Abstract
Subcutaneous immunoglobulin G (SCIG) infusions as life-long replacement therapy in patients with primary antibody deficiences (PAD) is being applied increasingly. However, only a few published pharmacokinetic studies are available for this route of administration. Therefore, the pharmacokinetics of a 16% immunoglobulin G (IgG) preparation intended for subcutaneous use were investigated in patients with common variable immunodeficiency and X-linked agammaglobulinaemia. SCIG infusions (200 mg/kg body weight) were administered to 12 adult patients every 14 days for 24 weeks (total of 144 infusions). Pharmacokinetic parameters were determined based on serum IgG trough levels and antibody levels against tetanus. The median half-life of the total serum IgG and for the tetanus antibodies was 40·6 and 23·3 days respectively. Median in vivo recovery of serum IgG and tetanus immunoglobulins were 36% and 46% respectively. Median, preinfusion serum IgG trough levels per patient were high without major variations between infusions and ranged from 7·24 to 7·86 g/l. Safety, in terms of adverse events including systemic adverse reactions and local tissue reactions at infusions sites, was monitored throughout the study. Six mild, local tissue reactions were observed during the study in one patient. No systemic adverse reactions related to the study drug were observed and no serious other adverse event occurred during the study. It is concluded that the bi-weekly SCIG therapy was well tolerated in the study and that it results in high and stable serum IgG levels, offering an alternative therapy regimen to patients suffering from PAD.
Keywords: gammaglobulin treatment, primary antibody immunodeficiency disorders, SCIG home therapy, self-infusions, subcutaneous IgG replacement therapy
Introduction
In patients suffering from primary antibody deficiencies (PAD), prophylactic replacement therapy with immunoglobulin G (IgG) is the method of choice to prevent infections [1–3].
The IgG preparations were formulated generally either for intramuscular or intravenous (IV) administration. Rapid subcutaneous administration of intramuscular preparations without preservatives [4] or, later, IgG preparations especially intended for subcutaneous use have become increasingly employed in both adults and children, and hundreds of thousands of subcutaneous IgG (SCIG) infusions have been given during the last decades [3–16]. The SCIG replacement therapy is effective, highly appreciated by the patients and families, leads to a better health-related quality of life, has a low risk of systemic adverse reactions and leads to high serum IgG concentrations [3–23]. Weekly infusions of SCIG have been found to prevent infections to the same degree as IV IgG (IVIG) therapy [11,15]. After adequate training in a hospital setting, subsequent infusions are provided as self-infusions at home, thus reducing markedly the yearly costs per patient [3,5]. The rapid SCIG infusions are given most often once every week. An extension of the interval between infusions may facilitate the lifelong replacement therapy in some patients and also be advantageous during travels and vacations.
In the current study we investigated the pharmacokinetic properties and safety in terms of adverse events (AEs), including systemic adverse reactions and local tissue reactions, of a novel immunoglobulin preparation (Subcuvia®) given every second week to patients with PAD.
Materials and methods
Patients
A total of 12 patients were included in the study under the following criteria: at least 18 years old and diagnosed with PAD manifesting as common variable immunodeficiency (CVID) or X-linked agammaglobulinaemia (XLA). At the time of enrolment, all patients were already on subcutaneous treatment (100 mg per kg body weight and week) and all of them were self-administering at home.
Patients with a history of severe systemic adverse reactions to immunoglobulin preparations were excluded from the study. The same was true for patients with severe acute infections requiring treatment with antibiotics at entry, pregnant patients or nursing mothers and patients with known positivity for human immunodeficiency virus, hepatitis C virus or hepatitis B virus. The first 12 consecutive patients who fulfilled the inclusion criteria agreed to participate in the study and signed an informed consent. The median age of the seven women and five men was 62 (range 22–72) years with a median weight of 68·9 (range 52·8–95·9) kg. Eleven patients suffered from CVID and one from XLA (Table 1).
Table 1.
Patient characteristics.
| Patient no. | Sex/age (years) | Diagnosis of patients entered | Pre-treatment IgG level (g/l) | Weeks on s.c. therapy before study start | Interval of s.c. therapy before study start | Trough IgG (g/l) before study start |
|---|---|---|---|---|---|---|
| 1 | F/49 | CVID | 0·7 | 458 | 7 days | 6·5 |
| 2 | F/57 | CVID | 1·6 | 334 | 7 days | 14·2 |
| 3 | M/54 | CVID | 1·5 | 425 | 7 days | 6·9 |
| 4 | F/70 | CVID | 1·5 | 121 | 7 days | 6·6 |
| 5 | M/67 | CVID | 0·7 | 533 | 7 days | 8·6 |
| 6 | M/76 | CVID | 0·6 | 481 | 7 days | 8·0 |
| 7 | M/69 | CVID | 2·8 | 47 | 7 days | 8·5 |
| 8 | M/22 | XLA | 1·6 | 317 | 7 days | 7·1 |
| 9 | F/56 | CVID | 2·0 | 364 | 7 days | 8·4 |
| 10 | F/72 | CVID | 2·9 | 50 | 7 days | 10·9 |
| 11 | F/71 | CVID | 1·4 | 469 | 7 days | 7·4 |
| 12 | F/44 | CVID | 1·2 | 481 | 7 days | 7·5 |
CVID, common variable immunodeficiency; s.c., subcutaneous; XLA, X-linked agammaglobulinaemia.
Infusion technique and immunoglobulin preparation
The study was conducted at the Immunodeficiency Unit, Karolinska University Hospital, Huddinge, Stockholm, Sweden. A solvent/detergent-treated immunoglobulin (160 mg/ml) preparation (Subcuvia®, Baxter AG, Vienna, Austria) was infused. Per patient, a total of 12 infusions of 200 mg of the IgG preparation per kilogram of body weight were administered subcutaneously once every 2 weeks. This corresponds to the recommended dose of 400 mg/kg body weight per month according to the International Union of Immunological Societies Scientific Committee Guidelines [1]. Based on individual body weight the infusion volumes ranged between 66 and 120 ml, divided between multiple infusion sites, corresponding to a dose range of 10·6–19·2 g IgG every second week. All infusions were, for practical reasons, administered at the hospital. The SCIG infusions were administered as described previously [4] using infusion pumps (Medis Infusa T1, Milan, Italy), together with infusion sets with butterfly needles (25 gauge, 0·5 mm, Vycon, Écouen, France). The infusions were given as rapid infusions [4] at a speed of 20 ml/h using 10 ml syringes (Once, Asik, Denmark). Two to four (usually two) pumps were used simultaneously. The infusions were given either in the lower part of the abdominal wall, in the upper, lateral parts of the thighs, or both. A volume of 10–15 ml was given at each infusion site. The infusion time per patient varied from 50 min to 2 h 30 min depending on body weight and number of pumps used.
Assessment of pharmacokinetic parameters
Pharmacokinetic parameters were determined based on serum IgG trough levels (turbidimetry; using the Olympus analytical system and reagents) and antibody levels to tetanus toxoid (enzyme-linked immunosorbent assay; Enzyquick Tetanus, Immuno Diagnostika, Vienna, Austria). Other pharmacokinetic parameters assessed were in vivo recovery (IVR), incremental recovery (IR), maximum concentration (Cmax), time to reach Cmax, area under the curve and clearance.
For determination of pharmacokinetics, a batch with a high tetanus antibody titre (≥ 550 IU/ml) was administered to all patients for the first four infusions. Serum samples for determination of IgG levels were always collected immediately prior to each bi-weekly infusion. For calculation ofIVR and half-life for total IgG and for antibodies to tetanus, serum samples were obtained immediately prior to the third infusion (day 0 of the pharmacokinetics, and on days 1, 2, 4, 6, 8, 10, 12 and 14 (i.e. immediately prior to the next infusion) after the third infusion of the study drug. After the first four infusions, the patients received eight infusions of a batch with a normal tetanus antibody level.
Calculations of pharmacokinetic parameters
The determination of half-life was performed according to the two-phase, log-linear regression model of Lee et al. [24]. For each patient, the terminal elimination rate bt of the best-fitted one- or two-phase model was used to calculate the half-life by the formula:
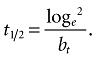 |
IVR was corrected for plasma volume (PV) according to:
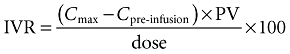 |
where PV preinfusion was calculated using the patient's preinfusion haematocrit (Ht) with the formula:
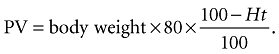 |
Incremental recovery (IR-value) in terms of increases of tetanus anti-toxin/total serum IgG level was calculated as follows:
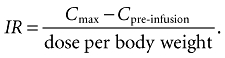 |
Assessment of AEs
Safety in terms of AEs including systemic adverse reactions and local tissue reactions was monitored throughout the study and blood and urine analyses were also performed. Vital signs were assessed at intervals of 30 min for 4 h after initiation of each infusion and thereafter at 12 and 24 h. AEs were recorded daily in a diary by the patients for 2 weeks after each infusion and were graded in five levels of severity corresponding to mild (1), moderate (2), severe (3), life-threatening (4) and death (5). Levels 3, 4 and 5 correspond to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines [25]. ‘Mild’ was defined as showing transient or mild discomfort with no limitation on activities and no therapy needed. ‘Moderate’ was defined as an impact on activities, but patients being able to work full-time with minimal or no medical intervention required.
Statistics
Pharmacokinetic parameters of serum IgG and tetanus antibody titres including IVR and half-life were summarized in the patients where the parameter was available by medians and 95% non-parametric confidence intervals for medians.
Ethics
The study was approved by the local ethical committee at Karolinska University Hospital, Huddinge, Stockholm.
Results
Pharmacokinetics
All patients completed the whole study period and received the planned 12 bi-weekly infusions and subsequent follow-ups. Thus, 144 infusions were administered during the study.
The median half-life determined for total serum IgG was 40·6 days, whereas the median half-life for tetanus antibodies was 23·3 days (Table 2). Median IVR of total serum IgG was 36%, and median IVR of tetanus antibodies 46% (Table 2). Median serum IgG trough levels ranged from 7·24 to 7·86 g/l, being constantly high without major variations. The IgG trough levels over the study period of 24 weeks are shown in Fig. 1 and the increase in total serum IgG levels after infusion in Fig. 2.
Table 2.
Medians of half-life, IVR, IR, Cmax, Tmax, AUC and CL for total serum IgG and tetanus antibody titre with 95% confidence intervals.
| Parameter | Median total serum IgG (with 95% CI) | Median tetanus antibody titre (with 95% CI) |
|---|---|---|
| Half-life days | 40·6 days (CI from 20·1 to 56·1 days) | 23·3 days (CI from 12·7 to 31·3 days) |
| IVR % | 35·8% (CI from 18·1 to 56·4%) | 45·6% (CI from 38·1 to 55·6%) |
| IR g/ml | 7·3 g/ml (CI from 3·8 to 12·4 g/ml) | 9·1 g/ml (CI from 8·4 to 11·3 g/ml) |
| Cmax mg/dl | 8·82 g/l (CI from 8·31 to 10·32 g/l) | 15·5 IU/ml (CI from 14 to 17 IU/ml) |
| Tmax days | 4 days (CI from 4 to 8 days) | 4 days (CI from 2 to 8 days) |
| AUC days × mg/dl | 11 400 days × mg/dl (CI from 10 200 to 11 800 day × mg/dl) | 179 days × IU/ml (CI from 165 to 199 day × IU/ml) |
| CL ml/kg/day | 1·7 ml/kg/day (CI from 1·6 to 1·9 ml/kg/day) | 4·0 ml/kg/day (CI from 3·6 to 4·3 ml/kg/day) |
CI, confidence intervals; IVR, in vivo recovery; IR, incremental recovery; Cmax, maximum concentration; Tmax, time to reach maximum concentration; AUC, area under the curve; CL, clearance. IgG, immunoglobulin G.
Fig. 1.
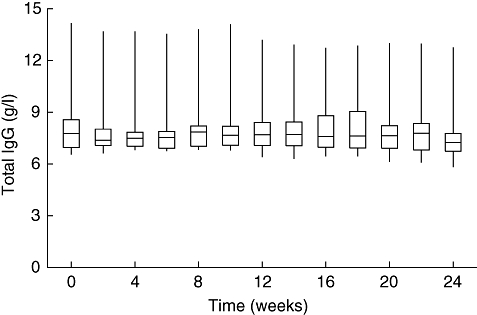
Serum immunoglobulin G (IgG) trough levels (medians, interquartile spreads and ranges) over the study period of 24 weeks.
Fig. 2.
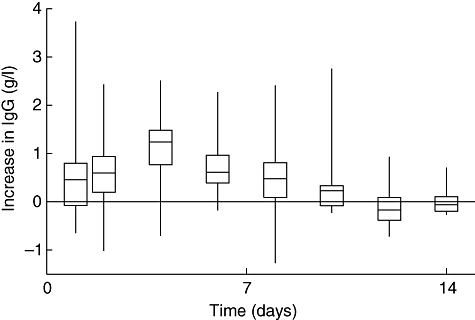
Increase in total serum immunoglobulin G (IgG) trough level over baseline after subcutaneous infusion (medians, interquartile spreads and ranges) for the pharmacokinetic phase of 14 days (normalized values shown).
The medians of the other pharmacokinetic parameters are shown in Table 2.
Safety
One patient suffered mild local tissue reactions at the infusion sites that were related to the study drug. These reactions manifested as local pain, soreness and swelling at the infusion site after the first six infusions. The local tissue reactions subsided spontaneously thereafter. No systemic reactions or other AEs occurred. No major bacterial infections occurred during the study period.
Discussion
The kinetic properties of IVIG in patients with PAD have been evaluated previously and the half-lives for IgG have been reported to be normal [26,27] or prolonged [28–30]. However, there is a paucity of pharmacokinetic data after SCIG administration to patients suffering from PAD and so far only one study has been published [31].
Immunoglobulin G has a half-life of about 3–4 weeks in normal individuals. Immediately after IVIG infusion, the maximum amount of the applied dose is found in the intravascular space, whereas this is not seen until 3–4 days after SCIG or intramuscular administration [32]. The immunoglobulin catabolism is Fc-receptor-mediated and is dependent, at least partly, upon serum IgG concentrations [33]. Following IVIG administration to hypogammaglobulinaemic patients, the half-life of IgG has been reported to be either normal [26,27] or increased [28–30]. Two clinical studies on the pharmacokinetics of IVIG infusions in patients with PAD have been published showing similar results. Alyanakian et al. administered 280 (±60) mg/kg of IVIG every 3 weeks and found a half-life of IgG of 36 (±10·8) days [34] and Ballow et al. used three different IVIG preparations and found the IgG half-lives to range from 34 to 36 days [35]. A half-life of 40·6 days for total IgG was found after SCIG administration in the current study and is well within the reported range in patients with PAD.
For the specific tetanus antibodies, a median half-life of 23·3 days was observed. This is shorter than that of IgG but this finding was not unexpected, as half-lives of specific antibodies have been described frequently to be shorter than the half-life of total IgG. For tetanus immunoglobulin in patients with PAD half-lives of 21–27 days have been reported [36]. In another report, half-lives of 37 and 21 days, respectively, for total IgG and tetanus antibodies after administration of an unmodified IV immunoglobulin preparation have been described [37]. A possible explanation for the differences between the half-lives of total IgG and tetanus antibodies might be that most patients with PAD have a residual intrinsic IgG synthesis which affects serum IgG concentrations and alters the final slope of the IgG decay curve [36], as hypogammaglobulinaemic patients show a consistent failure to produce specific antibodies following test immunizations [38]. A lack of synthesis of specific anti-tetanus antibodies might result in a shorter half-life compared with that of total serum IgG.
Waniewski et al. reported stable IgG serum concentrations between consecutive weekly SCIG infusions with a small peak of the serum IgG level at day 4 [31]. This is consistent with our pharmacokinetic data for total serum IgG, showing a peak at day 4 following a pharmacokinetic phase of 14 days after SCIG infusion (Fig. 2). The fractional catabolic rate of IgG was reported by Waniewski et al. to be 4·1–5·9% per day for CVID patients given SCIG infusions assuming 100% bioavailability of the subcutaneously infused IgG [31]. In contrast, it has been suggested that large boluses of IVIG infusions may result in increased catabolism of IgG [14].
The median IVR of the study drug was 36% for the total IgG and 46% for the tetanus antibodies, which is in agreement with Smith et al., who reported an IVR for serum IgG of 33% after subcutaneous injection of an anti-Rh immunoglobulin [32]. Individual variations of pharmacokinetic parameters among the patients have to be considered for IVR, similar to individual values observed with regard to half-life [27,28,30].
Using the SCIG infusion therapy, serum IgG trough levels have been found to be constantly high without major variations, thus resembling normal physiological conditions [3–5,15,31]. This was confirmed by the data obtained in this study, where serum IgG trough levels after SCIG infusions every second week remained high and without major variations throughout the study period. Data from a retrospective analysis in children have shown that serum IgG trough levels using weekly SCIG treatment are comparable with those from IVIG-treated children [10]. A randomized, cross-over study comparing efficacy and safety of SCIG and IVIG therapy demonstrated that the SCIG administrative route led to as high serum IgG trough levels as the IVIG therapy [11]. Moreover, two recent studies have demonstrated an increase in serum IgG trough levels in patients switching from IVIG to SCIG therapy using the same dosing of IgG [15,16].
In terms of local tissue reactions, Gardulf et al. [5,18] reported that 87% of 152 patients participating in a long-term survey had at least once experienced some form of tissue reaction at the infusion site after subcutaneous administration. However, most patients did not perceive these reactions to be troublesome [18]. Furthermore, local tissue reactions have been reported to decline over time in patients starting with SCIG therapy, most distinctively after 8–10 weeks [15]. The data generated in the current study show that the safety profile of the study drug is consistent with published data on the safety of SCIG replacement using other preparations [3–16].
In the current study, the obtained data showed that SCIG infusions every second week result in the same serum IgG concentrations as weekly infusions [3–16]. For patients with PAD this opens yet another possibility to adapt more effectively the IgG replacement therapy to individual needs during, e.g. vacations or travelling.
In conclusion, the bi-weekly subcutaneous administration of the immunoglobulin preparation investigated in this study was found to be safe, well tolerated and showed favourable pharmacokinetic data leading to high and stable IgG concentrations in the sample of 12 study subjects.
Acknowledgments
This work was supported by Baxter AG, Vienna, Austria. Dr Gustafson was investigator at the Immunodeficiency Unit, Karolinska University Hospital, Huddinge, Stockholm, Sweden at the time of the study conduct and is employed currently by Baxter Medical AB, Stockholm, Sweden. Drs Leibl and Engl are employees of Baxter AG, Vienna, Austria and Dr Müller was an employee of Baxter AG, Vienna, Austria at the time of the conduct of the study.
References
- 1.Primary Immunodeficiency Diseases. Report of an IUIS Scientific Committee. Clin Exp Immunol. 1999;118:1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eibl MM, Wedgwood RJ. Intravenous immunoglobulin: a review. Immunodef Rev. 1989;1(Suppl.):1–42. [PubMed] [Google Scholar]
- 3.Gardulf A. Immunoglobulin treatment for primary antibody deficiencies: advantages of the subcutaneous route. Biodrugs. 2007;21:105–16. doi: 10.2165/00063030-200721020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gardulf A, Hammarström L, Smith CIE. Home treatment for hypogammaglobulinaemia with subcutaneous gammaglobulin by rapid infusion. Lancet. 1991;338:162–6. doi: 10.1016/0140-6736(91)90147-h. [DOI] [PubMed] [Google Scholar]
- 5.Gardulf A, Andersen V, Björkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345:365–9. doi: 10.1016/s0140-6736(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 6.Gardulf A, Björvell H, Gustafson R, et al. Safety of rapid subcutaneous gammaglobulin infusions in patients with primary antibody deficiency. Immunodeficiency. 1993;4:81–4. [PubMed] [Google Scholar]
- 7.Thomas MJ, Brennan VM, Chapel HH. Rapid subcutaneous immunoglobulin infusions in children. Lancet. 1993;342:1432–3. doi: 10.1016/0140-6736(93)92798-x. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsen TG, Sandersen H, Bustnes A. Home therapy with subcutaneous immunoglobulin infusions in children with congenital immunodeficiencies. Pediatrics. 1996;98:1127–31. [PubMed] [Google Scholar]
- 9.Gustafson R, Gardulf A, Granert C, et al. Prophylactic therapy for selective IgA deficiency. Lancet. 1997;350:865. doi: 10.1016/S0140-6736(05)62034-X. [DOI] [PubMed] [Google Scholar]
- 10.Gaspar J, Gerritsen B, Jones A. Immunoglobulin replacement treatment by rapid subcutaneous infusion. Arch Dis Child. 1998;79:48–51. doi: 10.1136/adc.79.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapel HM, Spickett GP, Ericson D, et al. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. doi: 10.1023/a:1006678312925. [DOI] [PubMed] [Google Scholar]
- 12.Hansen S, Gustafson R, Smith CIE, et al. Subcutaneous IgG infusions in patients with primary antibody deficiencies: decreased time of delivery with maintained safety. Clin Immunol. 2002;104:237–41. doi: 10.1006/clim.2002.5215. [DOI] [PubMed] [Google Scholar]
- 13.Grunebaum E, Levy Y, Shoenfeld Y. Novel aspects of hypogammaglobulinemic states: subcutaneous immunoglobulin treatment. Isr Med Assoc J. 2002;4:288–9. [PubMed] [Google Scholar]
- 14.Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. doi: 10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies − a prospective, multi-national study. J Clin Immunol. 2006;26:177–85. doi: 10.1007/s10875-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 16.Ochs HD, Gupta S, Kiessling P, et al. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 17.Gardulf A, Björvell H, Gustafson R, et al. The life situations of patients with primary antibody deficiency untreated or treated with subcutaneous gammaglobulin infusions. Clin Exp Immunol. 1993;92:200–4. doi: 10.1111/j.1365-2249.1993.tb03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardulf A, Björvell H, Andersen V, et al. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients' experiences of subcutaneous self-infusions and home therapy. J Adv Nurs. 1995;21:917–27. doi: 10.1046/j.1365-2648.1995.21050917.x. [DOI] [PubMed] [Google Scholar]
- 19.Gardulf A, Nicolay U, Asensio O, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114:936–41. doi: 10.1016/j.jaci.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life in patients with primary immunodeficiencies in North America receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26:65–72. doi: 10.1007/s10875-006-8905-x. [DOI] [PubMed] [Google Scholar]
- 21.Gardulf A, Nicolay U. Replacement IgG therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:434–42. doi: 10.1097/01.all.0000246619.49494.41. [DOI] [PubMed] [Google Scholar]
- 22.Kittner JM, Grimbacher B, Wulff W, et al. Patients' attitude to subcutaneous immunoglobulin substitution as home therapy. J Clin Immunol. 2006;26:400–5. doi: 10.1007/s10875-006-9031-5. [DOI] [PubMed] [Google Scholar]
- 23.Gardulf A, Borte M, Ochs HD, et al. Prognostic factors for health-related quality of life in adults and children with primary antibody deficiencies receiving SCIG home therapy. Clin Immunol. 2007;126:81–8. doi: 10.1016/j.clim.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Lee ML, Poon WY, Kingdon HS. A two-phase linear regression model for biologic half-life data. J Lab Clin Med. 1990;115:745–8. [PubMed] [Google Scholar]
- 25.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) London: European Medicines Agency (EMEA); ICH Guideline for clinical safety data management: definitions and standards for expedited reporting (III/3375/93) and US regulations (FDA 21 CFR, 312.32) [Google Scholar]
- 26.Mankarious S, Lee M, Fischer S, et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med. 1988;112:634–40. [PubMed] [Google Scholar]
- 27.Fischer SH, Ochs HD, Wedgwood RJ, et al. Monogr Allergy. Vol. 23. 1988. Survival of antigen-specific antibody following administration of intravenous immunoglobulin in patients with primary immunodeficiency diseases; pp. 225–35. [PubMed] [Google Scholar]
- 28.Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- 29.Lever AM, Yap PL, Cuthbertson B, et al. Increased half-life of gammaglobulin after prolonged intravenous replacement therapy. Clin Exp Immunol. 1987;67:441–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Thampakkul S, Ballow M. Replacement intravenous immune serum globulin therapy in patients with antibody immune deficiency. Immunol Allergy Clin North Am. 2001;21:165–84. [Google Scholar]
- 31.Waniewski J, Gardulf A, Hammarström L. Bioavailability of gammaglobulin after subcutaneous infusions in patients with common variable immunodeficiency. J Clin Immunol. 1994;14:90–7. doi: 10.1007/BF01541341. [DOI] [PubMed] [Google Scholar]
- 32.Smith GN, Griffiths B, Mollison D, et al. Uptake of IgG after intramuscular and subcutaneous injection. Lancet. 1972;1:1208–12. doi: 10.1016/s0140-6736(72)90926-9. [DOI] [PubMed] [Google Scholar]
- 33.Yu ZY, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody mediated autoimmune diseases. N Engl J Med. 1999;340:227–8. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 34.Alyanakian MA, Bernatowska E, Scherrmann JM, et al. Pharmacokinetics of total immunoglobulin G and immunoglobulin G subclasses in patients undergoing replacement therapy for primary immunodeficiency syndromes. Vox Sang. 2003;84:188–92. doi: 10.1046/j.1423-0410.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 35.Ballow M, Berger M, Bonilla FA, et al. Pharmacokinetics and tolerability of a new intravenous immunoglobulin preparation, IGIV-C, 10% (Gamunex(TM), 10%) Vox Sang. 2003;84:202–10. doi: 10.1046/j.1423-0410.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 36.Morell A. Pharmacokinetics of intravenous immunoglobulin preparations. In: Lee M, Strand V, editors. Intravenous immunoglobulins in clinical practice. New York: M. Dekker Inc.; 1997. pp. 1–18. [Google Scholar]
- 37.Schiff RI. Half-life and clearance of pH 6.8 and pH 4·25 immunoglobulin G intravenous preparations in patients with primary disorders of humoral immunity. Rev Infect Dis Suppl. 1986;4:449–56. doi: 10.1093/clinids/8.supplement_4.s449. [DOI] [PubMed] [Google Scholar]
- 38.Webster AD, Latif AA, Brenner MK, et al. Evaluation of test immunization in the assessment of antibody deficiency syndromes. Br Med J (Clin Res Ed) 1984;288:1864–6. doi: 10.1136/bmj.288.6434.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]


