Abstract
We quantified the variation and plasticity in cold tolerance among four larval stages of four laboratory strains of Drosophila melanogaster in response to both acute (<2 hours of cold exposure) and chronic (∼7 hours of cold exposure) cold exposure. We observed significant differences in basal cold tolerance between the strains and among larval stages. Early larval instars were generally more tolerant of acute cold exposures than 3rd instar larvae. However, wandering larvae were more tolerant of chronic cold exposures than the other stages. Early stages also displayed a more pronounced rapid cold-hardening response than the later stages. Heat pre-treatment did not confer a significant increase in cold tolerance to any of the strains at any stage, pointing to different mechanisms being involved in resolving heat- and cold-elicited damage. However, when heat pre-treatment was combined with rapid cold-hardening as sequential pre-treatments, both positive (heat first) and negative (heat second) effects on cold tolerance were observed. We discuss possible mechanisms underlying cold-hardening and the effects of acute and chronic cold exposures.
Keywords: Drosophila melanogaster, heat shock, rapid cold-hardening, cold tolerance
I. Introduction
Insects of the temperate, alpine and boreal regions experience physiologically challenging climatic conditions during winter, and the adaptations used to overcome these have been reviewed many times (e.g. Duman et al., 1991; Leather et al. 1993, Bale, 2002). However, organisms not adept at surviving low temperatures suffer lethal damages that are less well-understood (Hawes and Bale, 2007). Physiological studies in various organisms show that mild stress can increase the hardiness of the organism to the same but harsher stress (Bowler, 2005). Rapid Cold Hardening (RCH), an increase in cold tolerance after a prior (sub-lethal) exposure to cold, first described by Lee et al. (1987) has been observed in numerous insect groups (Meats, 1973; Chown and Terblanche, 2007) including isolated tissue and cells (Yi and Lee, 2003).
RCH differs from the heat shock response in that it doesn't appear to require time for the production of chaperone proteins (Michaud and Denlinger, 2006; Sinclair & Roberts 2005). Both adult and larval Drosophila melanogaster show a strong RCH response (Czajka and Lee 1990), and this RCH response is also elicited during slow cooling, pointing to ecological relevance (Kelty and Lee 1999, 2001). The mechanisms of RCH remain obscure, but seem to include changes in membrane fatty acid saturation and whole-body glucose concentration (Overgaard et al. 2005, 2007), and there is no clear implication of heat shock proteins in the RCH process of D. melanogaster (Nielsen et al., 2005).
Cold tolerance of D. melanogaster is expected to vary with developmental stage (Rako and Hoffman, 2006). However, previous studies (e.g., Jensen et al., 2007; Czajka and Lee, 1990) that have investigated the ontogeny of cold hardiness have used developmental times, rather than discrete stages. Variation in development time within populations means that this method can result in experiments being performed on a mix of instars (Welbergen and Sokolowski, 1994; Roberts, 1998). Although geographic variation in cold tolerance has been shown in the adult Drosophila species (Gibert and Huey, 2001), no study to our knowledge has directly compared cold tolerance among common laboratory strains (Prout, 1958; Salgado, 1984; Chakir et al., 2007). There have, however, been numerous studies demonstrating the rapid loss of field-relevant traits in insects in culture (Hoffmann, 2000; Sgrò and Hoffmann, 1998), and this phenomenon probably applies equally to D. melanogaster cold tolerance.
Sinclair and Roberts (2005) reviewed the cold tolerance literature for Drosophila and pointed out significant variation in the traits measured as ‘cold tolerance’. In particular, cold may be divided into acute (short, intense) and chronic (longer and less intense) exposures, which seem to result in different forms of injury – through membrane phase transition in acute, intense exposures, and through ion equilibration in more chronic exposures (although we note that phase transition would also result in ion equilibration). Most RCH investigations have involved acute cold exposure, although Shintani and Ishikawa (2007) reported a reduced RCH effect in Psacothea hilaris eggs exposed to a relatively mild temperature (which necessitates a longer exposure time), compared to a more acute exposure to a lower temperature. This implies that RCH may not impart increased tolerance to chronic cold exposure, although this hypothesis has not been explicitly tested.
In this paper, we investigate tolerance of four life stages of four different strains of Drosophila melanogaster to both acute and chronic cold exposure. Because common lab strains of D. melanogaster have been reared under near-constant thermal conditions for thousands of generations, we hypothesised (1) that the tolerance of common lab strains would be equivalent, and that this relationship would not change when considering chronic and acute cold exposure. However, we predicted that a recently-collected wild-type strain would have improved cold tolerance compared to the lab strains. Secondly, in accordance with Krebs and Loeschcke (1995) who noted that heat tolerance is lowest among the first and last stage of larval development, we hypothesised (2) that larval cold tolerance will also increase with age (developmental stage) and decrease in the later stages (wandering larvae) in all strains of D. melanogaster and for both acute and chronic cold tolerance. Because there are apparent differences in the means of cold injury from acute and chronic cold exposure (Sinclair and Roberts, 2005), we hypothesised (3) that rapid cold-hardening (and heat shock) pre-treatments will not elicit equivalent protection to both acute and chronic cold exposure. Given the apparent mode of action of RCH, through membrane desaturation (Overgaard et al., 2005, 2006), we predicted that RCH would afford better protection against acute cold exposure than against chronic cold exposure. We expected this to be uniform across the strains. Finally, we hypothesised (4) that the modes of heat shock and RCH protection against cold differ; from this, we predicted that RCH and heat shock protection against cold exposure would be additive (or interactive).
II. Methods
Drosophila melanogaster strains and larval collection
Four wild-type strains of D. melanogaster (Oregon R-S, Canton S and Berlin K) were sourced from the Bloomington Drosophila Stock Centre, Bloomington, Indiana, USA (stock numbers 4269, 1 and 8522, respectively). A recently-collected (2005) wild strain was provided by Dr. Michael Dillon, University of Washington (W5N; established from 10 isofemale lines from Washington State, USA). All the flies were reared in the lab at 21 ± 0.7 °C, 65−75% humidity and 14:10 L:D. Flies were reared in 50 ml plastic vials initiated with ∼ 50 − 75 eggs/vial or 150 eggs per 250 ml polypropylene bottles. Flies were reared on a cornmeal-yeast medium (1.7% active yeast (w/v), 1% soy flour, 7.0% cornmeal, 4.5 % malt extract, 0.5% agar, 7.5% (v/v) corn syrup and 0.5 % propionic acid). To our knowledge, all of these strains have been reared at near-constant temperature and light conditions since collection.
Flies were transferred at seven days post-eclosion to population cages made from 3.7 L PET plastic jars for mass breeding and egg collection. For egg collection, flies were provided with food in a Petri dish topped with live yeast paste (1.5:1 parts baker's yeast to water (w/v)) for two days, after which the food was replaced with a fresh food plate sprinkled with active yeast granules. After 12 hours, the plates were incubated in a humidified 21 °C incubator and the larvae were isolated after 24 hours, 48 hours, and 72 hours of incubation. Larvae of equal sizes were collected and sub-samples were checked for appropriate stage by observing the mandible and spiracle morphology described in Roberts (1998). Wandering stages were isolated after 80−90 hours by their distinctive roaming behaviour outside of the food.
Cold and heat pre-treatments
Prior to all treatments the larvae were rinsed in distilled water and surface water was blotted off using a filter paper. Larvae were then placed in aluminium foil packets (15mm w x 10 mm h) and placed in 2 mm thick slots in an aluminium block cooled by circulating propylene glycol solution from a refrigerated circulator (VWR-1157P, Mississauga, Canada). Cold pre-treatment (cold hardening) procedure involved exposing the larvae in aluminium foil packets to 0 °C for 1 hour in an identical apparatus. Pre-treated larvae were used immediately for various treatments. Heat pre-treatment was done by exposing the larvae in aluminium foil packets to 36.5 °C for 1 hour (Feder and Krebs, 1998). The larvae were then permitted to recover for 1 hour at 22 °C before being used for various treatments. Larvae were also treated to a combination of heat followed by cold shock and vice versa. The heat pre-exposures were always separated by 1h at room temperature prior to cold exposure.
Cold exposure
Cold tolerance was assessed at −2 °C, and −7 °C for periods ranging from 30 to 1440 minutes (0.5 to 24 hours). “Acute” and “chronic” chilling exposures [defined by Sinclair and Roberts (2005), as <3h and > 6h, respectively] were defined for each species and stage from the LT90, the time required for 90 % mortality, which was calculated from a logistic regression of time on survival at −2 and −7 °C. The lethal time for 90% mortality was then used for subsequent assessment of the effects of pre-treatments on acute and chronic cold exposure. At least three replicates each consisting of 10−25 larvae were used per time-temperature combination. Supercooling points were assessed for larvae exposed to −7 °C for up to 150 minutes and no freezing exotherms were observed, confirming that mortality during the cold exposure is due to chilling injury rather than freezing. Lethal time for 10% mortality and 50% mortality were also calculated from these data to provide comparisons with other studies and indicate the temperature where chilling injury begins (analogous to the Upper Limit of Cold Injury Zone (ULCIZ) described in Nedved et al. (1998)).
After cold exposure, larvae were transferred immediately to a Petri dish containing larval rearing food at 21 °C, and the ability to move and feed, coupled with an absence of dark spots indicating necrosis due to chill damage were assessed after 24 h. Third instar and wandering larvae that pupated in the 24 h following cold exposure were scored as alive if they had pupated and the pupa did appear to contain necrotic tissue. Pupae that were black or larva-shaped (incomplete pupation) were considered dead. All individuals were followed to pupation, and the eclosing adults were sexed and the sex ratio was noted.
Data analysis
Comparison of survival between stages within strains and between strains was conducted separately for chronic and acute exposures using a generalized linear model for binomial distribution with logit link function using the ‘glm procedure’ in R (R version 2.4.1, 2006). For these analyses, a ‘replicate’ would be a single foil packet with 10−25 larvae. See Quinn and Keough (2002) for an explanation of the reasons for using generalised linear modelling in this case. Comparisons of the treatments were performed on the R package ‘multcomp’ with function ‘glht’ (Hothorn et al., 2007). Treatment was nested within stage and treatment (stage) within strain, where ‘treatments’ included each of the pre-treatments described above. Raw data and fitted curves are available from the authors upon request.
III. Results
i. Basal cold tolerance in different strains and larval stages
Basal cold tolerance of the four stages and strains is shown in Tables 1 and 2. Drosophila melanogaster larvae survived 8 − 13 h at −2 °C (Table 1); and 50 − 135 min at −7 °C (Table 2). Berlin K 1st instars had the highest chronic LT90s (781 minutes, Table 1), while second instar Oregon R were the least tolerant of chronic exposure (402 minutes; Table 1). In general, early instars had lower LT10 compared to later instars for both chronic and acute exposure, but in both cases, early stages had greater LT90s (i.e., better overall cold tolerance) than later stages. Oregon R was least tolerant and Berlin K most tolerant to acute cold exposure (Table 2). There were fewer significant differences between stages within strains in response to acute cold, but the strains themselves exhibited substantial variation (Table 2).
Table 1.
Mean (± s.e.m.) time required for 10, 50 and 90 % mortality (LT10, LT50 and LT90, respectively) at −2 °C for four larval stages (instars I, II and III and wandering 3rd instars, w) of four wild-type strains of D. melanogaster. Values were derived from a generalized linear model of survival. Comparisons of models between stages within a strain and between strains within a stage were via a post hoc analysis (general linear hypothesis testing — glht in R; see methods for further details). Asterisks indicate significant differences between stages within strains and between strains within stages:
| Strain / Stage | LT10 (minutes) | LT50 (minutes) | LT90 (minutes) | Nonlinear comparison between stages | Nonlinear comparison between strains | |
|---|---|---|---|---|---|---|
| Oregon R | I | 209.4±95.20 | 425.7±84.20 | 636.0±196.30 | II** III* W* | be** wn*** |
| II | 178.3±31.50 | 242.5±68.91 | 402.4±172.30 | I** III* W** | be** wn** | |
| III | 162.1±56.12 | 344.8±72.52 | 522.3±124.80 | I* II* | be** wn** | |
| W | 240.2±85.20 | 418.6±72.00 | 588.1±95.40 | I* II** | be** wn** | |
| Berlin K | I | 214.8±99.33 | 497.9±121.20 | 781.1±184.21 | II*** W** | or** ca* wn** |
| II | 219.3±107.20 | 456.5±100.60 | 711.1±139.50 | I*** W** | or** wn* | |
| III | 161.2±74.50 | 437.1±102.50 | 701.8±162.01 | or** ca** wn*** | ||
| W | 241.6±112.70 | 382.7±103.57 | 711.6±195.00 | I** II** | or** ca** wn* | |
| Canton S | I | 111.1±48.90 | 334.4±82.10 | 557.4±134.60 | II*** III** | be* wn** |
| II | 132.3±34.00 | 349.8±80.89 | 566.8±101.00 | I*** II*** III*** | wn** | |
| III | 129.5±62.70 | 337.8±107.47 | 663.0±203.55 | I** II*** | be** wn* | |
| W | 105.1±47.20 | 343.6±9.90 | 581.1±197.20 | be** wn* | ||
| W5N | I | 187.6±84.32 | 411.5±9.40 | 635.1±171.64 | II** | or*** be** ca* |
| II | 214.5±102.80 | 340.8±59.10 | 467.2±102.50 | I** W** | or** be* ca** | |
| III | 250.0±116.20 | 435.5±76.33 | 620.1±162.40 | or** be*** ca* | ||
| W | 228.3±109.50 | 492.5±112.24 | 756.1±154.30 | II** | or** be* ca* |
p < 0.05
p < 0.01
p < 0.001.
Table 2.
Mean (± s.e.m.) time required for 10, 50 and 90 % mortality (LT10, LT50 and LT90, respectively) at −7 °C for four larval stages (instars I, II and II and wandering 3rd instars, W) of four wild-type strains of D. melanogaster. Values were derived from a generalized linear model of survival. Comparisons of models between stages within a strain and between strains within a stage were via a post hoc analysis (general linear hypothesis testing — glht in R; see methods for further details). Asterisks indicate significant differences between stages within strains and between strains within stages:
| Strain/Stage | LT10 (minutes) | LT50 (minutes) | LT90 (minutes) | Nonlinear comparison between stages | Nonlinear comparison between strains | |
|---|---|---|---|---|---|---|
| Oregon R | I | 11.12±6.23 | 42.22±10.3 | 63.66±21.45 | II*** III** | be*** wn*** |
| II | 25.1±7.85 | 49.27±7.85 | 73.3±21.30 | I*** | be*** wn** | |
| III | <10 | 39.04±12.45 | 74.6±33.00 | I** | be** wn*** | |
| W | <10 | 52.53±11.97 | 96.1±35.52 | be*** wn** | ||
| Berlin K | I | 27.52±8.93 | 57.13±15.82 | 124.7±31.98 | or*** ca* wn*** | |
| II | 29.6±11.01 | 76.12±15.37 | 122.62±42.0 | or*** ca* wn** | ||
| III | 27.7±4.88 | 77.28±16.04 | 126.8±49.31 | or** ca*** wn*** | ||
| W | 46.23±7.98 | 90.54±15.38 | 135.56±56.2 | or*** ca** wn** | ||
| Canton S | I | <10 | 28.29±13.23 | 103.8±38.77 | II*** III** W ** | be* wn*** |
| II | 13.1±6.2 | 48.5±21.88 | 68.27±24.5 | I** | be* wn** | |
| III | <10 | 26.56±7.41 | 53.6±12.43 | I** W ** | be*** wn** | |
| W | 22.32±7.811 | 51.5±24.24 | 83.83±20.4 | I** III** | be** wn** | |
| W5N | I | 45.82±12.33 | 66.58±17.06 | 100.22±38.6 | or*** be*** ca*** | |
| II | 47.43±10.96 | 71.5±31.62 | 96.27±32.01 | III** W** | or** be** ca** | |
| III | 45.11±18.2 | 65.36±17.83 | 81.68±19.4 | II** | or*** be*** ca** | |
| W | 28.8±9.02 | 52.81±35.72 | 99.33±41.54 | II** | or** be** ca** |
p < 0.05
p < 0.01
p < 0.001.
ii. Rapid cold-hardening and acute and chronic cold tolerance
With the exception of Oregon R stages I and W, Berlin K first instar, and W5N stages III and W, a rapid cold-hardening pre-treatment resulted in a significant improvement in survival of acute cold exposure (Fig. 1). All stage/strain combinations with the exception of second instars of Oregon R and Berlin K showed improved chronic cold survival after a rapid cold-hardening pre-treatment (Fig. 1).
Figure 1.
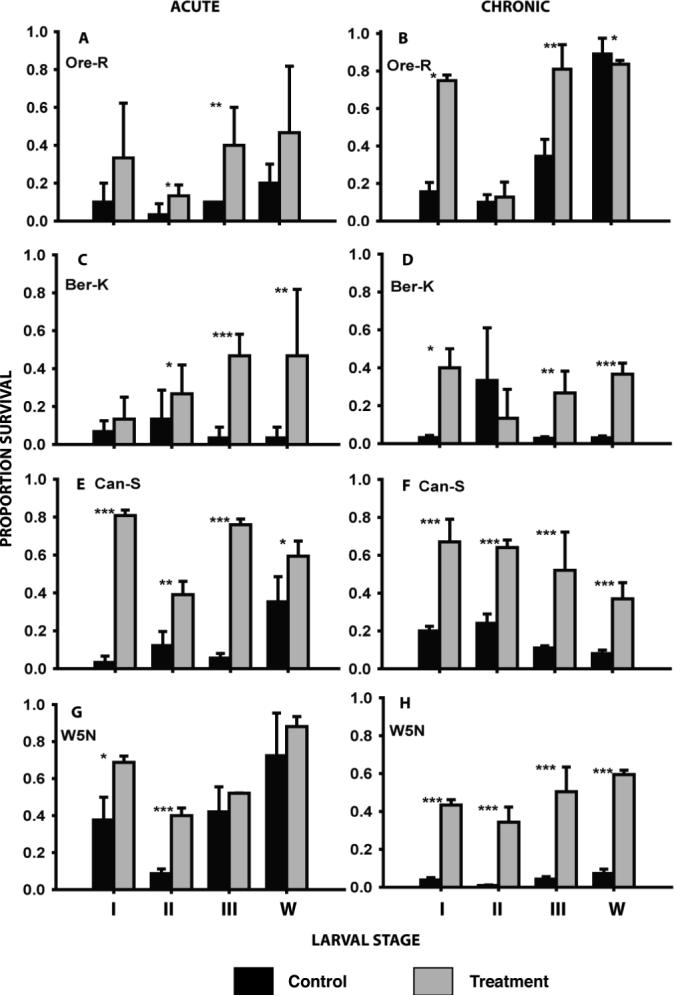
The effect of rapid cold hardening on the acute and chronic cold tolerance in four larval stages (instars I, II and III and wandering 3rd instars - w) of four strains of Drosophila melanogaster. Black bars are control animals, grey bars represent animals pre-treated at 0 °C for 1 hour. Acute exposures were at the previously-determined LT90 at −7 °C; chronic exposures were at the LT90 at −2 °C (see Tables 1 and 2 for details). Mean survival (± s.d.) was derived from 3 to 5 replicates each consisting of 10 − 20 larvae. Asterisks indicate significant differences between control and pre-treated animals: *p<0.05; **p<0.01; ***p<0.001.
iii. Heat pretreatment and cold tolerance
With the exception of Oregon R stage II and III, Berlin K III and W, and W5N stages I and W heat pre-treatment did not significantly elevate survival of the larvae after acute cold exposure (Fig. 2). In several cases such as Oregon R Stage W, Berlin K stage II and Canton S stage W, heat pretreatment resulted in significantly decreased acute cold tolerance (Fig. 2). With the exception of small increases in survival in Oregon R stage W and W5N stage W and significant decreases in Berlin K stages II and III, Canton S stages I and II, and W5N stage II, the heat shock pre-treatment did not result in significant changes in tolerance to chronic cold exposure (Fig. 2).
Figure 2.
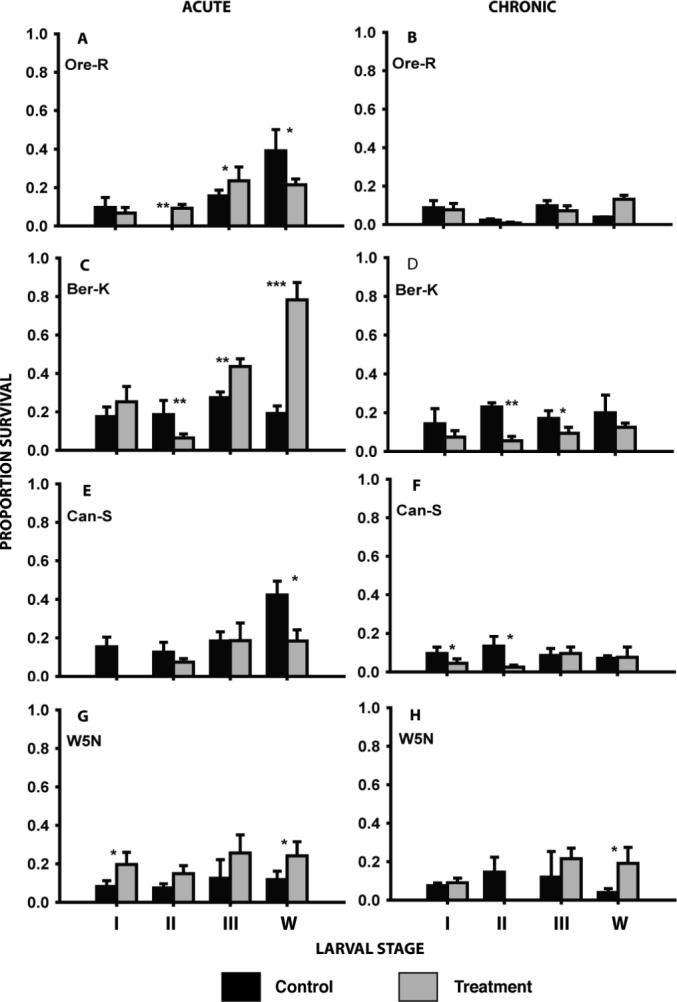
The effect of heat treatment on the acute and chronic cold tolerance in four larval stages (instars I, II, III and wandering late 3rd instars - w) of four strains of Drosophila melanogaster. Black bars represent control animals and grey bars represent animals pre-treated at 36.5 °C for 1 hour and recuperated for 1 more hour at 21 °C. Acute exposures were at the previously-determined LT90 at −7 °C and chronic exposures were at the LT90 for −2 °C (see Tables 1 & 2 for further details). Mean survival (± s.d.) was derived from 3 to 5 replicates each consisting of 10 − 20 larvae. Asterisks represents significant differences between the control and pre-treated animals: *p<0.05; **p<0.01; ***p<0.001.
iv. Combined Heat pretreatment and cold-hardening
Sequential heat shock and RCH pre-treatments resulted in a significant increase in survival of both acute and chronic cold exposure across most stage/strain combinations when compared to controls or one of the pre-treatments individually (Fig. 3). Conversely, RCH followed by heat shock resulted in less of an improvement in cold tolerance, with no effect at all in some cases. In these trials, the effect of RCH pre-treatments on cold survival was somewhat diminished compared to earlier data (Fig. 1), but still indicated a significant improvement in most cases (Fig. 3).
Figure 3.
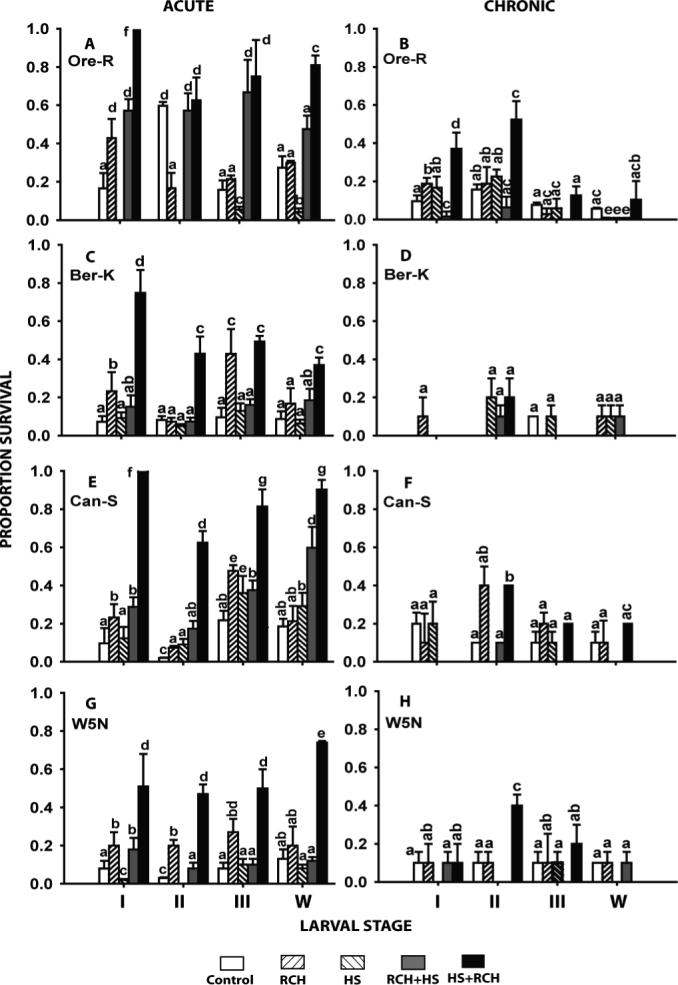
The effect of a combination of heat shock and rapid cold-hardening pre-treatments in four larval stages (instars I, II, III and wandering late 3rd instars - w) of four strains of Drosophila melanogaster. Acute exposures were at the previously-determined LT90 at −7 °C and chronic exposures were at the LT90 for −2 °C (see Tables 1 & 2 for further details). All bars represent mean survival (± s.d.) derived from 3−5 replicates each consisting of 10−20 larvae. Bars sharing common letters are not statistically different from each other.
IV. Discussion
Cold tolerance of D. melanogaster larvae
We found significant differences between strains in tolerance to both acute and chronic cold exposure, although, contrary to our expectations, the recently-collected W5N was less cold-hardy than Berlin K. This does not support our hypothesis that all laboratory strains would be approximately equal, nor does it verify our prediction that W5N would be the most cold hardy. There is considerable support in the literature for our hypothesis that older developmental stages would be more cold tolerant (e.g. Bowler, 1967; Kelty and Lee, 2001), and we did find that 3rd instar (and wandering) larvae were often more tolerant of acute cold exposure (when measured at the LT90). However, instars I and II were most tolerant of chronic cold exposure, suggesting that this hypothesis does not hold true for all ‘kinds’ of cold exposure (see also Jensen et al., 2007). Our comparisons were made of the entire model, rather than simply of the lethal temperature values. As a result, our results are fairly conservative, as stage/strain combinations need to have different responses to cold (as well as different survival) to be considered. As a result, our conclusions of differences are likely conservative.
The wild-type laboratory strains we used (Oregon R, Canton S and Berlin K) have all been in laboratory culture for many decades, and, to our knowledge, the cultures have not been exposed to low temperature stress over this time. W5N is a recently-collected strain and never more cold tolerant than the Berlin K lab strain. Berlin K has been noted for its tolerance to other environmental stressors (e.g. Vogel, 1980; Magnusson and Ramel, 1986). Cold tolerance is both heritable and adaptive (e.g., Heino and Lumme, 1989; Magiafoglou and Hoffmann, 2003), so it is interesting that our strains have been in laboratories and stock centers for decades without selective pressure and yet still exhibit variation in cold tolerance. This suggests that there may not be a long-term fitness cost to maintaining cold tolerance under lab conditions or that cold tolerance is fixed as a result of historical population bottlenecks in the laboratory populations.
The developmental difference in responses to acute and chronic cold exposure implies not only that there is a difference between the kinds of damage imparted by acute and chronic cold exposure (or in recovery processes from the two kinds of cold injury), but that there is a developmental difference in ability to survive such damage. It is possible that this could have some ecological implications if, for example, early instars are more likely to experience transient cold events, whereas later instars are more likely to experience chronic exposure. Feder (1997) suggested that ovipositing Drosophila might be able to sense regions of fruit that have been exposed to temperature extremes. If different developmental stages experience different thermal conditions, then early stage larvae close to the surface might be more prone to exposure to rapid thermal changes, and this could be reflected in heterogeneous responses across instars.
We found relatively high variance in our estimates of cold tolerance, which we attribute to physiological differences between larvae in the same stage but in different developmental states (as we show in early and late – wandering- 3rd instars). Such variation has also been described by other authors (e.g. Tucic, 1979; Rako and Hoffmann, 2006; Jensen et al., 2007), and may be of similar origin. Heino and Lumme (1989) show that there is variation in low temperature tolerance between sexes of adult D. virilis and D. lummei at the same age. If this were true of the larvae then sex differences may also contribute to the variation in our results. However, we found no consistent sex bias in cold-exposed larvae that survived to eclosion that would suggest sex-specific survival (data not shown; χ2 tests p>0.1 in 9/10 cases, p<0.001 in RCH + HS acute exposure, where there was a male bias), suggesting that there is no direct effect of gender in our data.
Previous studies on cold tolerance in D. melanogaster larvae have often concentrated on studying a single developmental stage viz. wandering 3rd instar larvae (e.g. Burton et al. 1988; Czajka and Lee, 1990). However, Jensen et al. (2007) recently studied both 48 and 96 hour old larvae (determined as time after oviposition). Drosophila melanogaster embryonic and larval development can be highly variable as in numerous insect species (Howe, 1967), and we found that larvae that were of the same age (48 & 96 h) were often of heterogeneous stages: there were equal proportions of larvae in both stages II and III at 48 hours and mostly wanderers and very few stage III among them at 96 hours (results not shown). Our basal cold tolerance studies indicate that on several occasions, 3rd instar and wandering larvae had longer LT90s (indicating greater tolerance) to acute cold than the first two instars. If larvae are aged by time, rather than by morphology, these differences between 2nd and 3rd instars may account for some of the variability in the data reported by Jensen et al. (2007). Burton et al. (1988) and Czajka and Lee (1990) both examined cold tolerance of wandering larvae, concluding that larval stages of D. melanogaster are less cold-hardy than eggs, pupae and adults. Our data show that instars I and II may be as tolerant of acute cold exposure as the pupa and some of younger adults in the study by Czajka and Lee (1990). All the strains we studied showed an LT90 >6 h at −2 °C. However, Czajka and Lee (1990) reported ∼20 % survival of 2 h at 0 °C among 4−5 day old larvae (probably 3rd instar). Another possible explanation for discrepancies between our results and those of Burton et al. (1988) and Czajka and Lee (1990) is that we used bare larvae in small foil packets, whereas other researchers have exposed larvae while in food vials. Why this latter approach might result in reduced survival is unclear, although it could be associated with anoxia during recovery of larvae in chill coma, or inoculative freezing if the food freezes.
The effect of RCH alone on cold tolerance in Figure 3 was not as strong as observed in some of our previous experiments, but we are unable to explain this discrepancy. This set of experiments was conducted on a single generation of flies, and it is possible that there were some unanticipated maternal effects in that generation, although we have no evidence of a source for this variation. Alternately, the reduced amplitude of the RCH response may arise from increased handling time during the execution of this rather complex set of experiments. However, the sequential and RCH pre-treatments shown in Figure 3 were conducted simultaneously, so the effects of the sequential treatments do represent a significant improvement, rather than simply an RCH effect.
Rapid Cold-hardening and Heat Shock
As in most insects, a rapid cold-hardening pre-treatment imparted increased tolerance to subsequent cold exposure (Sinclair et al., 2003, Sinclair and Roberts, 2005). To our knowledge, this is the first study to show that RCH applies to both acute and chronic cold exposure, in spite of the postulated differences between the damage caused by these two stressors (Sinclair and Roberts, 2005; Rako and Hoffmann, 2006). Acute cold injury is hypothesised to be mediated via cell membrane conformational changes, with changes in membrane fluidity the proposed local mode of action for RCH (e.g. Overgaard et al., 2005). Chronic cold injury is hypothesised to arise from loss of membrane ion pump activity and loss of osmotic functionality (Košt'ál et al., 2004; Overgaard et al., 2006), and a mode of action for RCH to prevent this is less clear. However, recent work by Yi and Lee (2007) has shown that RCH may also be associated with a blocking of apoptosis-related pathways, and this may be an explanation for a common RCH effect on both acute and chronic cold tolerance.
Heat shock pretreatment in our studies did not generally elevate the tolerance of larvae to acute or chronic cold exposure. Acute heat shock as well as prolonged (5−16 h) cold exposure at 0 °C elicit heat shock protein expression which subsequently has been shown to increase cold tolerance of wandering larvae of D. melanogaster (Burton et al., 1988; Petersen et al., 1990), although Sejerkilde et al. (2003) showed that while cold hardening increases heat resistance in adult females, heat hardening did not affect cold tolerance. Induction of the heat shock response resulted in a decrease in cold tolerance in D. buzzatii larvae (Sørensen et al., 1999). While heat shock proteins have been implicated in cold tolerance of a number of insects (e.g. Yocum, 2001; Chen et al., 2005; Rinehart et al., 2007), there is no evidence that Hsp70 is associated with cold hardening at least in adult D. melanogaster (Kelty and Lee, 2001), and Sinclair et al. (2007) found that any heat shock gene expression in response to cold exposure in adult D. melanogaster occurs during recovery, and would not be able to account for the rapid cold-hardening phenomenon. However, Sejerkilde et al., (2003) and Nielsen et al., (2005) noted that long term cold exposures can induce Hsp70 expression.
Although a heat shock pre-treatment did not improve survival, it resulted in a significant increase in acute cold tolerance if it was followed by an RCH pre-treatment. In most tests we performed this improvement was significantly greater than that elicited by an RCH pre-treatment alone. This sequential treatment had less of an impact on tolerance of chronic cold exposure, although it did improve chronic cold tolerance in several stage/strain combinations. The converse pre-treatment, where RCH was followed by heat shock, resulted in improved survival of acute cold exposure in only a few cases, and did not affect chronic cold tolerance. Current hypotheses for the mechanisms of RCH (membrane remodeling, apoptosis prevention and glucose increase, Lee et al., 2006; Yi and Lee, 2007; Overgaard et al., 2007) and heat shock protection (prevention of harmful protein aggregation, Feder and Krebs, 1998; Bowler, 2005) suggest that the two pre-treatments should have complementary effects on physiological tolerances, and this is indeed the case here. We assume that our heat shock pre-treatment will result in production of near-maximal quantities of heat shock proteins, particularly Hsp70 (Feder and Krebs, 1998). Although membrane perturbations have been reported to influence heat shock protein synthesis (Carratu et al., 1996; Feder and Hofmann, 1999), there is no suggestion that heat shock proteins may be modifying membrane fluidity.
We hypothesize that the heat shock pretreatment results in the production of heat shock proteins, and that the RCH pre-treatment results in changes to membrane composition, and then there is an additive effect of the two treatments when applied sequentially. It is possible that when the reverse treatment is applied (RCH then heat shock), the membrane remodeling is rapidly reversed in response to the high temperature, and the residual protection is a result of the heat shock response and any effects of RCH that do not affect membrane fluidity, like the blocking of apoptotic pathways (Yi et al., 2007).
In this study, we have shown that not all long-term laboratory reared strains of Drosophila melanogaster or even the stages within the strains are equal in their response to cold. The variation in cold tolerance among the various stages of larval development seemed to be related to the larval age, although not in a sequential manner. While heat hardening did not increase tolerance to acute or chronic cold exposure, it does seem to act synergistically with rapid cold hardening, and this may provide some avenue towards integrating the various hypotheses for the mode of action of rapid cold-hardening. Finally, we note that these sequential pre-treatments may facilitate cryopreservation of D. melanogaster larvae by increasing survival of chilling before freezing is initiated.
Acknowledgements
We thank Heather Tarnowski for technical assistance, and Heather Tarnowski and Caroline Williams for comments on an earlier version of the ms. This research was supported by grant number RR022885 to BJS from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
V. References
- Bale JS. Insects and low temperatures: From Molecular Biology to Distribution and Abundance. Philosophical Transactions of the Royal Society of London B. 2002;357:849–862. doi: 10.1098/rstb.2002.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler K. Changes in temperature tolerance with adult age in Tenebrio molitor. Entomologia Experimentalis et Applicata. 1967;10:16–22. [Google Scholar]
- Bowler K. Acclimation, heat shock and hardening. Journal of Thermal Biology. 2005;30:125–130. [Google Scholar]
- Burton V, Mitchell HK, Young P, Petersen NS. Heat Shock Protection against Cold Stress of Drosophila melanogaster. Molecular and Cellular Biology. 1988;8:3550–3552. doi: 10.1128/mcb.8.8.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carratù L, Franceschelli S, Pardini CL, Kobayashi GS, Horvath I, Vigh L, Maresca B. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakir M, Moreteau B, Capy P, David JR. Phenotypic variability of wild living and laboratory grown Drosophila: Consequences of nutritional and thermal heterogenity in growth conditions. Journal of Thermal Biology. 2007;32:1–11. [Google Scholar]
- Chen B, Kayukawa T, Monteiro A, Ishikawa Y. The expression of the Hsp90 gene in response to winter and summer diapauses and thermal-stress in the onion maggot, Delia antiqua. Insect Molecular Biology. 2005;14:697–702. doi: 10.1111/j.1365-2583.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Chown SL, Terblanche JS. Physiological diversity in insects: Ecological and evolutionary contexts. Advances in Insect Physiology. 2007;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajka MC, Lee RE. A rapid cold hardening response in Drosophila melanogaster. Journal of Experimental Biology. 1990;148:245–254. doi: 10.1242/jeb.148.1.245. [DOI] [PubMed] [Google Scholar]
- Duman JG, Wu WD, Xu L, Tursman D, Olsen MT. Adaptations of insects to subzero temperatures. Quarterly Review of Biology. 1991;66:387–410. [Google Scholar]
- Feder ME. Necrotic fruit: A novel model system for thermal ecologists. Journal of Thermal Biology. 1997;22:1–9. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feder ME, Krebs RA. Natural and genetic engineering of the heat-shock protein hsp70 in Drosophila melanogaster: Consequences for thermotolerance. American Zoologist. 1998;38:503–517. [Google Scholar]
- Gibert JM, Huey RB. Chill-Coma Temperature in Drosophila: Effects of Developmental Temperature, Latitude, and Phylogeny. Physiological and Biochemical Zoology. 2001;74:429–434. doi: 10.1086/320429. [DOI] [PubMed] [Google Scholar]
- Hawes TC, Bale JS. Plasticity in arthropod cryotypes. Journal of Experimental Biology. 2007;210:2585–2592. doi: 10.1242/jeb.002618. [DOI] [PubMed] [Google Scholar]
- Heino R, Lumme J. Inheritance of cold shock in hybrids of Drosophila virilis and Drosophila lummei. Genetica. 1989;79:17–25. doi: 10.1007/BF00056061. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA. Laboratory and field heritabilities: some lessons from Drosophila. In: Mousseau TA, editor. Adaptive Genetic Variation in the Wild. Oxford University Press; New York: 2000. pp. 200–218. [Google Scholar]
- Hothorn T, Bretz F, Westfall P, Heiberger RM. multcomp: Simultaneous Inference for General Linear Hypotheses. 2007 with contributions by. R package version 0.991-9, URL http://cran.r-project.org/src/contrib/Descriptions/multcomp.html.
- Jensen D, Overgaard J, Sørensen JG. The influence of developmental stage on cold shock resistance and ability to cold-harden in Drosophila melanogaster. Journal of Insect Physiology. 2007;53:179–186. doi: 10.1016/j.jinsphys.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Kelty JD, Lee RE. Induction of rapid cold hardening by cooling at ecologically relevant rates in Drosophila melanogaster. Journal of Insect Physiology. 1999;45:719–726. doi: 10.1016/s0022-1910(99)00040-2. [DOI] [PubMed] [Google Scholar]
- Kelty JD, Lee RE. Rapid cold hardening of Drosophila melanogaster (Diptera: Drosophilidae) during ecologically based thermoperiodic cycles. Journal of Experimental Biology. 2001;204:1659–1666. doi: 10.1242/jeb.204.9.1659. [DOI] [PubMed] [Google Scholar]
- Košt'ál V, Vambera J, Bastl J. On the nature of pre-freeze mortality in insects: water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. Journal of Experimental Biology. 2004;207:1509–1521. doi: 10.1242/jeb.00923. [DOI] [PubMed] [Google Scholar]
- Leather SR, Walters KFA, Bale JS. The Ecology of Insect Overwintering. University Press; Cambridge: 1993. [Google Scholar]
- Lee RE, Chen C, Denlinger DL. A rapid cold-hardening process in insects. Science. 1987;238:1415–1417. doi: 10.1126/science.238.4832.1415. [DOI] [PubMed] [Google Scholar]
- Lee RE, Damodaran K, Yi S-X, Lorigan GA. Rapid cold-hardening increases membrane fluidity and cold tolerance of insect cells. Cryobiology. 2006;52:459–463. doi: 10.1016/j.cryobiol.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Magnusson J, Ramel C. Genetic Variation in the Susceptibility to Mercury and Other Metal Compounds in Drosophila melanogaster. Teratogenesis, Carcinogenesis, and Mutagenesis. 1986;6:289–305. doi: 10.1002/tcm.1770060405. [DOI] [PubMed] [Google Scholar]
- Magiafoglou A, Hoffmann AA. Cross-generational effects due to cold exposure in Drosophila serrata. Functional Ecology. 2003;17:664–672. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. 1st ed. Chapman and Hall; London: 1983. [Google Scholar]
- Meats A. Rapid acclimatization to low temperature in the Queensland fruit fly, Dacus tryoni. Journal of Insect Physiology. 1973;19:1903–1911. [Google Scholar]
- Michaud RM, Denlinger DL. Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapauses in the flesh fly, Sarcophaga crassipalpis. Journal of Insect Physiology. 2006;52:1073–1082. doi: 10.1016/j.jinsphys.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nedved O, Lavy D, Verhoef HA. Modeling the time-temperature relationship in cold injury and effect of high-temperature interruptions on survival in a chill-sensitive collembolan. Functional Ecology. 1998;12:816–824. [Google Scholar]
- Nielsen MM, Overgaard J, Sorensen JG, Holmstrup M, Justesen J, Loeschcke V. Role fof HSF activation for resistance to heat, cold and high-temperature knock-down. Journal of Insect Physiology. 2005;51:1320–1329. doi: 10.1016/j.jinsphys.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Sørensen JG, Petersen SO, Loeschcke V, Holmstrup M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. Journal of Insect Physiology. 2005;51:1173–1182. doi: 10.1016/j.jinsphys.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Sørenson JG, Petersen SO, Loeschscke V, Holmstrup M. Reorganization of membrane lipids during fast and slow cold hardening in Drosophila melanogaster. Physiological Entomology. 2006;31:328–335. [Google Scholar]
- Overgaard J, Melmendel A, Sørensen JG, Bundy JG, Loeschcke V, Nielsen NC, Holmstrup M. Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. Journal of Insect Physiology. 2007;53:1218–1232. doi: 10.1016/j.jinsphys.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Petersen NS, Young P, Burton V. Heat shock mRNA accumulation during recovery from cold shock in Drosophila melanogaster. Insect Biochemistry. 1990;20:679–684. [Google Scholar]
- Prout T. A possible difference in genetic variance between wild and laboratory population. Drosophila Information Service. 1958;32:148–149. [Google Scholar]
- Quinn G, Keough M. Experimental design and data analysis for biologists. 1st edn. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing. Vienna, Austria.: 2006. R: A language and environment for statistical computing. ISBN 3−900051−07−0, URL http://www.R-project.org. [Google Scholar]
- Rako L, Hoffmann A. Complexity of the cold acclimation response in Drosophila melanogaster. Journal of Insect Physiology. 2006;52:94–104. doi: 10.1016/j.jinsphys.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward AL, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DB, editor. Drosophila: A practical approach. 2nd Ed. Oxford University Press; 1998. p. 416. [Google Scholar]
- Salgado C. Quantitative genetic differences between populations of Drosophila melanogaster from diverse geographic origins. Genetica. 1984;65:215–226. [Google Scholar]
- Sejerkilde M, Sørensen JG, Loeschcke V. Effects of cold- and heat hardening on thermal resistance in Drosophila melanogaster. Journal of Insect Physiology. 2003;49:719–726. doi: 10.1016/s0022-1910(03)00095-7. [DOI] [PubMed] [Google Scholar]
- Sgrò CM, Hoffmann AA. Heritable variation for fecundity in field-collected Drosophila melanogaster and their offspring reared under different environmental temperatures. Evolution. 1998;52:134–143. doi: 10.1111/j.1558-5646.1998.tb05146.x. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Ishikawa Y. Relationship between rapid cold-hardening and cold acclimation in the eggs of the yellow-spotted longicorn beetle, Psacothea hilaris. Journal of Insect Physiology. 2007;53:1055–1062. doi: 10.1016/j.jinsphys.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Vernon P, Klok CJ, Chown SL. Insects at low temperatures: an ecological perspective. Trends in Ecology and Evolution. 2003;18:257–262. [Google Scholar]
- Sinclair BJ, Roberts SP. Acclimation, shock and hardening in the cold. Journal of Thermal Biology. 2005;30:557–562. [Google Scholar]
- Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Molecular Biology. 2007;16:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- Sørensen JG, Michalak P, Justesen J, Loeschcke V. Expression of the heat-shock protein HSP70 in Drosophila buzzatii lines selected for thermal resistance. Hereditas. 1999;131:155–164. doi: 10.1111/j.1601-5223.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- Tucic N. Genetic capacity for adaptation to cold resistance at different developmental stages of Drosophila melanogaster. Evolution. 1979;33:350–358. doi: 10.1111/j.1558-5646.1979.tb04688.x. [DOI] [PubMed] [Google Scholar]
- Vogel E. Genetical relationship between resistance to insecticides and procarcinogens in two Drosophila populations. Archives of Toxicology. 1980;43:201–211. doi: 10.1007/BF00297585. [DOI] [PubMed] [Google Scholar]
- Welbergen P, Sokolowski MB. Developmental time and pupation behavior in Drosophila melanogaster subgroup (Diptera: Drosophilidae). Journal of Insect Behavior. 1994;7:263–277. [Google Scholar]
- Yi S-X, Moore CW, Lee RE. Rapid cold-hardening protects Drosophila melanogaster from cold-induced apoptosis. Apoptosis. 2007;12:1183–1193. doi: 10.1007/s10495-006-0048-2. [DOI] [PubMed] [Google Scholar]
- Yocum GD. Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. Journal of Insect Physiology. 2001;47:1139–1145. doi: 10.1016/s0022-1910(01)00095-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


