Abstract
The involvement of excessive T helper 1 (Th1) cell functions in the pathogenesis of Behçet's disease (BD) has been reported. We therefore studied Toll-like receptor (TLR)-expressing cells, which play important roles in innate immunity in patients with BD. Peripheral blood mononuclear cells (PBMC) of BD and healthy controls, and tissue specimens of intestinal BD and Crohn's disease (CD) were analysed for messenger RNA (mRNA) and protein expressions by reverse transcription–polymerase chain reaction and immunostaining respectively. PBMC of BD expressed TLR-2 and TLR-4 mRNA almost comparable with healthy controls. Intestinal lesions of BD expressed TLR-2 and TLR-4 mRNA consistently. In contrast, TLR-4 mRNA was expressed preferentially and TLR-2 mRNA was expressed less frequently in CD lesions. In intestinal samples of BD, TLR-2 and TLR-4 mRNA were detected in ileocaecal ulcer lesions, but not in unaffected sites of the same sample, indicating the association of the TLR expression with the disease manifestation of intestinal BD. TLR-2-expressing cells which were simultaneously cluster of distribution (CD)68-positive produced interleukin (IL)-12 in the lesions, indicating the participation of TLR-2-expressing cells in the Th1 skewed responses in vivo. As a possible ligand of TLR-2, in BD self-heat shock protein 60 was expressed in peripheral blood lymphocytes and intestinal tissues. Collectively, TLR-2-expressing cells as well as TLR-4-expressing cells accumulated in the intestinal lesions of BD. IL-12 produced by TLR-2-expressing cells may contribute to the induction of Th1-dominant immune responses in intestinal BD.
Keywords: Behçcet's disease, heat shock protein, Toll-like receptor
Introduction
Behçet's disease (BD) is an inflammatory disorder of unknown cause, characterized by recurrent oral aphthous ulcers, skin lesions, genital ulcers and uveitis [1]. Both genetic factors and environmental factors play a role in the pathogenesis of this disease [2–4]. Immune dysfunction was reported in BD [5]. Active lesions of BD, including those induced during the pathergy test, are infiltrated by neutrophils in the absent of infection. At 48 h, the dermis in the pathergy reaction was infiltrated predominantly with mononuclear cells composed mainly of T lymphocytes and monocytes/macrophages, suggesting the involvement of T lymphocytes in BD progression [6,7].
Recent studies disclosed the involvement of excessive T helper 1 (Th1) cell functions and heat shock protein (HSP) expression in the pathogenesis of this disease [8]. We have shown previously that ectopic expression of self-HSP led to the excessive activation of self-HSP-reactive Th1 cells [9,10].
Intestinal BD is a subtype of BD, involving intestinal ulcers associated with abdominal pain and lower gastrointestinal bleeding [11]. Intestinal BD recurs frequently but its precise prevalence rate remains obscure, although it is relatively high in far eastern countries, especially in Japan [1].
Much remains unanswered about the pathogenesis of intestinal BD. Activated CD8+ T cell participation in its pathogenesis was reported using peripheral blood lymphocytes (PBL) of intestinal BD [12]. We have reported previously the participation of Th1 cells expressing Txk in its pathogenesis [9].
Recently, it has been revealed that Toll-like receptors (TLRs) are involved in the pathogenesis of inflammatory bowel diseases (IBD) [13,14]. In particular, the importance of TLR-4-expressing cells has been emphasized. The aims of this study were to elucidate whether cells expressing TLRs were involved in the pathogenesis of intestinal BD. To this end, we studied TLR messenger RNA (mRNA) expression in the PBL and intestinal lesions from patients with intestinal BD, compared with those from patients with Crohn's disease (CD). We analysed HSP60 expression of the same preparations in parallel, and found TLR-2-expressing cells which were CD68-positive with interleukin (IL)-12 production in the lesions of intestinal BD, which may contribute to Th1 cell accumulation in intestinal lesions.
Patients and methods
Patients
We studied 12 patients with BD [mean age ± standard deviation (s.d.)] of 37·8 + 8·3 year. (range 28–55) (Tables 1 and 2). Patients fulfilled the diagnostic criteria proposed by both the BD Research Committee of Japan and the International Study Group of BD [15,16]. Twelve gender- and age-matched healthy volunteer blood donors served as control subjects (mean age ± s.d.) of 37·5 ± 6·2 years (range 26–53). None of the patients were being treated with intermediate–high-dose corticosteroid therapy (more than 5 mg prednisone/day) or immunosuppressive therapy at the time of examination, except those shown in Table 2. Disease activity was evaluated by clinical symptoms and appropriate laboratory investigations [17]. Human study committee approval and individual informed consent from each patient were obtained before this study, which was conducted in compliance with the Declaration of Helsinki. Four BD patients underwent surgical resection and diagnostic biopsy of the intestines. Patients with CD served as a control. Clinical data of the patients whose intestinal specimens were used in reverse transcription–polymerase chain reaction (RT–PCR) and immunohistochemical study are summarized in Table 2 and the peripheral blood mononuclear cells (PBMCs) used are summarized in Table 1.
Table 1.
Characteristics of patients with Behçet's disease (BD) whose peripheral blood mononuclear cells were analysed in this study.
| Patients | Gender | Age (years) | Disease duration (years) | Disease type | Medication | |
|---|---|---|---|---|---|---|
| BD5 | M | 48 | 16 | C | (E, M) | PSL 3 mg/day |
| BD6 | F | 38 | 5 | I | (M, C, A) | PSL 5 mg/day |
| BD7 | M | 37 | 15 | C | (E, M, C, A) | COL 0·5 mg/day |
| BD8 | F | 45 | 4 | C | (E, M C, A) | COL 0·5 mg/day |
| BD9 | M | 30 | 1 | I | (M, C, A) | COL 1 mg/day |
| BD10 | F | 36 | 5 | I | (M, C, A) | COL 0·5 mg/day |
| BD11 | F | 28 | 8 | C | (E, M C, A) | COL 0·5 mg/day, PSL 5 mg/day |
| BD12 | M | 28 | 2 | I | (M, C, A) | PSL 5 mg/day |
C, complete type; I, incomplete type; E, eye symptom; M, mucosal symptom; C, cutaneous symptom; A, arthralgia; COL, colchicine; PSL, prednisone.
Table 2.
Characteristics of patients with inflammatory bowel diseases in this study.
| Patients | Gender | Age (years) | Disease duration (years) | Location of the lesion | Procedure | Medication |
|---|---|---|---|---|---|---|
| BD1 | F | 37 | 2 | IL | Resection | PSL 50 mg/day, AZP 100 mg/day |
| BD2 | M | 31 | 5 | CO | Biopsy | None |
| BD3 | M | 55 | 22 | CO | Biopsy | PSL 10 mg/day, SASP 3000 mg/day |
| BD4 | F | 40 | 1 | CO | Biopsy | PSL 30 mg/day |
| CD1 | F | 32 | 5 | IL/CO | Biopsy | Naproxen 300 mg/day |
| CD2 | M | 26 | 7 | CO | Biopsy | AZP50mg/day, mesalazine 3000 mg/day |
| CD3 | M | 32 | 1 | IL/CO | Biopsy | Mesalazine 3000 mg/day |
| CD4 | M | 29 | 1 | IL/CO | Biopsy | Mesalazine 1500 mg/day |
BD, Behçet's disease; IL, ileum; CO, colon; PSL, prednisone; AZP, azathioprine; SASP, salazosulphapyridine.
Separation of PBMC
Peripheral blood mononuclear cells were obtained by Ficoll-Hypaque centrifugation of heparinized blood from normal healthy donors and patients with BD. Freshly isolated PBMC were suspended in RPMI-1640 medium containing 10% fetal calf serum (MP Biomedicals, Inc., Aurora, OH, USA), penicillin and streptomycin [18].
Reverse transcription–polymerase chain reaction
Total RNA extraction and cDNA synthesis from PBL and intestinal specimens have been reported [19]. Cyclingparameters were denaturing at 94°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 60 s. The reaction was repeated 30 times, preceded by hot-starting at 94°C for 2 min and followed by elongation at 72°C for 10 min. Sequences of the primers were as follows: HSP60 [309 base pairs (bp)], sense TGTTTTGGGAGGGGGTTGTGC, anti-sense AACAGAGAGGCCACACCAGCA; beta-actin (314 bp), sense TCCTGTGGCATCCACGAAACT, anti-sense GAAGCATTTGCGGTGGACGAT. TLR-2 (478 bp), sense ATGAAAATTGATGTGGGCCTG, anti-sense TTACCCAAAATCCTTCCCGC; and TLR-4 (507 bp), sense TGGATACGTTTCCTTATAAG, anti-sense GAAATGGAGGCACCCCTTC.
Quantitative real-time PCR analysis was performed using the abi prismr 7000 Sequence Detection System. The PCR mixture consisted of 10 μL of TaqManR Universal PCR Master Mix (ABI), 1 μl of TLR-2 TaqManR gene expression assays (ABI) and the cDNA samples in a total volume of 20 μl, as recommended by the manufacture. The quantitative PCR data was calculated with the delta cycle threshold method. The threshold cycle (CT) for each sample was calculated and relative mRNA abundance was determined based on the control glyceraldehyde 3-phosphate dehydrogenase abundance.
Immunochemical and immunofluorescence staining
Cryostat sections (5 μm thick) of intestinal lesions were fixed in 4% paraformaldehyde for 15 min. Endogenous peroxidase activity was blocked by incubating sections with 0·03% hydrogen peroxide. All subsequent procedures were performed using Dako LSAB®2 Kit (Dako, Carpinteria, CA, USA). The samples were incubated with primary antibodies and followed by peroxidase conjugated secondary antibodies. The primary antibodies included anti-TLR-2 (eBioscience, San Diego, CA, USA) and anti-HSP60 (Affinity BioReagents, Golden, CO, USA). After colour development, tissue specimens were counterstained with haematoxylin and appropriate control antibodies were included in the staining. For the immunohistochemical procedures, adjacent sections served as negative controls and were processed using identical procedures, except for incubation with an isotype-matched control antibody in each case.
The expression of various antigens was examined by immunofluorescence staining. In this method, the sections were blocked for 2 h in 0·2% Tween 20, 0·2% gelatin in phosphate-buffered saline. The sections were incubated overnight with appropriate primary antibody, then with biotinylated second antibody and finally with Alexa488-conjugated streptavidin (Molecular Probes, Eugene, OR, USA) and Cy3-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA, USA). Fluorescence was recorded with a confocal laser microscope (Carl Zeiss, Jena, Germany). Appropriate control antibodies were included in all experiments.
Results
Toll-like receptor mRNA expression of mononuclear cells in patients with BD
In order to elucidate the involvement of TLRs in the pathogenesis of intestinal BD, we investigated the expression of TLR mRNA.
We first analysed mRNA of TLR-2 and TLR-4 in PBMC from patients with BD and healthy controls by RT–PCR. TLR-2 mRNA and TLR-4 mRNA were detected in four of eight samples from patients with BD and in four of eight samples from healthy control under this PCR condition. BD-specific expressions of TLR-2 and TLR-4 were not found in PBMC.
We performed pathological examination of the intestinal lesions in patients with BD. All specimens showed variable dense focal mononuclear cell infiltration in haematoxylin and eosin-stained frozen sections (Fig. 1a–c). Ulcer formation was noted and mononuclear cell infiltration was observed in mucosal and submucosal tissue in intestinal lesions in BD. We then analysed mRNA expressions of TLR-2 and TLR-4 in intestinal lesions, using intestinal tissue samples from a patient with BD who underwent surgical resection. Expressions of TLR-2 and TLR-4 mRNA were detected in the ulcer lesion, while there was no TLR expression in the normal limb of the ulcer (Fig. 1d, normal and ulcer).
Fig. 1.
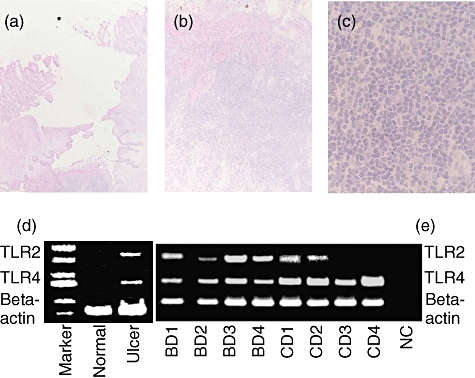
Histological examination and reverse transcription– polymerase chain reaction (RT–PCR) analysis of the intestinal lesions of Behçet's disease (BD). (a) Haematoxylin and eosin staining of an intestinal lesion of BD. An intestinal ulcer of BD was infiltrated by inflammatory cells. Results of a representative case of BD are shown. Magnification, × 2. (b) Higher magnification of (a). Most mononuclear cells. A very small number of neutrophils also infiltrated the site. Magnification, × 10. (c) Higher magnification of the (a). Magnification ×100. (d) RT–PCR analysis of Toll-like receptor (TLR) mRNA expression of the intestinal lesion of BD. TLR-2 and TLR-4 mRNA expressions were analysed. As control diseases, intestinal lesions of patients with Crohn's disease (CD) were included. NC, negative control (DDW).
We further analysed TLR-2 mRNA and TLR-4 mRNA expressions in intestinal lesions from three additional patients who underwent diagnostic biopsy. To investigate whether TLR expression was specific for BD, intestinal lesions of four CD patients were analysed similarly. TLR-4 mRNA was detected in all intestinal samples, including BD and CD. On the other hand, TLR-2 mRNA was detected in all samples from patients with BD. Half the samples from CD expressed TLR-2 mRNA in this PCR condition, suggesting that TLR-2 expression predominated in intestinal lesions of BD (Fig. 1e). To confirm the finding, we conducted quantitative real-time PCR and calculated delta CT values. Delta CT of BD patient 1 in Fig. 1e was −1·33.
Delta CT of CD patients 2, 3 and 4 was 1·96, 0·56 and 0·99 respectively. Thus, TLR-2 mRNA expression was less abundant in patients with CD compared with that in patients with intestinal BD.
We then characterized cells infiltrating the intestinal lesions by immunostaining. Almost all were mononuclear cells in the intestinal lesions of BD (Fig. 1). To confirm TLR-2 protein expression in the intestinal lesions of patients with BD, we analysed intestinal lesions from a BD patient by immunohistochemistry. Consistent with TLR-2 mRNA expression, TLR-2 protein was detected in infiltrating mononuclear cells in intestinal lesions (Fig. 2c). Intestinal lesions of patients with CD were analysed similarly. There were many infiltrating cells in the lesion of CD (Fig. 2b); however, TLR-2 cells were scarcely detected in the lesion of a CD patient (Fig. 2d).
Fig. 2.
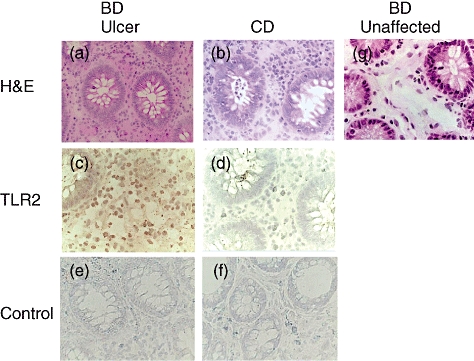
Characterization of Toll-like receptor (TLR)-2-expressing cells in the affected site of intestinal Behçet's disease (BD). (a) Haematoxylin and eosin (H&E) staining of an intestinal lesion of BD. (b) H&E staining of an intestinal lesion of Crohn's disease (CD). (c) Immunohistochemical staining with anti-TLR-2 monoclonal antibodies (mAb) of the affected site of intestinal BD. (d) Immunohistochemical staining with anti-TLR-2 mAb of the affected site of CD. We found hardly any TLR-2-positive cells in the lesion in this CD patient. (e) Immunohistochemical staining with control mouse immunoglobulin G (IgG) of the affected site of intestinal BD. (f) Immunohistochemical staining with control mouse IgG of the affected site of CD. (g) H&E staining of the unaffected site of resected intestine in patients with intestinal BD, where infiltrating mononuclear cells were detected marginally.
To characterize TLR-2-expressing cells in intestinal lesions of BD we conducted two-colour staining, and found that TLR-2-expressing cells were CD68-positive (Fig. 3). More than 98% of CD68-positive cells were TLR-2-positive in the intestinal lesions of BD. There were many CD68-positive cells in the intestinal lesions of CD, and some were also TLR-2-positive. Indeed, 64·7 + 22·3% (mean ± s.d. of four samples) of CD68-positive cells were TLR-2-positive in patients with CD.
Fig. 3.
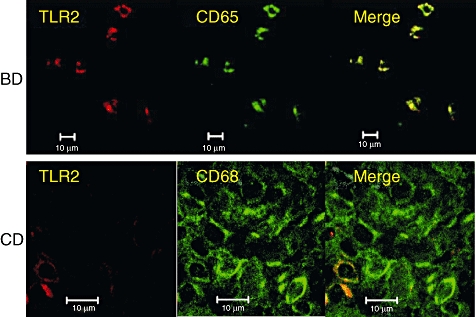
Two-colour analysis of intestinal lesions of Behçet's disease (BD) and Crohn's disease (CD) with anti-Toll-like receptor (TLR)-2 and anti-CD68 monoclonal antibodies (mAbs). We found that more than 98% of CD68-positive cells were TLR-2 positive in four patients with intestinal BD. There were many CD68-positive cells in the intestinal lesions of CD, and some were TLR-2-positive. Indeed, 64·7 ± 22·3% (mean ± standard deviation of four samples) of CD68-positive cells were TLR-2-positive in patients with CD. Scale bars represent 10 μm.
When we studied intestinal lesions of BD, TLR-2-expressing cells produced IL-12 (Fig. 4), important for Th1 cell-dominant immune responses, and TLR-2-positive cells were located in neighbouring CD3-positive cells (Fig. 4).
Fig. 4.
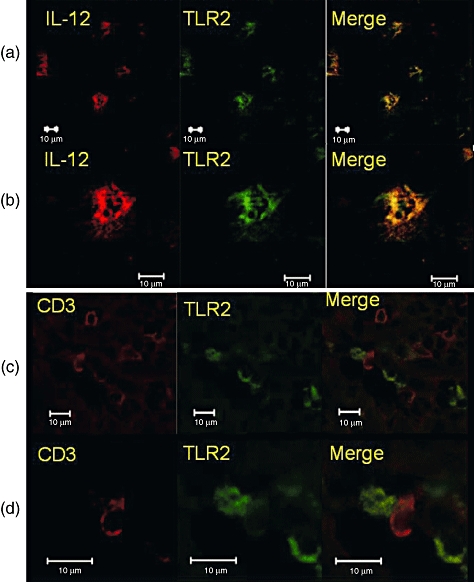
Two-colour analysis of cells infiltrating an intestinal lesion of Behçet's disease (BD). (a) Two-colour analysis of an intestinal lesion of BD with anti-Toll-like receptor (TLR)-2 and anti-interleukin (IL)-12 monoclonal antibodies (mAbs). Lower magnification. (b) Two-colour analysis of an intestinal lesion of BD with anti-TLR-2 and anti-IL-12 mAbs. Higher magnification. (c) Two-colour analysis of an intestinal lesion of BD with anti-TLR-2 and anti-CD3 mAbs. Lower magnification. (d) Two-colour analysis of an intestinal lesion of BD with anti-TLR-2 and anti-CD3 mAbs. Higher magnification. Scale bars represent 10 μm.
Expression of HSP60 in intestinal lesions in patients with BD
Several reports have mentioned the expression of self-HSP in BD. We detected the expression of self-HSP60 in the skin lesions and intestinal lesions of patients with BD [9,10], and reconfirmed the above findings. Human HSP60 mRNA and protein were expressed in intestinal lesions of BD (Fig. 5), especially cells accumulating in ulcerative lesion. HSP60 may become a trigger of TLR-associated immune responses leading to Th1 skewed responses in the intestine.
Fig. 5.
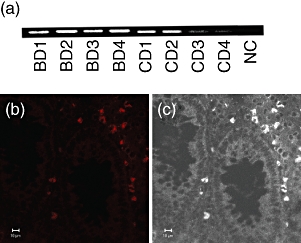
Heat shock protein (HSP) expression of intestinal lesions of Behçet's disease (BD). (a) Reverse transcription–polymerase chain reaction (RT–PCR) analysis of HSP60 expression of the intestinal lesions of BD patients. Intestinal specimens were used to extract total RNA, and the RNA was reverse-transcribed for PCR amplification. Intestinal lesions of four BD patients and four Crohn's disease (CD) patients were analysed. Beta-actin PCR was the same as Fig. 1d, and was thus omitted. NC, negative control. (b) Confocal microscopic analysis of HSP60 expression of the intestinal lesions of BD patients. (c) White/black image of (b) shows the location of HSP-expressing cells. Scale bars represent 10 μm for (b) and (c).
Discussion
Accumulating evidence suggests the pathophysiological role of Th1 skewed T cell immunity in BD [20–22]; however, the mechanisms responsible for Th1 skewed immunity remain obscure.
In this study, we examined TLR expression in the intestinal lesions of intestinal BD to study whether innate immunity has some association with Th1 skewed immunity in this disease. In addition, HSP60 expression and IL-12 production were studied similarly in the intestinal lesions of this disease.
It has been reported that plasma levels of IL-12 correlate with disease activity in BD [23]. TLR-expressing macrophages produce IL-12 and induce Th1 cell responses by suppressing Th2 cell responses [24].
A major inducer of the inflammatory response to Gram-negative bacteria is lipopolysaccharide (LPS), derived from the outer envelope of these bacteria. TLR-2 and TLR-4 are important receptors of pathogen-associated molecular patterns and, in humans, TLR- and TLR-4 may be activated by LPS [25,26].
Gram-negative enteropathogens such as Salmonella minnesota or Escherichia coli activate TLR-4 predominantly in human monocytes [27], whereas LPS from the Gram-negative oral pathogen Porphyromonas gingivalis may stimulate TLR-2 and TLR-4 [28]. We found that both TLR-2 and TLR-4 mRNA expressions were detected in intestinal lesions of BD. TLR-2 mRNA expressions in intestinal lesions of CD were less frequent than TLR-4 mRNA expressions of the same lesions. It is possible that an unidentified pathogen in intestinal BD stimulates both TLR-2 and TLR-4 almost equally. To characterize the lesions, we counted and calculated TLR-2+/CD68+ cells by two-colour immunofluorescence analysis, and found that almost all the infiltrating CD68-positive cells were TLR-2-positive in patients with BD.
Toll-like receptors have been described as sensors for pathogen-associated molecules crucial for the initiation of an innate immune response. Exogenous TLR ligands include lipoteichoic acid, LPS, cytosine–phosphate–guanosine motifs of bacterial DNA and viral DNA [29–32]. Recently, many studies have addressed the role of endogenous ligands in TLR-mediated cell activation [33–36]. Intriguingly, such endogenous ligands, including HSP, are released upon tissue damage and cell stress, events that are likely to occur in inflammatory conditions. It is tempting to speculate that an immune response to endogenous components plays a role in the initiation of autoimmune diseases. TLRs are implicated in the breakdown of tolerance, as shown in experimental models of CD, diabetes and multiple sclerosis [37–39]. In our preliminary experiments, using enzyme-linked immunosorbent assay we measured IL-12 production by PBMC stimulated with bacterial components. We found that anti-TLR-2 blocking antibody partly inhibited IL-12 production, suggesting further the importance of TLR-2 in the pathogenesis of BD.
Recent studies of the innate immune system have shown that HSP60/65 is a ligand for TLR-2 and TLR-4 [40,41]. It has been reported that not only bacterial but also mammalian HSPs interact with TLRs [42]. Human HSP60 is the first example of non-pathogen-derived ligands of TLRs. It is possible that HSP60/65 acts as an endogenous and/or exogenous signal to trigger immune responses, including the induction of proinflammatory cytokine release and the enhancement of adaptive Th1-type responses. We have shown here the co-presence of HSP60, TLR-2 and IL-12 in the lesions, and we should be aware that mononuclear cells activated with various antigens might express both TLR-2 and IL-12. When we looked for HSP60 expression in the normal intestine of the BD patients, we found that HSP60 was not expressed in the normal intestine of the BD patients.
Activation of both innate and adaptive responses with HSPs may be involved in the development of the clinical features of intestinal BD. These results suggest that TLR-2-expressing cells play a pivotal role in priming for destructive Th1-type responses at sites of local HSP60 release in this disease. It should be noted that there is huge variation in the duration of disease within the BD group and control group. It is possible that TLR-2/HSP60 interaction plays distinctive roles in disease initiation, the established disease and the disease during treatment. In addition, the numbers of IBD are too low to make definite conclusions. We therefore need to study further its role in various disease processes.
In this study, we revealed the dominant infiltration of TLR-2- and TLR-4-expressing cells in ileocecal mucosa. TLR-2/HSP60 interaction may be important for the pathogenesis and progression of ileocecal inflammation of BD. Modalities inhibiting self-HSP60 expression may be applicable for intestinal BD. We hope that our findings of the pathogenesis of intestinal BD may contribute to the development of a new therapeutic strategy for this disease in the near future.
References
- 1.Sakane T, Takeno M, Suzuki N, et al. Behçet's disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 2.Lehner T. Immunopathogenesis of Behçet's disease. Ann Med Interne. 1999;150:483–7. [PubMed] [Google Scholar]
- 3.Karasneh J, Gul A, Ollier WE, et al. Whole-genome screening for susceptibility genes in multicase families with Behçet's disease. Arthritis Rheum. 2005;52:1836–42. doi: 10.1002/art.21060. [DOI] [PubMed] [Google Scholar]
- 4.Yurdakul S, Hamuryudan V, Yazici H. Behçet syndrome. Curr Opin Rheumatol. 2004;16:38–42. doi: 10.1097/00002281-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Zouboulis CC. HLA-independent antibacterial host response toward Th1 immunity mediated by IL-12: a new concept for the pathogenesis of Adamantiades–Behçet's disease. J Invest Dermatol. 2006;126:1444–7. doi: 10.1038/sj.jid.5700281. [DOI] [PubMed] [Google Scholar]
- 6.Gul A, Esin S, Dilsen N, et al. Immunohistology of skin pathergy reaction in Behçet's disease. Br J Dermatol. 1995;132:901–7. doi: 10.1111/j.1365-2133.1995.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 7.Melikoglu M, Uysal S, Krueger JG, et al. Characterization of the divergent wound-healing responses occurring in the pathergy reaction and normal healthy volunteers. J Immunol. 2006;177:6415–21. doi: 10.4049/jimmunol.177.9.6415. [DOI] [PubMed] [Google Scholar]
- 8.Direskeneli H, Saruhan-Direskeneli G. The role of heat shock proteins in Behçet's disease. Clin Exp Rheumatol. 2003;21:S44–8. [PubMed] [Google Scholar]
- 9.Imamura Y, Kurokawa MS, Yoshikawa H, et al. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behçet's disease. Clin Exp Immunol. 2005;139:371–8. doi: 10.1111/j.1365-2249.2005.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagafuchi H, Takeno M, Yoshikawa H, et al. Excessive expression of Txk, a member of the Tec family of tyrosine kinases, contributes to excessive Th1 cytokine production by T lymphocytes in patients with Behçet's disease. Clin Exp Immunol. 2005;139:363–70. doi: 10.1111/j.1365-2249.2004.02688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takada Y, Fujita Y, Igarashi M, et al. Intestinal Behçet's disease − pathognomonic changes in intramucosal lymphoid tissues and effect of a ‘rest cure’ on intestinal lesions. J Gastroenterol. 1997;32:598–604. doi: 10.1007/BF02934108. [DOI] [PubMed] [Google Scholar]
- 12.Naganuma M, Iwao Y, Inoue N, et al. Analysis of clinical course and long-term prognosis of surgical and nonsurgical patients with intestinal Behçet's disease. Am J Gastroenterol. 2000;95:2848–51. doi: 10.1111/j.1572-0241.2000.03198.x. [DOI] [PubMed] [Google Scholar]
- 13.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 14.Hart AL, Al-Hassi HO, Rigby RJ, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima Y. Behçet's Disease Research Committee of Japan. Annual report of Behçet's Disease Research Committee of Japan, 1986. Tokyo: Ministry of Health and Welfare of Japan; 1987. Criteria for diagnosis of Behçet's disease; pp. 11–17. [Google Scholar]
- 16.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 17.Sakane T. Annual report of Behçet's Disease Research Committee of Japan, 1994. Tokyo: Ministry of Health and Welfare of Japan; 1995. Criteria for activity of Behçet's disease; pp. 188–90. Behçet's Disease Research Committee of Japan. [Google Scholar]
- 18.Suzuki N, Kaneko S, Ichino M, et al. In vivo mechanisms for the inhibition of T lymphocyte activation by long-term therapy with tacrolimus (FK-506): experience in patients with Behçet's disease. Arthritis Rheum. 1997;40:1157–67. doi: 10.1002/art.1780400622. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwakura J, Suzuki N, Nagafuchi H, et al. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. J Exp Med. 1999;190:1147–54. doi: 10.1084/jem.190.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koarada S, Haruta Y, Tada Y, et al. Increased entry of CD4+ T cells into the Th1 cytokine effector pathway during T-cell division following stimulation in Behçet's disease. Rheumatology (Oxf) 2004;43:843–51. doi: 10.1093/rheumatology/keh195. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Yang P, Zhou H, et al. T-bet expression is upregulated in active Behçet's disease. Br J Ophthalmol. 2003;87:1264–7. doi: 10.1136/bjo.87.10.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamzaoui K, Hamzaoui A, Guemira F, et al. Cytokine profile in Behçet's disease patients. Relationship with disease activity. Scand J Rheumatol. 2002;31:205–10. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 23.Turan B, Gallati H, Erdi H, et al. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in Behçet's disease; soluble TNFR-75 as a biological marker of disease activity. J Rheumatol. 1997;24:128–32. [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 25.Kirschning CJ, Wesche H, Merrill Ayres T, et al. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–7. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow JC, Young DW, Golenbock DT, et al. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 27.Tapping RI, Akashi S, Miyake K, et al. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–7. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 28.Darveau RP, Pham TT, Lemley K, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–51. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwandner R, Dziarski R, Wesche H, et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 31.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi K, Burkart V, Flohe S, et al. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 34.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 36.Kariko K, Ni H, Capodici J, et al. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi M, Kweon MN, Kuwata H, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zipris D, Lien E, Xie JX, et al. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J Immunol. 2005;174:131–42. doi: 10.4049/jimmunol.174.1.131. [DOI] [PubMed] [Google Scholar]
- 39.Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J Clin Invest. 2004;113:990–7. doi: 10.1172/JCI19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohashi K, Burkart V, Flohe S, et al. Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 41.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 42.Warger T, Hilf N, Rechtsteiner G, et al. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J Biol Chem. 2006;281:22545–53. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]


