Abstract
Airway inflammation is characterized by selective recruitment of mononuclear and granulocytic cells. This recruitment is mediated by the action of chemotactic cytokines, such as chemokines. A number of chemokines and their receptors have been identified and proposed as potential therapeutic agents in allergic airway inflammation. One of these chemokines is chemokine (C-C motif) ligand 13 (CCL13), a CC chemokine that has been associated with allergic inflammatory diseases such as asthma and allergic rhinitis. To investigate alternative therapeutic agents to alleviate allergic inflammatory diseases, a number of chemokine-derived synthetic peptides were designed and tested for their ability to modulate in vitro and in vivo chemokine-mediated functions. Our results show that one of these peptides, CDIP-2, displayed antagonist functions in in vitro chemotaxis assays using monocytic cell lines. In addition, we found that CDIP-2 significantly reduced peribronchial, perivascular infiltrate and mucus overproduction in an ovalbumin-induced allergic lung inflammation murine model. Thus, CDIP-2 may be considered as part of a novel group of anti-inflammatory agents based on chemokine-derived synthetic peptides.
Keywords: allergy, chemokine, inflammation, peptide
Introduction
Chemokines are a large family of low molecular weight basic proteins involved in several biological functions, including recruitment of cells to sites of inflammation, cellular differentiation and lymphoid organogenesis [1–4] Chemokine-mediated responses in leucocytes are triggered by their interaction with seven transmembrane G protein coupled receptors. Currently, a model of ligand–receptor interaction involves a two-step mechanism, where the chemokine initially interacts with the amino-terminus of the chemokine receptor [5]. This first interaction does not promote signalling, and probably allows the chemokine to reach the inner region of the chemokine receptor. The amino-terminus of the chemokine then activates the receptor, promoting several responses including actin polymerization, calcium flux and degranulation [6].
Chemokine (C-C motif) ligand 13 (CCL13) is a member of the CCL chemokines that binds CCR1, CCR2 and CCR3 receptors expressed in eosinophils, basophils, dendritic cells, monocytes and T lymphocytes [2,7]. This chemokine is up-regulated in several chronic inflammatory diseases, including asthma, rhinitis, glomerulonephritis and atherosclerosis [8–12]. Interestingly, CCL13 also displays anti-microbial activities [13,14].
However, the residues of CCL13 involved in receptor binding and activation are not known. Recently, it was shown that truncated variants of CCL13, generated by MMPs or pathogen cysteine proteases, have antagonistic activities on monocytic cells [15,16]. These results provide new insights into the mechanisms involved in the ligand–receptor interactions and aims to seek for molecules that may modulate chemokine-mediated biological responses.
As an approach to find novel therapeutic agents, we have analysed a number of synthetic peptides based on the primary sequence of CCL chemokines and tested them for either agonist or antagonist functions. In this report, data are presented regarding the biological functions of a short CCL13-derived peptide (CDIP-2). This peptide was capable of antagonizing chemokine-mediated functions in vitro and to reduce leucocyte recruitment in vivo, using a murine model of allergic airway inflammation. CDIP-2 may be included within a novel group of recently described chemokine-derived agents with broad antagonist functions and considered as a good anti-inflammatory candidate.
Materials and methods
Reagents
Peptides (18–19 aa length) were synthesized using solid-phase synthesis and obtained from AnaSpec (San Jose, CA, USA). The amino acid sequence of the peptides based on the CCL13 mature protein (1–75) is as follows: chemokine-derived peptides (CDP)-1 (1–18): qpdalnvpstccftfssk, CDIP-2 (19–37): kislqrlksyvittsrcpq. Human and murine recombinant chemokines were purchased from Peprotech (Rocky Hill, NJ, USA). Hank's balanced salt solution was obtained from Invitrogen (Piscataway, NJ, USA), supplemented with 0·5% endotoxin-free bovine albumin (Sigma Chemical, St Louis, MO, USA). Calcein-AM, Fura-Red and Fluo-3 were from Molecular Probes (Eugene, OR, USA).
Cell lines
The human monocytic cell line tumour human peripheral blood (THP-1) (ATCC TIB-202) was cultured in RPMI-1640 media (Life Technologies, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FCS), sodium pyruvate, non-essential amino acids and penicillin/streptomycin (Life Technologies).
Chemotaxis assays
Chemotaxis assays were carried out using modified Boyden's chambers (NeuroProbe, Inc., Cabin John, MD, USA), as described previously [17]. In experiments using peptides, cells were preincubated with different concentrations of peptide (0–4 μM) for 15 min at 4°C before the assay. Chemokines (0–0·1 μM) were loaded in the lower chamber separated by a 5-μm polycarbonate membrane from the cells (5 × 106/ml), which are loaded in the upper chamber. After a 90-min incubation at 37°C, the membrane was separated and non-migrating cells were removed with a scraper. The membrane was air-dried, and migrating cells were quantified by detecting fluorescence in a molecular imager (FX; Bio-Rad, Hercules, CA, USA).
Calcium flux assays
Cells (1 × 107/ml) were loaded with a solution containing 6 μg/ml Fura-Red (Molecular Probes), 10 μg/ml Fluo-3 and 0·1% pluronic acid in RPMI-1640/2% FCS media for 45 min at 37°C, in the dark. After incubation, cells were centrifuged, washed twice with RPMI-1640 2% FCS and kept at 4°C until they were acquired using a fluorescence activated cell sorter (FACScan) cytometer (Becton Dickinson, San Jose, CA, USA). For acquisition, cells were diluted in RPMI-1640 to a final density of 1 × 106 cells/ml, and data were analysed using the FACS Assistant software (Beckton Dickinson). Changes of cytosolic free calcium were monitored after addition of chemokines (0·1–0·5 μM) or peptides (4·2–8·4 μM). Fluo-3 and Fura Red were excited at 488 nm with Fluo-3 emission detected at 515–535 nm and Fura Red emission detected at 665–685 nm. The data are presented as the relative ratio of fluorescence of Fluo3/Fura Red.
Murine peritonitis model
BALB/c mice (4–6 weeks old) were injected with 1 ml of a 3% thioglycolate solution; 24 h later, 1 mg/kg of peptide in saline solution (SS) (final volume of 100 μl) was administered intraperitoneally (i.p.). Mice were killed 72 h post-thioglycolate injection. Total peritoneal cells were harvested by washing the peritoneal cavity with 4 ml of prewarmed sterile RPMI-1640 medium. The collected fluid was centrifuged, cells were separated and supernatant was kept frozen at −20°C. An aliquot of the cell suspension was fixed on a coverslip using a Cytofuge-2 cytocentrifuge system (Statspin, Norwood, MA, USA), stained by Wright–Giemsa staining and cells counted differentially under a light microscope.
Ovalbumin-sensitization and challenge
We have followed a protocol of immunization and challenge that has been reported previously, with minor modifications [18]. Briefly, groups of age-matched (6–8 weeks) female, BALB/c mice were immunized i.p. with 100 μl of a 10 μg ovalbumin (OVA) suspension in 1 mg of alum (Pierce, NJ, USA) on days 0 and 5. After immunization, mice were challenged intratracheally (i.t.) with a solution of 0·75% OVA in SS, on day 12. Control groups received SS on the same day. On day 15, mice were treated i.t. either with the peptides (2 mg/kg) or with SS. After treatment, mice were killed on day 16. Bronchoalveolar lavages (BAL) were obtained using 4 ml of SS. Cell suspension from BAL was centrifuged and fixed on a coverslip by citospin. Cells were stained by Wright–Giemsa staining and counted differentially at 40× under a light microscope.
Lung tissue processing and histochemical analysis
Mice were killed and the lungs were fixed by i.t. instillation of 1 ml of 90% ethanol. After fixation, lungs were embedded in paraffin and processed for analysis. Tissue sections were stained with haematoxylin and eosin (H&E) staining to assess cellular infiltrate. Mucus production was revealed by periodic acid Schiff (PAS) staining. Transversal lung tissue sections, embedded in paraffin and stained with H&E, were photographed at 100×. Eight animals from each group (five fields from each animal) were measured using the Motic Image Plus 2·0 software (Motic Instruments Inc., British Columbia, CA, USA). Bronchioles were identified by their structure, characterized by ducts covered by tall cubical ciliated epithelia surrounded by smooth muscle, while pulmonary vessels were covered by its tunica intimae, media and adventitia. From each bronchiole, the length in μm2 from the basal lamina to the external muscle layer was measured, while in the case of pulmonary vessels the space between the adventitia and the connective tissue was analysed.
Experimental protocols involving animals were approved and conducted in accordance with the Instituto de Investigaciones Biomédicas's Animal Safety Committee guidelines.
Enzyme-linked immunosorbent assays
An enzyme-linked immunosorbent assays (ELISA) assay to determine levels of anti-immunoglobulin E (IgE) OVA-specific antibodies in serum and bronchoalveolar fluid (BALF) was developed using a biotinylated anti-IgE antibody (BioLegend, San Diego, CA, USA), following the manufacturer's instructions. To determine serum levels of IgG1 and IgG2a, an ELISA assay was developed using secondary anti-IgG1 and anti-IgG2a peroxidase-labelled antibodies (Zymed, Carlsbad, CA, USA), according to the manufacturer's instructions. Cytokine levels in BAL and serum were quantified using commercially available ELISA kits (Opt EIA; BD Pharmigen, San Diego, CA, USA).
Statistical analysis
Data were analysed by using the one-way anova test to determine between-groups significance. P-values < 0·05 were considered significant.
Results
Analysis of the agonistic activity of CCL13-derived peptides
To determine whether the CDP-1 and CDIP-2 have agonistic activities, in vitro chemotaxis assays were evaluated using the THP-1 monocytic cells. These cells were stimulated with each of the peptides using CCL13 as a control. None of the peptides was able to induce a chemotactic response in THP-1 cells. Figure 1a shows a representative experiment using 0·42 μM of CDP-1 and CDIP-2 peptides, and the corresponding CCL13 control used at 0·1 μM (which corresponds to the peak of dose–response in chemotaxis for THP-1 cells, data not shown). To characterize further the potential chemotactic response to CDPs, we tested different concentrations of these peptides (0·004–42 μM). In Fig. 1b representative data are shown of the chemotactic response of CDIP-2. None of these concentrations induced a significant chemotactic response on THP-1 cells. In addition, to determine whether these peptides were capable of inducing intracellular calcium fluxes, different peptide concentrations (0–4·2 μM) were used to stimulate THP-1 cells and calcium responses were measured using a flow cytometer (Fig. 1c). As shown, both peptides, CDP-1 and CDIP-2 (at 4·2 μM) were incapable of inducing calcium flux responses in THP-1 cells compared with CCL13 (0·1 μM), which induced significant calcium mobilization.
Fig. 1.
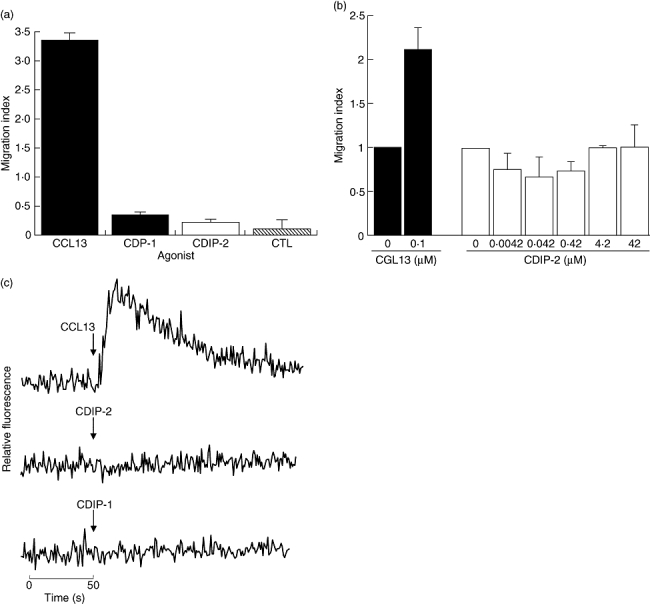
Analysis of the agonistic properties of chemokine (C-C motif) ligand 13 (CCL13)-derived peptides (CDPs) in tumour human peripheral blood monocytic (THP-1) cells. (a) Chemotaxis assays. THP-1 monocytic cells were used in a chemotaxis assay using the CDPs as agonists, as described in Methods. Peptides were used at a concentration of 0·42 μM. The chemokine CCL13 (0·1 μM) was used as control. Data are expressed as the mean migration index as described in Methods. (b) Dose–response analysis of CDIP-2 chemotaxis assays using different concentrations of CDIP-2 peptide (0·004–42 μM). CCL13 (0·1 μM) was used as control. (c) Calcium flux assays. Calcium flux responses induced by CDPs was monitored by cytometry and displayed as relative fluorescence ratio. THP-1 cells were stimulated with 8·4 μM of CDPs and 0·1 μM of the chemokine CCL13 at different time-points (arrow). Tracings are from a representative three independent experiments.
Analysis of the antagonistic activities by CCL13-derived peptides
Because no chemotactic responses were induced by CDPs, we explored the possibility that these peptides could be acting as antagonists, possibly by binding the chemokine receptors without inducing activation or by blocking the binding of CCL13 to their receptors. First, to evaluate their antagonistic effect, THP-1 cells were preincubated with different concentrations of each peptide (1–4·2 μM) for 15 min at 4°C, prior to chemotaxis assays using CCL13 (1 μM) as agonist. Interestingly, CDIP-2 (<2·1 μM) induced more than 40% of inhibition of the chemotactic response of THP-1 cells compared with other CDP. At lower concentrations (1 μM), it had little or no effect on CCL13-mediated chemotaxis (Fig. 2a). In addition, we determined the effect of CDIP-2 on both human peripheral blood mononuclear cells and murine peritoneal mononuclear cells, showing a similar antagonistic effect (data not shown). Furthermore, we determined whether the CDPs might have an inhibitory effect on the calcium flux response. Peptides (4·2–8·4 μM) were added to monocytic cells before adding CCL13 (0·1 μM) (Fig. 2b) and calcium fluxes were determined by FACS analysis. As shown, none of the tested peptides (CDIP-2 and CDP-1) inhibited the calcium flux induced by CCL13.
Fig. 2.
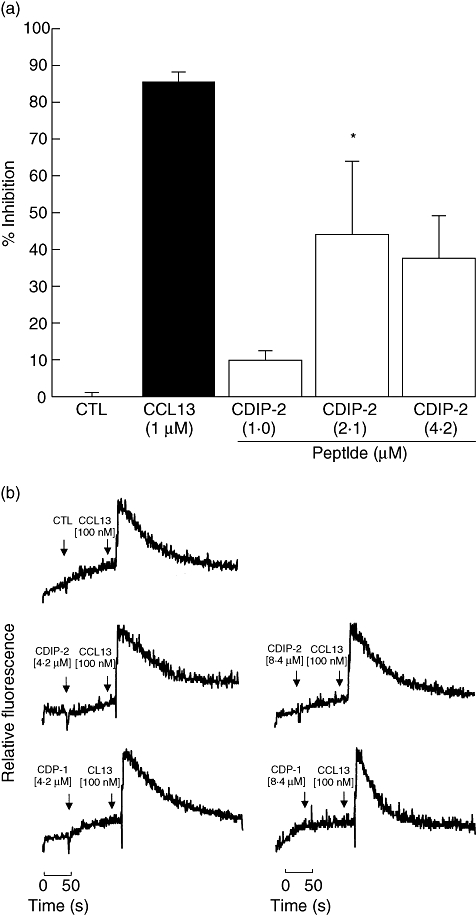
Antagonistic activities induced by chemokine (C-C motif) ligand 13 (CCL13)-derived peptides (CDPs) in tumour human peripheral blood monocytic (THP-1) cells. (a) CDIP-2 peptide inhibits CCL13-mediated chemotactic responses of THP-1 cells. Cells were preincubated with different concentrations of either peptide CDIP-2 (1–4·2 μM) or CCL13 (1 μM) previous to the chemotaxis assay using CCL13 (1 μM) as a chemoattractant. Results are shown as percentage of inhibition ± standard error (*P < 0·01). (b) Analysis of the antagonist activities of CDIP-2 in calcium flux responses. Calcium flux responses induced by CDPs was monitored by cytometry and displayed as relative fluorescence ratio. CDIP-2 and CDP-1 (4·2–8·4 μM) peptides were added to THP-1 cells (arrow 1) before stimulation (arrow 2) with human CCL13 (0·1 μM). The data show a representative experiment (n = 3).
Specificity of CDIP-2-mediated antagonism
To analyse further the specificity of this antagonist effect, CDIP-2 was tested on two other members of the monocyte chemoattractant protein (MCP) subfamily, CCL2 (MCP-1) and CCL8 (MCP-3). These chemokines bind to chemokine receptors CCR2 and CCR1, 2 and 3 respectively. Preincubation with CDIP-2 or CDP-1 peptides (2·1–4·2 μM) had little or no effect on the chemotactic responses induced by CCL2 (0·05 μM) and CCL8 (0·05 μM) on THP-1 cells (Fig. 3a). In addition, we analysed the antagonist activity of CDIP-2 on calcium flux assays. As tested in chemotaxis assays, CDIP-2 (2·1–4·2 μM) had no antagonist effect on CCL2 (0·1 μM) and CCL8 (0·1 μM) functions (Fig. 3b). The same response was observed when CDP-1 (2·1–4·2 μM) peptide was tested (not shown). These results may suggest that the antagonist effect of CDIP-2 on monocytic cells may be specific for CCL13-mediated responses.
Fig. 3.
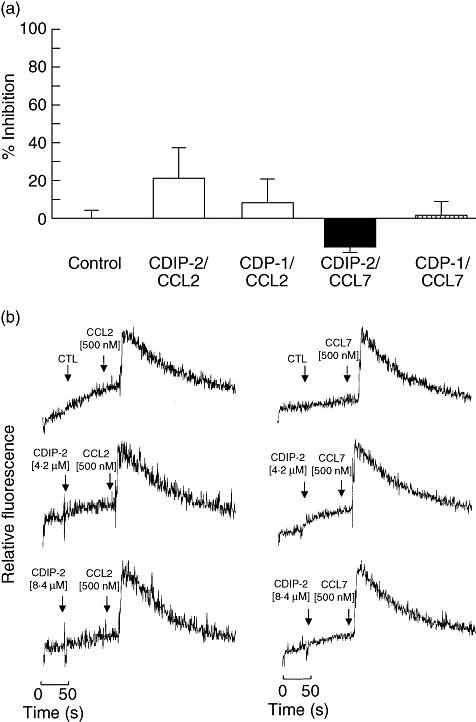
Analysis of the antagonist activities of CDIP-2 on other chemokine (C-C motif) ligand (CCL) chemokines. (a) Chemotaxis assays. Tumour human peripheral blood monocytic (THP-1) cells were preincubated with either CDIP-2 (4·2 μM) or CDP-1 (4·2 μM) peptides prior to the chemotaxis assay using CCL2 (0·1 μM) or CCL7 (0·1 μM) as agonists. Results are shown as percentage of inhibition (% ± standard error) (P > 0·2). (b) Calcium flux induced by CCL13-derived peptides (CDPs) and chemokines was monitored by flow cytometry and displayed as relative fluorescence ratio. CDIP-2 and CDP-1 (4·2–8·4 μM) peptides were added to THP-1 cells (arrow 1) before stimulation (arrow 2) with human CCL7 (0·1 μM) and CCL2 (0·1 μM). The data show a representative experiment (n = 3).
Murine peritonitis model
As a first approach to investigate the in vivo antagonist effects of CDIP-2, we used a well-characterized murine model of peritoneal inflammation induced by thioglycolate medium. Inflammation was induced after i.p. injection of a 3% thioglycolate as described in Methods. After 72 h of treatment, the inflammatory response is characterized by recruitment of a high percentage (70–80%) of mononuclear cells. Mice treated with CDIP-2 peptide showed consistently reduced numbers of total cell infiltration (76%) when compared with controls (no peptide treatment or SS) (Fig. 4). CDIP-2 affected the recruitment of macrophages compared with other cell types such as lymphocytes (data not shown). CDP-1 also had a reduction (45%) on the inflammatory cell recruitment, although it was always lower than with CDIP-2.
Fig. 4.
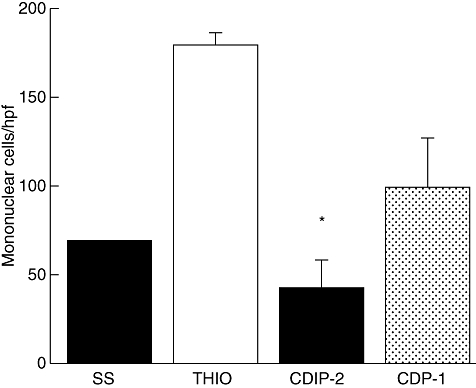
Analysis of the in vivo antagonist activities of CDIP-2 using a murine peritonitis model. BALB/c mice were immunized intraperitoneally with 3% thioglycolate broth, and were treated 24 h later with peptides either CDIP-2 or CDP-1 (1 mg/kg). Mice were killed 48 h later, peritoneal washes were obtained, and number of total cells counted under light microscope. The data are presented as number of migrating cells per high-power field. (*P < 0·02).
Allergic lung inflammation model
In addition, an in vivo model of OVA-induced allergic lung inflammation was analysed. Mice were immunized and challenged i.t. with OVA as described in Methods. Seventy-two hours after the last challenge (day 15), mice were treated i.t. either with peptides (2 mg/kg weight) or with SS. Mice were killed 24 h after peptide treatment (day 16). BAL fluids and tissues were obtained and analysed by ELISA and histochemistry. As shown in Fig. 5, this model of allergic lung inflammation induced a typical recruitment of inflammatory cells around the vessels (Fig. 5a–d), as well as the peribronchial areas (Fig. 5e–h). The production of mucin by goblet cells was also observed by PAS staining (Fig. 5i–l). CDIP-2 treatment (Fig. 5c,g) reduced significantly the inflammatory process induced by administration of OVA (Fig. 5b,f), in particular the recruitment of eosinophils and mononuclear cells to the peribronchial and perivascular area (Fig. 6). In addition, the production of mucin by goblet cells was also diminished (Fig. 5k) in the CDIP-2 peptide-treated animals in comparison with untreated animals (Fig. 5j). Treatment with other peptides such as CDP-1 had a null effect on cell recruitment (Fig. 5d,h,l).
Fig. 5.
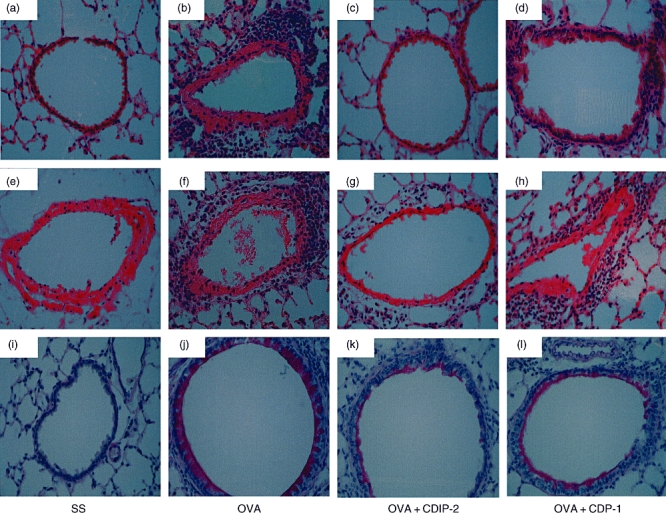
Analysis of the effect of CDIP-2 in an ovalbumin (OVA)-induced lung inflammation murine model. BALB/c mice were sensitized and challenged with OVA following the protocol described in Methods. Controls using saline solution (SS) and chemokine (C-C motif) ligand 13 (CCL13)-derived peptide (CDP)-1 treatment and challenge were performed as described. Histological analysis of haematoxylin and eosin-stained lung tissue sections of (a,e) SS-treated mice, (b,f) OVA-sensitized, OVA-challenged and SS-treated, (c,g) OVA-sensitized, OVA-challenged and CDIP-2-treated mice, (d,h) OVA-sensitized, OVA-challenged and CDP-1-treated mice. Histological analysis of periodic acid Schiff-stained lung tissue sections. (i) SS-treated mice, (j) OVA-sensitized, OVA-challenged and SS-treated, (k) OVA-sensitized OVA-challenged and CDIP-2-treated mice, (l) OVA-sensitized, OVA-challenged and CDP-1 treated.
Fig. 6.
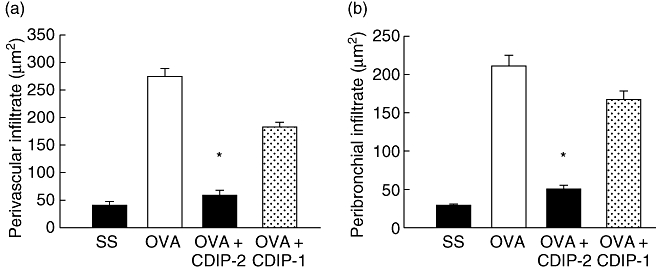
Quantitative analysis of inflammatory cells in haematoxylin and eosin-stained lung tissues. Quantitative analysis of the recruited inflammatory cells in the (a) perivascular and (b) peribronchial regions. Cell infiltrate area (μm2) from selected areas is shown in the histograms and analysed as described in Methods. A representative experiment is shown (n = 5) (*P < 0·001).
When we analysed the BAL fluid of the CDIP-2-treated mice, our results displayed similar features to those observed in the tissues. A decrease in total cell numbers (Fig. 7a) as well as in the number of mononuclear cells and eosinophils (Fig. 7b) was observed in CDIP-2-treated mice, compared with SS-treated mice, while mice treated wth CDP-1 peptide showed a less pronounced effect (data not shown).
Fig. 7.
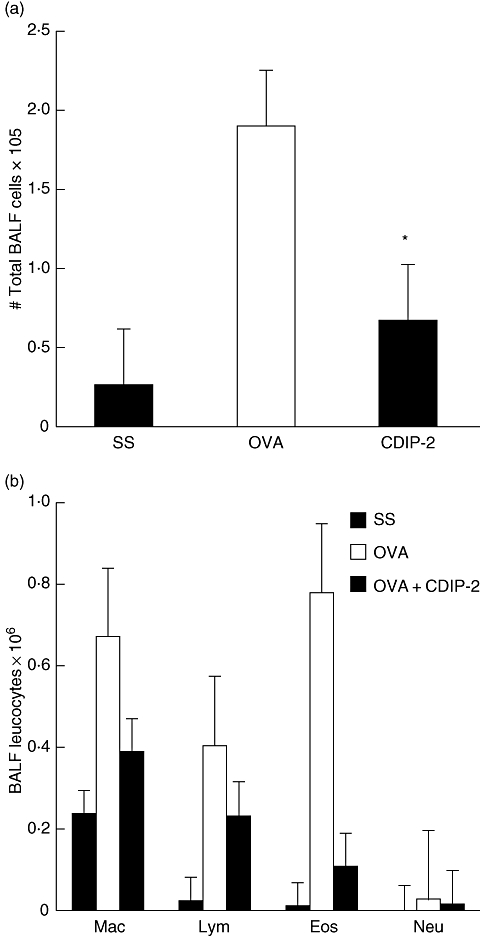
Analysis of the bronchoalveolar fluid (BALF) cells of the CDIP-2-treated and untreated mice. (a) Analysis of the total BALF cell numbers. Cells were separated from the BALF and counted under microscope as described in Methods. (b) Differential cell counting of the BALF in the CDIP-2-treated mice, compared with those treated with chemokine (C-C motif) ligand 13 (CCL13)-derived (CDP)-1 peptide or saline solution (SS) treatment. Data represent total number of cells ×105 (n = 5) (*P < 0·01). Mac, macrophages; Lym, lymphocytes; Eos, eosinophils; Neu, neutrophils.
Finally, we analysed whether peptide treatment might have an effect on the production of IgE antibodies and T helper 2 (Th2)-associated cytokines in both, serum and BALF. Using ELISA assays, it was found that serum levels of anti-OVA IgE-specific antibodies diminished but not IgG1 or IgG2a, which are part of the hallmarks of the allergic inflammatory response in the lung (Table 1). Interestingly, we also found a decrease in the levels of interleukin (IL)-4 but not interferon-γ or tumour necrosis factor-α cytokines in the BALF of the CDIP-2 treated mice compared with non-treated or control mice (Table 2). Altogether, this may indicate that, under these conditions of treatment, CDIP-2 may be affecting the amplification of the Th2 responses generated during the allergic airway inflammation.
Table 1.
Effect of CDIP-2 on antibody levels by enzyme-linked immunosorbent assays (ELISAs). Ag-specific immunoglobulin G1 (IgG1), IgG2a and IgE in serum obtained from of untreated (n = 5) and peptide-treated (n = 5) mice was quantified by ELISA as described in Methods. Mean values are reported as A450 nm units (±standard error).
| SS | OVA | OVA + CDIP-2 | OVA + CDP-1 | |
|---|---|---|---|---|
| IgG1 | 0·10 (0·014) | 2·20 (0·013) | 2·00 (0·004) | 2·10 (0·05) |
| IgG2a | 0·02 (0·001) | 0·48 (0·08) | 0·56 (0·25) | 0·50 (0·20) |
| IgE | 0·02 (0·001) | 1·07 (0·21) | 0·80 (0·17) | 1·09 (0·31) |
CDP, chemokine (C-C motif) ligand 13 (CCL13)-derived peptides; OVA, ovalbumin; SS, saline solution.
Table 2.
Effect of CDIP-2 on cytokine levels by enzyme-linked immunosorbent assays (ELISAs). Determination of interleukin (IL)-4, interferon (IFN)-α, and tumour necrosis factor (TNF)-α cytokines in the bronchoalveolar fluid of untreated (n = 5) and peptide treated (n = 5) mice by ELISA as described in Methods. Values reported as pg/ml (±standard error).
| SS | OVA | OVA + CDIP-2 | OVA + CDP-1 | |
|---|---|---|---|---|
| IFN-α | 30·5 (3·95) | 31·5 (5·20) | 30·00 (11·54) | 30·5 (2·93) |
| TNF-β | 28·5 (2·73) | 31·0 (1·78) | 27·00 (1·86) | 28·0 (0·94) |
| IL-4 | 56·5 (6·33) | 161·1 (12·84) | 56·38 (3·21) | 86·3 (2·85) |
CDP, chemokine (C-C motif) ligand 13 (CCL13)-derived peptides; OVA, ovalbumin; SS, saline solution.
Discussion
Understanding the mechanisms by which chemokines bind and activate their receptors is crucial for the design and development of therapeutic tools to be used in inflammatory and infectious diseases [19]. As an initial attempt to investigate these interactions, we designed several chemokine-derived synthetic peptides based on published data regarding the agonist and antagonist activities of several members of the CCL chemokine family on leucocytes. In particular, we select and tested several CCL13-derived peptides and found that none of them displayed agonistic activities. This lack of agonism could be explained by the fact these peptides might lack the crucial residues required for induction of functions such as migration or induction of calcium fluxes in mononuclear cells. Indeed, the mature N-terminal amino acid sequence is thought to be important for the biological activity and leucocyte selectivity of some CCL chemokines. For example, addition or deletion of the first residue in the amino terminal region of CCL2 reduces its biological activity on monocytes by 100–1000-fold [20]. Additionally, an N-terminal extension in CCL13 and CCL11 decreases their biological functions [21]. Hence, it was proposed that the residues, which would allow binding and activation of CCL2 to chemokine receptor CCR2, might be distributed throughout the entire protein [22].
This may not be the case for all chemokines, as it was shown that peptides (1–10 aa length) from the NH2-terminus of chemokine CCL5 induced chemotaxis of THP-1 cells [23]. Similarly, a peptide (1–17) derived from CXCL12 promotes chemotaxis of T cells [24]. This peptide competes with complete CXCL12 for binding its CXCR4 receptor, suggesting that its NH2-terminal region is important for binding and activation. Furthermore, these data are supported by mutational analysis of CXCL12, in which the first two NH2-terminal residues are required for activation, while 12–18 amino acids may be involved in binding to CXCR4 [25].
Moreover, analysis of the biological effect of metalloproteases on MCP chemokines such as CCL7 and 13 resulted in the finding that NH2-terminal truncated chemokines were inactive [15,26]. This finding indicated that the NH2-terminal region might be important for agonist functions in these chemokines.
In this report, we have investigated the agonist functions of two CCL13-derived peptides, CDP-1 and CDIP-2, and demonstrated that the residues in these peptides are not sufficient to elicit agonistic responses in monocytic cells expressing CCR1 and CCR2. This lack of activity is probably not due to low peptide affinity, because even at higher (42 μM) concentrations of peptides we were unable to induce chemotaxis of THP-1 cells. Instead, other regions of the protein may be required for the activation of these chemokine receptors.
In addition, as part of our analysis of biological functions modulated by CCL13-derived synthetic peptides, we analysed for antagonism mediated by these peptides and found that CDIP-2 antagonized some of the biological responses mediated by CCL13. Interestingly, CDIP-2 could inhibit in vitro monocyte chemotaxis, but did not inhibit calcium flux mobilization. This finding suggests that several functional responses elicited by chemokines can be inhibited selectively at the chemokine–receptor interaction level, making it possible to design inhibitors that target one or several responses at a time. This antagonistic effect was observed on CCL13, but not on other related CC chemokines that share chemokine receptors, such as CCL2 and CCL7. These findings suggest that it is possible to design antagonists, which can target specific ligands even if they share chemokine receptors with other ligands. Our results suggest that CDIP-2 may be acting on chemokine receptors CCR1, 2 and 3. We are currently investigating which of these receptors is involved specifically in its antagonist function. Our data also imply that there are important differences in the mechanisms of activation used by closely related chemokines. Interestingly, CDIP-2 is a novel antagonistic peptide which spans a short region of the NH2-terminal and part of the β-sheet region of CCL13 (19–37). This region has not been investigated as an antagonist in other members of the MCP/CCL family of chemokines.
Several small molecule inhibitors of chemokine receptors are now being developed for the treatment of asthma and chronic obstructive pulmonary disease. CCR3 antagonists, which block eosinophil chemotaxis, are now in clinical development for asthma therapy [27]. Using an allergic inflammation murine model, we have shown that a single dose of CDIP-2 peptide can inhibit the inflammatory response in the lungs of treated mice. CDIP-2 was capable of reducing both the peribronchial and perivascular recruitment of mononuclear and eosinophil cells. In addition, production of mucus by goblet cells was also diminished. Both inflammatory cell recruitment and mucus production are important signs of the physiopathology of asthma.
Additionally, serum and BALF levels of proinflammatory Th2-like cytokines such as IL-4 were affected by CDIP-2 treatment. This correlates with the role of IL-4 in the promotion of eosinophilic inflammation and inhibition of eosinophil apoptosis. Indeed, a reduction in the number of eosinophils obtained from the BALF is accompanied by a reduction in the levels of IL-4. This could also be associated with a reduction in the levels of IL-5 or chemokines such as CCL11 (data not shown). As already known, IL-4 is involved in the production of IgE by B lymphocytes. In this context, CDIP-2 treatment also had an effect on the levels of anti-OVA-specific IgE antibodies. It was proposed that preventing eosinophilia in the lung must be beneficial to the asthmatic patients. Therefore, using modified or truncated chemokines aimed at reducing the recruitment of these cells may be of relevance in the treatment of allergic inflammatory diseases such as asthma.
Altogether, our results demonstrate that a CCL13-derived peptide, CDIP-2, is capable of inhibiting both in vitro chemotaxis and inflammation in two different in vivo murine models. CDIP-2 reduced airway eosinophilia as well as Th2 cytokines, suggesting that it has a potential use as a therapeutic agent in lung diseases such as asthma.
Acknowledgments
We would like to thank Veronica Rodríguez and Judith Reyes for processing tissue samples for histological analysis. Thanks are also due to Dr P. Ostoa for providing some reagents and G. Dupont for technical assistance. This work was supported by a CONACYT Grant No. 33365-N (EAGZ). F. M. was supported by a Doctoral Fellowship from CONACYT. E. M. E. was supported by a pre-Doctoral Fellowship from DGAPA-UNAM.
References
- 1.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 3.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–99. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 5.Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J Biol Chem. 1996;271:19084–92. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- 6.Tenscher K, Metzner B, Hofmann C, Schopf E, Norgauer J. The monocyte chemotactic protein-4 induces oxygen radical production, actin reorganization, and CD11b up-regulation via a pertussis toxin- sensitive G-protein in human eosinophils. Biochem Biophys Res Commun. 1997;240:32–5. doi: 10.1006/bbrc.1997.7601. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613–26. [PubMed] [Google Scholar]
- 8.Taha RA, Minshall EM, Miotto D, et al. Eotaxin and monocyte chemotactic protein-4 mRNA expression in small airways of asthmatic and nonasthmatic individuals. J Allergy Clin Immunol. 1999;103:476–83. doi: 10.1016/s0091-6749(99)70474-4. [DOI] [PubMed] [Google Scholar]
- 9.Lamkhioued B, Garcia-Zepeda EA, Abi-Younes S, et al. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. Induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am J Respir Crit Care Med. 2000;162:723–32. doi: 10.1164/ajrccm.162.2.9901080. [DOI] [PubMed] [Google Scholar]
- 10.Chakravorty SJ, Howie AJ, Girdlestone J, Gentle D, Savage CO. Potential role for monocyte chemotactic protein-4 (MCP-4) in monocyte/macrophage recruitment in acute renal inflammation. J Pathol. 2001;194:239–46. doi: 10.1002/path.877. [DOI] [PubMed] [Google Scholar]
- 11.Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG, Lipoxin A. 4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci USA. 2002;99:13266–71. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto T, Okamoto H, Iikuni N, et al. Monocyte chemoattractant protein-4 (MCP-4)/CCL13 is highly expressed in cartilage from patients with rheumatoid arthritis. Rheumatology (Oxf) 2006;45:421–4. doi: 10.1093/rheumatology/kei209. [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Chen Q, Hoover DM, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–55. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Becerra F, Silva DA, Dominguez-Ramirez L, et al. Analysis of the antimicrobial activities of a chemokine-derived peptide (CDAP-4) on Pseudomonas aeruginosa. Biochem Biophys Res Comms. 2007;355:352–8. doi: 10.1016/j.bbrc.2007.01.188. [DOI] [PubMed] [Google Scholar]
- 15.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–7. [PubMed] [Google Scholar]
- 16.Pertuz Belloso S, Ostoa Saloma P, Benitez I, Soldevila G, Olivos A, Garcia-Zepeda E. Entamoeba histolytica cysteine protease 2 (EhCP2) modulates leucocyte migration by proteolytic cleavage of chemokines. Parasite Immunol. 2004;26:237–41. doi: 10.1111/j.0141-9838.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- 17.Soldevila G, Licona I, Salgado A, Ramirez M, Chavez R, Garcia-Zepeda E. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3. Immunology. 2004;112:191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huerta-Yepez S, Hernandez-Pando R, Santos-Argumedo L, et al. Therapeutic efficacy of an E. coli strain carrying an ovalbumin allergenic peptide as a fused protein to OMPC in a murine model of allergic airway inflammation. Vaccine. 2003;21:566–78. doi: 10.1016/s0264-410x(02)00244-x. [DOI] [PubMed] [Google Scholar]
- 19.Proudfoot AE, Power CA, Rommel C, Wells TN. Strategies for chemokine antagonists as therapeutics. Semin Immunol. 2003;15:57–65. doi: 10.1016/s1044-5323(02)00128-8. [DOI] [PubMed] [Google Scholar]
- 20.Clark-Lewis I, Kim KS, Rajarathnam K, et al. Structure–activity relationships of chemokines. J Leukoc Biol. 1995;57:703–11. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613–26. [PubMed] [Google Scholar]
- 22.Hemmerich S, Paavola C, Bloom A, et al. Identification of residues in the monocyte chemotactic protein-1 that contact the MCP-1 receptor, CCR2. Biochemistry. 1999;38:13013–25. doi: 10.1021/bi991029m. [DOI] [PubMed] [Google Scholar]
- 23.Wells TN, Guye-Coulin F, Bacon KB. Peptides from the amino-terminus of RANTES cause chemotaxis of human T-lymphocytes. Biochem Biophys Res Commun. 1995;211:100–5. doi: 10.1006/bbrc.1995.1783. [DOI] [PubMed] [Google Scholar]
- 24.Loetscher P, Gong JH, Dewald B, Baggiolini M, Clark-Lewis I. N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem. 1998;273:22279–83. doi: 10.1074/jbc.273.35.22279. [DOI] [PubMed] [Google Scholar]
- 25.Crump MP, Gong JH, Loetscher P, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–6. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 27.De Lucca GV. Recent developments in CCR3 antagonists. Curr Opin Drug Discov Devel. 2006;9:516–24. [PubMed] [Google Scholar]


