Abstract
INITIO is an open-labelled randomized trial evaluating first-line therapeutic strategies for human immunodeficiency virus-1 (HIV-1) infection. In an immunology substudy a tetanus toxoid booster (TTB) immunization was planned for 24 weeks after initiation of highly active antiretroviral therapy (HAART). All patients had received tetanus toxoid immunization in childhood. Generation of proliferative responses to tetanus toxoid was compared in two groups of patients, those receiving a protease inhibitor (PI)-sparing regimen (n = 21) and those receiving a PI-containing (n = 54) regimen. Fifty-two participants received a TTB immunization [PI-sparing (n = 15), PI-containing (n = 37)] and 23 participants did not [PI-sparing (n = 6) or PI-containing (n = 17)]. Cellular responses to tetanus antigen were monitored by lymphoproliferation at time of immunization and every 24 weeks to week 156. Proportions with a positive response (defined as stimulation index ≥ 3 and Δ counts per minute ≥ 3000) were compared at weeks 96 and 156. All analyses were intent-to-treat. Fifty-two participants had a TTB immunization at median 25 weeks; 23 patients did not. At weeks 96 and 156 there was no evidence of a difference in tetanus-specific responses, between those with or without TTB immunization (P = 0·2, P = 0·4). There was no difference in the proportion with response between those with PI-sparing or PI-containing regimens at both time-points (P = 0·8, P = 0·7). The proliferative response to tetanus toxoid was unaffected by initial HAART regimen. Anti-tetanus responses appear to reconstitute eventually in most patients over 156 weeks when treated successfully with HAART, irrespective of whether or not a TTB immunization has been administered.
Keywords: HAART, HIV-1, NNRTI, PI, tetanus toxoid
Introduction
Highly active antiretroviral therapy (HAART), typically a combination of two nucleoside analogues with either a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI), has been shown to be equally effective at reducing levels of plasma human immunodeficiency virus-1 (HIV-1). The INITIO trial is an open-labelled randomized trial to evaluate different therapeutic strategies of combination therapy for HIV-1 infection. The immunology substudy of INITIO compared regimens utilizing two NRTI (didanosine + stavudine) combined with NNRTI (efavirenz: noPI); or combined with a PI (nelfinavir ± efavirenz: PI), on the generation of tetanus toxoid-specific immune responses following tetanus toxoid boost (TTB) immunization. The patients were naive for antiretroviral therapy (ART) at the time of entry into the trial and were followed up for a period of 196 weeks (3 years) [1,2]. TTB immunization is recommended in all individuals every 10 years to maintain protection, although efficacy data in HIV-1+ individuals are limited [3–6].
Lymphoproliferative responses (LPR) to various antigenic stimuli are lost during HIV-1 infection as the CD4 count decreases, leading to an increased incidence of opportunistic infections [7]. HAART can restore CD4 levels and reduce HIV-1 load, leading to a reduction in the incidence of opportunistic infections [8–10]. LPR to recall antigens are restored in many individuals following viral suppression [11–13]. Previous in vitro studies have suggested that the presentation and processing of antigen in the context of major histocompatibility complex (MHC) classes I and II could be defective in the presence of PIs [14]. Therefore, this study compared LPR to tetanus toxoid in patients initiating HAART either with or without a PI.
Materials and methods
Trial design
Trial participants initiated HAART with two NRTI drugs (didanosine + stavudine) and in addition were randomized to receive either an NNRTI (efavirenz), a PI (nelfinavir), or both [1,2]. Fifty-two participants received TTB immunization at 25 weeks (noPI n = 15; PI n = 37) and 23 participants did not (noPI n = 6; PI n = 17). The decision to receive TTB immunization or not was made by the patient and clinician and was not a structured randomization process. We compared the generation of tetanus toxoid-specific responses in those receiving TTB immunization and those not boosted in participants receiving either a noPI regimen or a PI regimen. The data were reported as proportion responding, as this was the criterion set at the commencement of this trial by the co-ordinating committee to remove any interlaboratory variation or bias of the results. The Institutional Review Boards of all clinical centres approved the study, and all patients provided their written informed consent. Samples were taken at randomization (week 0) and every 24 weeks until week 156 (3 years).
Proliferation assays
Proliferation assays were carried out using standardized reagents and protocols in Australia, France, Italy, Switzerland and the United Kingdom, as described previously [2]. Briefly, freshly isolated peripheral blood mononuclear cells (105/well) were cultured in 10% AB plasma/RPMI-1640 (Sigma, Poole, UK) with either 5 μg/ml tetanus toxoid (Pasteur Merieux, Marcy I'Etoile, France), 5 μg/ml phytohaemagglutinin mitogen control (Sigma) or 10% AB plasma/RPMI-1640 only (negative control), in round-bottomed microtitre plates (Greiner, Stonehouse, UK) for 5 days. Each well was then pulsed with 1 μCi [3H]-methyl thymidine ([3H]-TdR; Amersham International, Amersham, UK) and 16 h later cells were harvested onto glass fibre filtermats (Wallac Oy, Turku, Finland). Proliferation was measured by liquid scintillation spectroscopy using a 1205 Betaplate counter (Wallac Oy). A positive response is defined as a stimulation index score of ≥ 3 coupled with a Δ counts per minute of > 3000.
Statistical analysis
All participants with data available for immunization and response status at week 24 were included in the analysis. The effect of immunization, week 24 CD4 count, pre-existing week 24 tetanus response and class of HAART received, on the proportion of positive tetanus responders and odds of a positive response at weeks 48, 96 and 156, were investigated using χ2 tests and logistic regression, respectively. Changes in CD4 counts from baseline were calculated using quartile regression. All analyses are intention-to-treat.
Results
Patients
Of the 911 participants of the INITIO trial, 120 were enrolled into the Immunology Substudy. LPR results were available for 102 of these individuals. One participant did not have TTB immunization data recorded, 10 were seen only at baseline and 16 did not have a week 24 result. Therefore, tetanus responses in the remaining 75 participants, enrolled in Australia (23%), France (55%), Italy (5%), Switzerland (11%) and the United Kingdom (7%), were investigated. The decision as to whether to receive a TTB or not was the choice of the patient and their physician and was not a structured randomization process. Participants randomized to receive a PI-sparing regimen (n = 21) were compared with pooled data from all patients receiving a PI, either with or without an NNRTI (n = 54). Fifty-two participants had a TTB immunization at a median of 25 (interquartile range 24–26) weeks from randomization and 23 patients did not. Median CD4 count and the proportion of patients with HIV-1 RNA plasma levels < 50 copies/ml at 24 weeks were similar to those of the whole trial population (Table 1).
Table 1.
CD4 T cell count and plasma human immunodeficiency virus-1 (HIV-1) RNA load at week 24, in the different tetanus toxoid booster immunization (TTB), antiretroviral therapy (ART) and study groups.
| Immunization | Randomized initial treatment | ART | Tetanus substudy | Main trial | |||||
|---|---|---|---|---|---|---|---|---|---|
| TTB | No TTB | EFV | NFV | EFV/NFV | Non-PI | PI | |||
| Number of patients (n) | 52 | 23 | 21 | 27 | 27 | 21 | 54 | 75 | 779 |
| Median CD4 T cell count/μl blood (IQR) | 297 (155–432) | 266 (180–480) | 210 (100–410) | 270 (139–479) | 305 (195–483) | 210 (100–410) | 295 (177–483) | 280 (157–456) | 300 (168–446) |
| % with plasma HIV-1 RNA ≤ 50 copies/ml | 94 | 96 | 95 | 96 | 93 | 95 | 94 | 95 | 93 |
EFV: efavirenz; IQR: interquartile range; NFV: nelfinavir; PI: protease inhibitor.
Effect of HAART
Within the substudy group at the time of TTB immunization, the median CD4 count was higher in participants receiving PI-based regimens; however, this difference was not statistically significant (P = 0·1) (Table 1). There was no evidence of a difference in the magnitude of the increase in CD4 count from randomization between the noPI and PI groups at week 24 (P = 0·2; Fig. 1a). The proportion of participants with HIV-1 RNA plasma levels below the limit of detection (BLD; 50 copies/ml) at the time of TTB immunization was also similar in each treatment group (Table 1). The proportion of participants with a tetanus response did not differ significantly according to initial HAART regimen received at week 24 (noPI 14% versus PI 28%; P = 0·2), week 96 (noPI 35% versus PI 57%; P = 0·8) or week 156 (noPI 50%, PI 59%; P = 0·7; Fig. 1b). A similar proportion of participants who did and did not receive a TTB immunization started HAART with a PI-based regimen [37 (71%) versus 17 (74%), P = 0·8]. There was no evidence of a difference in the proportion with responses to tetanus antigen at weeks 96 or 156 according to immunization status and class of HAART (Fig. 1c).
Fig. 1.
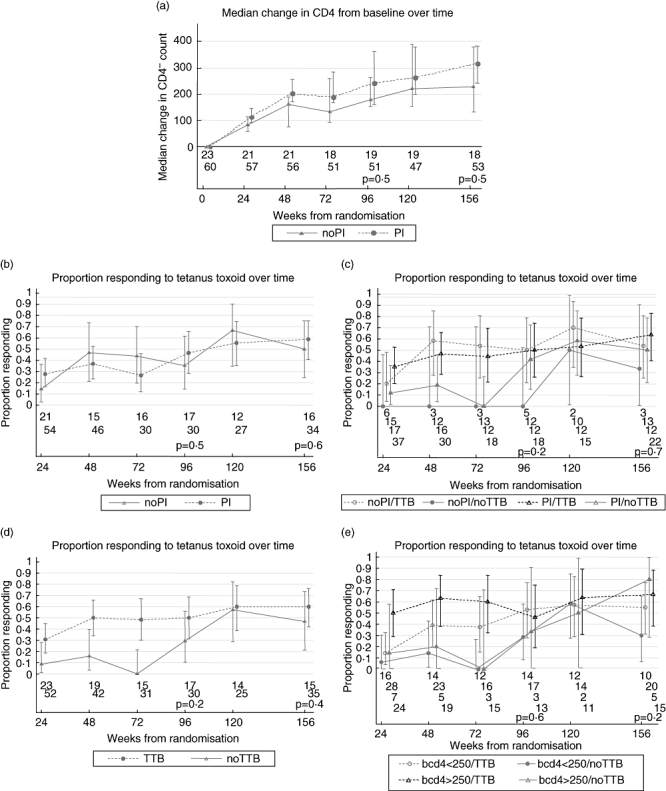
Median change from baseline in CD4 T cell count over the study period (a). Proportion of T cell responders to tetanus toxoid split by: ART group (b); antiretroviral therapy group and immunization group (c); immunization group (d); and baseline CD4 T cell count and immunization group (e). Error bars indicate the 95% confidence intervals.
Effect of TTB immunization
The median CD4 count and proportion with a HIV-1 RNA plasma load BLD at week 24 were similar in those who did and did not receive a TTB immunization (Table 1). Sixteen (31%) participants receiving TTB immunization already had a positive response to tetanus toxoid prior to immunization, compared with two (9%) participants in the no TTB immunization group (P = 0·04). The odds of proliferative response at week 24 in the TTB immunization group were 4·67 [95% confidence interval (CI): 0·98–22·33; P = 0·05] times that of those in the no TTB immunization group. This may be driven by a CD4 count effect, as the odds ratio (OR) was reduced slightly to 4·34 (95% CI: 0·89–21·21; P = 0·07) after adjustment.
By week 48 significantly more TTB immunization recipients made responses to tetanus antigen than those not boosted [21 (50%) versus 3 (16%); P = 0·01]. The odds of response at week 48 were 5·33 (95% CI: 1·35–21·06; P = 0·02) times higher in those receiving TTB immunization than those not, but the difference between the arms was reduced after adjustment for CD4 counts and tetanus response at week 24 (OR 3·81, 95% CI: 0·82–17·72; P = 0·09).
The proportion of non-immunized participants responding to tetanus toxoid increased over time and by week 96 the difference in tetanus-specific responses between those who had received TTB immunization and those who had not had lessened, and the proportion responding no longer differed significantly [15 (50%) versus 5 (29%), P = 0·2]. By week 156 the difference in proportion responding between those who had received TTB immunization and those who had not had reduced further [21 (60%) versus 7 (47%), P = 0·4] (Fig. 1d).
Effect of CD4 count on TTB response
The proportion of participants with week 24 CD4 counts above 250 cells/μl was similar in those with and without a TTB immunization [30 (58%) versus 12 (52%), P = 0·7]. There was no evidence of a difference between responses at week 156 by TTB immunization status and week 24 CD4 count (P = 0·4), although the lowest response was seen in participants with CD4 count ≤ 250 cells/μl who had started a PI-sparing regimen (29% versus 56%, 63%, 63%) (Fig. 1e).
Discussion
This INITIO [1] substudy compared the effect of initiating HAART using/sparing PI and the effect of TTB immunization on the generation of immune responses to tetanus toxoid. The presence of a PI in the HAART regimen had no effect on LPR to tetanus toxoid, whether or not patients received TTB immunization. This concurs with the findings of the main INITIO immunology substudy, which showed no difference between regimens using/sparing PIs in terms of levels of long-term immune reconstitution to a panel of recall antigens [2], despite previous observations that PIs interfere with cell-cycle progression with consequential concentration-dependent inhibition of LPR [15]. Presentation and processing of antigen in the context of MHC classes I and II could be defective in the presence of PIs [14], although this effect appears to occur only at suprapharmacological doses in human cells [16]. Data presented here were obtained while using the PI nelfinavir, and we cannot discount that a ritonavir-boosted PI regimen, now used more commonly, may have an effect on these results, because of the differing pharmacokinetics [17].
By week 156 post-initiation of HAART, no difference in the proportion responding to tetanus toxoid was observed between the TTB immunization and non-TTB groups after 96 weeks, in spite of the initial lower LPR in those not boosted. However, these data suggest that there may be a difference in temporal responses before week 156, as more boosted patients had LPR at week 48 (although higher week 24 LPR in this group suggests that this could be due to a larger proportion of tetanus toxoid-specific memory T cells prior to boost, rather than the immunization itself), whereas non-TTB immunization patients showed a more delayed response, dependent on immunological recovery with time on ART [11–13].
Regimens containing NNRTIs have been shown to provide at least similar improvements in the surrogate markers of HIV-1 infection when compared with PI-containing regimens [18]. Although there was a higher CD4 count at week 24 in the PI group, there was no difference in CD4 T cell number recovery between the two groups. Exploratory analyses suggest a trend towards a lower proportion of responders in those with the lowest CD4 counts at time of immunization, although this study lacks power to address this question definitively, which concurs with data showing that in HIV-1-infected individuals the amount of antibodies formed after immunization with T cell-dependent recall antigens such as tetanus toxoid is proportional to CD4 counts [19]. Moreover, it has been demonstrated that formation of IgG anti-tetanus toxoid antibodies after immunization is impaired in HIV-1-infected adults with CD4 counts below 300 cells/μl blood [20]. We acknowledge that memory T cell responses may not correlate directly with protection from tetanus toxin [21]. However, we illustrate here that the reconstitution of memory immune responses is equivalent under both PI and NNRTI regimens in patients with advanced HIV-1 disease, and that TTB immunization during the initial weeks of HAART does not enhance this level of immune recovery. Indeed, our data concur with previous findings that immune responses to recall antigens are improved with PI-based HAART [22,23]. Here we propose that memory responses to tetanus toxoid are present in many individuals and will eventually regenerate in time, irrespective of HAART regimen choice or TTB immunization.
Acknowledgments
We would like to thank Professor Maxime Seligman for his strong involvement in the organization and co-ordination of this study, which would not have been performed without his determination. The authors would like to thank the investigators and staff at the co-ordinating centres and participating sites, which are listed in full in the main trial report [1]. We would also like to thank all patients who participated in the INITIO trial. This trial was funded by BMS and Dupont (now BMS), and Roche with additional support from GSK. BMS wholly funded the immunology substudies. DuPont and Merck supplied efavirenz and GSK supplied abacavir during the early period of the trial before these drugs were marketed in various countries. Roche Molecular Systems provided products and support for centralized HIV RNA assays. In the United Kingdom the immunology substudy work has also been supported by the Medical Research Council (grant number G0501957), St Stephen's AIDS Trust and AVIP EU Programme (grant number LSHP-CT-2004-503487). In Australia the immunology substudy work has also been supported by the National Centre in HIV Epidemiology and Clinical Research and is supported by the Commonwealth Department of Health and Ageing, Australia. A. D. K. is supported by a programme grant and a practitioner fellowship from the Australian National Health and Medical Research Council.
References
- 1.INITIO Trial International Co-ordinating Committee. Yeni P, Cooper DA, Aboulker JP, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet. 2006;368:287–98. doi: 10.1016/S0140-6736(06)69074-0. [DOI] [PubMed] [Google Scholar]
- 2.Samri A, Goodall R, Burton C, et al. Durable long-term immune reconstitution in PI-sparing and PI-containing antiretroviral regimens in advanced HIV-1 disease. Antivir Ther. 2007;12:553–8. [PubMed] [Google Scholar]
- 3.Gazzard B, Bernard AJ, Boffito M, et al. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 2006;7:487–503. doi: 10.1111/j.1468-1293.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 4.Pozniak A, Gazzard B, Anderson J, et al. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 2003;4(Suppl. 1):1–41. [PubMed] [Google Scholar]
- 5.Gazzard B. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 2005;6(Suppl. 2):1–61. doi: 10.1111/j.1468-1293.2005.0311b.x. [DOI] [PubMed] [Google Scholar]
- 6.Galbraith NS, Forbes P, Tillett H. National surveillance of tetanus in England and Wales 1930–79. J Infect. 1981;3:181–91. doi: 10.1016/s0163-4453(81)91586-3. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. HIV Outpatient Study Investigators. [DOI] [PubMed] [Google Scholar]
- 9.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 10.Pakker NG, Roos MT, van Leeuwen LR, et al. Patterns of T-cell repopulation, virus load reduction, and restoration of T-cell function in HIV-infected persons during therapy with different antiretroviral agents. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:318–26. doi: 10.1097/00042560-199712150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–16. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 12.Li TS, Tubiana R, Katlama C, Calvez V, Ait MH, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 13.Hardy GA, Imami N, Sullivan AK, et al. Reconstitution of CD4+ T cell responses in HIV-1 infected individuals initiating highly active antiretroviral therapy (HAART) is associated with renewed interleukin-2 production and responsiveness. Clin Exp Immunol. 2003;134:98–106. doi: 10.1046/j.1365-2249.2003.02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andre P, Groettrup M, Klenerman P, et al. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci USA. 1998;95:13120–4. doi: 10.1073/pnas.95.22.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavan S, Kodoth S, Pahwa R, Pahwa S. The HIV protease inhibitor indinavir inhibits cell-cycle progression in vitro in lymphocytes of HIV-infected and uninfected individuals. Blood. 2001;98:383–9. doi: 10.1182/blood.v98.2.383. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher AD, Booth BL, Jr, Sewell AK, et al. Effects of retroviral protease inhibitors on the proteasome function and processing of HIV-derived MHC class I restricted cytotoxic T lymphocyte epitopes. AIDS Res Hum Retroviruses. 2001;17:1063–6. doi: 10.1089/088922201300343744. [DOI] [PubMed] [Google Scholar]
- 17.Moyle G. Use of HIV protease inhibitors as pharmacoenhancers. AIDS Read. 2001;11:87–98. [PubMed] [Google Scholar]
- 18.Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 19.Kroon FP, van Tol MJ, Jol-van der Zijde CM, van FR, van Dissel JT. Immunoglobulin G (IgG) subclass distribution and IgG1 avidity of antibodies in human immunodeficiency virus-infected individuals after revaccination with tetanus toxoid. Clin Diagn Lab Immunol. 1999;6:352–5. doi: 10.1128/cdli.6.3.352-355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroon FP, van Dissel JT, Labadie J, van Loon AM, van FR. Antibody response to diphtheria, tetanus, and poliomyelitis vaccines in relation to the number of CD4+ T lymphocytes in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:1197–203. doi: 10.1093/clinids/21.5.1197. [DOI] [PubMed] [Google Scholar]
- 21.Saikh KU, Sesno J, Brandler P, Ulrich RG. Are DNA-based vaccines useful for protection against secreted bacterial toxins? Tetanus toxin test case. Vaccine. 1998;16:1029–38. doi: 10.1016/s0264-410x(97)00280-6. [DOI] [PubMed] [Google Scholar]
- 22.Pontesilli O, Kerkhof-Garde S, Notermans DW, et al. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldo CR, Jr, Liebmann JM, Huang XL, et al. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–36. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]


