AR, not long-term graft function. Although higher post-transplant neopterin levels were suggestive of subsequent rejection within our study, the level that defines an individual patient being at risk is uncertain, as there was overlapping of neopterin levels between patients with and without rejection (Fig. 1). Further prospective studies involving a larger population are needed to explore the levels of neopterin that can predict rejection before this non-invasive marker can be used to tailor individual immunosuppression regimens. Subclinical rejections could be detected by protocol biopsy. Examining the correlation between subclinical rejection and post-transplant neopterin levels would provide further insight into the practicality of this immune marker. We are currently in the process of a much larger study to answer these questions.
In summary, an increase in early post-transplant neopterin levels might be used as a clinical adjunct tool to select a subgroup of patients who are at risk of AR. It provides a post-transplant immunological marker, in addition to pretransplant HLA mismatching and/or PRA, for rejection risk stratification, therefore tailoring individual immunosuppression therapy in the early post-transplant period. IL-12B genotyping does not seem to provide additional information and its routine evaluation in either the donors or recipients cannot be recommended currently. Finally, in comparison with our earlier studies that demonstrated a predictive value for pretransplant IFN-γ levels measured in vitro, pretransplant in vivo serum levels of IFN-γ measured were not predictive of rejection.
References
- 1.Matas AJ, Gillingham KJ, Payne WD, Najarain JS. The impact of an acute rejection episode on long-term renal allograft survival. Transplantation. 1994;57:857–79. doi: 10.1097/00007890-199403270-00015. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright N, Demaine A, Jahromi M, Sanders H, Kaminski ER. A study of cytokine protein secretion, frequencies of cytokine expressing cells and IFN-γ gene polymorphisms in normal individuals. Transplantation. 1999;68:1546–52. doi: 10.1097/00007890-199911270-00019. [DOI] [PubMed] [Google Scholar]
- 4.Kaminski ER, Kaminski A, Bending MR, et al. In vitro cytokine profiles and their relevance to rejection following renal transplantation. Transplantation. 1995;60:703–6. doi: 10.1097/00007890-199510150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright NH, Demaine AG, Hurlock NJ, et al. Cytokine secretion in mixed lymphocyte culture: a prognostic indicator of renal allograft rejection in addition to HLA mismatching. Transpl Immunol. 2000;8:109–14. doi: 10.1016/s0966-3274(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 6.Suresh V, Carey BS, Shaw S, et al. Retrospective study of the prognostic impact of cytokine secretion in mixed lymphocyte culture on long-term graft function following allogeneic renal transplantation. Transplant Int. 2005;18:1067–71. doi: 10.1111/j.1432-2277.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 7.Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnot PJ, Hutchinson IV. In vitro production of IFN-γ correlates with CA repeat polymorphism in the human IFN-γ gene. Eur J Immunogenet. 1999;26:1–3. doi: 10.1046/j.1365-2370.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright NH, Keen LJ, Demaine AG, et al. A study of cytokine gene polymorphisms and protein secretion in renal transplantation. Transplant Immunol. 2001;8:237–44. doi: 10.1016/s0966-3274(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 9.Collinson J. University of Plymouth: The use of γ-interferon secretion in mixed lymphocyte culture and responsiveness to suppression by cyclosporin A as indicators of renal allograft rejection. MSc dissertation. [Google Scholar]
- 10.Wirleitner B, Hoffmann G, Fuchs D. Neopterin and 7,8-dihydroneopterin in TH1-type immune response. Mod Asp Immunobiol. 2005;15:36–40. [Google Scholar]
- 11.Bishop GA, Hall BM, Dugin G, Horvath JS, Sheil AR, Tiller DJ. Immunopathology of renal allograft rejection analysed with monoclonal antibodies to mononuclear cell markers. Kidney Int. 1986;29:708–17. doi: 10.1038/ki.1986.56. [DOI] [PubMed] [Google Scholar]
- 12.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015–22. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 13.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from nucleated cells. Nucleic Acids Res. 1998;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 16.Peng JC, Abu Baker S, Richardson MM, et al. IL10 and IL12B polymorphisms each influence IL-12p secretion by dendritic cells in response to LPS. Immunol Cell Biol. 2006;84:227–32. doi: 10.1111/j.1440-1711.2006.01419.x. [DOI] [PubMed] [Google Scholar]
- 17.Vilmaz V, Yentur SP, Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine. 2005;30:188–94. doi: 10.1016/j.cyto.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Stanilova S, Miteva L. Taq-1 polymorphism in 3′UTR of the IL−12B and association with IL-12p40 production from human PBMC. Genes Immun. 2005;6:364–6. doi: 10.1038/sj.gene.6364213. [DOI] [PubMed] [Google Scholar]
- 19.Ghafari A, Makhdoomi K, Ahmadpour P, Afshari AT, Lak SS, Fakhri L. Serum T-lymphocyte cytokines cannot predict early acute rejection in renal transplantation. Transplant Proc. 2007;39:958–61. doi: 10.1016/j.transproceed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi M, Daniel V, Weimer R, Wiesel M, Hergesell O, Opelz G. Pre-transplant Th1 and post-transplant Th2 cytokine patterns are associated with early acute rejection on renal transplant recipients. Clin Transplant. 2003;17:151–7. doi: 10.1034/j.1399-0012.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 21.Tinckam K, Rush D, Hutchinson I, et al. The relative importance of cytokine gene polymorphisms in the development of early and late acute rejection and six-month renal allograft pathology. Transplantation. 2005;79:836–41. doi: 10.1097/01.tp.0000155187.81806.df. [DOI] [PubMed] [Google Scholar]
- 22.Loucaidou M, Stitchbury J, Lee J, et al. Cytokine polymorphisms do not influence acute rejection renal transplantation under tacrolimus-based immunosuppression. Transplant Proc. 2005;37:1760–1. doi: 10.1016/j.transproceed.2005.03.151. [DOI] [PubMed] [Google Scholar]
- 23.Verma N, He XY, Chen J, et al. Interleukin 12 delays allograft rejection: effect mediated via nitric oxide. Transplant Proc. 2001;33:416–17. doi: 10.1016/s0041-1345(00)02074-1. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald JT, Johnson JR, Perez RV. Pre-transplant elevations of interleukin-12 and interleukin-10 are associated with acute rejection after renal transplantation. Clin Transplant. 2004;18:434–9. doi: 10.1111/j.1399-0012.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 25.Butani L, Johnson J, Troppmann C, McVicar J, Perez RV. Predictive value of pretransplant inflammatory markers in renal allograft survival and rejection in children. Transplant Proc. 2005;37:679–81. doi: 10.1016/j.transproceed.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann SC, Stanley EM, Cox ED, et al. Ethnicity greatly influences cytokine gene polymorphism distribution. Am J Transplant. 2002;2:560–7. doi: 10.1034/j.1600-6143.2002.20611.x. [DOI] [PubMed] [Google Scholar]
- 27.Goriely S, Goldman M. The interleukin-12 family: new player in transplantation immunity? Am J Transplant. 2007;7:278–84. doi: 10.1111/j.1600-6143.2006.01651.x. [DOI] [PubMed] [Google Scholar]
- 28.Grebe SO, Mueller TF. Immune monitoring in organ transplantation using neopterin. Curr Drug Metab. 2003;3:189–202. doi: 10.2174/1389200024605109. [DOI] [PubMed] [Google Scholar]
- 29.Margreiter R, Fuchs D, Hausen A, et al. Neopterin as a new biochemical marker for diagnosis of allograft rejection. Experience based upon evaluation of 100 consecutive cases. Transplantation. 1983;36:650–3. doi: 10.1097/00007890-198336060-00013. [DOI] [PubMed] [Google Scholar]
- 30.Schafer AJ, Daniel V, Dreikorn K, Opelz G. Assessment of plasma neopterin in clinical kidney transplantation. Transplantation. 1986;41:454–9. doi: 10.1097/00007890-198604000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Reibnegger G, Aichberger C, Fuchs D, et al. Postransplant neopterin excretion in renal allograft recipients − a reliable diagnostic aid for acute rejection and a predictive marker of long-term graft survival. Transplantation. 1991;52:58–63. doi: 10.1097/00007890-199107000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Lee PH, Huang MT, Chung YC, et al. Monitoring of serum neopterin in renal transplant recipients. J Formos Med Assoc. 1992;91:1209–12. [PubMed] [Google Scholar]
- 33.Weimer R, Susal C, Yildiz S, et al. Post-transplant sCD30 and neopterin as predictors of chronic allograft nephropathy: impact of different immunosuppressive regimens. Am J Transplant. 2006;6:1865–74. doi: 10.1111/j.1600-6143.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- 34.Brandacher G, Cakar F, Winkler C, et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int. 2007;71:60–7. doi: 10.1038/sj.ki.5002023. [DOI] [PubMed] [Google Scholar]
- 35.Jungraithmayr TC, Reschke M, Grebe SO, Lange H, Radsak K, Mueller TF. Assessment of cytomegalovirus infections using neopterin and a new immunoblot. Clin Chim Acta. 2001;310:63–9. doi: 10.1016/s0009-8981(01)00528-9. [DOI] [PubMed] [Google Scholar]


