Abstract
Erythrocytes are generally expected to passively transit microvascular beds, carrying oxygen but not delivering ROS. In contrast, Kiefmann and colleagues report in this issue of Blood that, in hypoxic lungs, erythrocytes release ROS, activate endothelium, and trigger leukocyte accumulation.
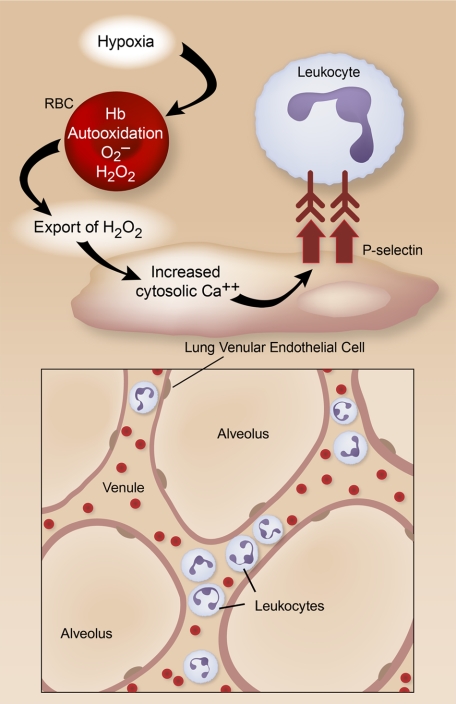
The study utilized elegant real-time fluorescent imaging of venular and capillary endothelial cells (ECs) to show that, when isolated rat and mouse lungs are perfused with red blood cells (RBCs), hypoxia triggers increased endothelial reactive oxygen species (ROS) and elevated levels of EC cytosolic calcium. Few laboratories are performing analysis of in situ endothelium in the pulmonary microvasculature with this precision. Biochemical and inhibitor studies, and interesting experiments using RBCs and lungs from genetically altered mice, indicate that the mechanism involves auto-oxidation of hemoglobin (Hb) with resultant generation of hydrogen peroxide (H2O2), likely from superoxide. Membrane-bound Hb appears to be particularly important, and this localization may partially isolate ROS generated by Hb auto-oxidation from cytosolic RBC antioxidant enzymes. H2O2 is then exported to EC by the hypoxic RBCs, inducing entry of external Ca+. This leads to translocation of the adhesion molecule P-selectin to endothelial surfaces, and rolling and tight adhesion of leukocytes in lung venules and septal capillaries (see figure). Because P-selectin display, rolling, and “sticking” are initiating events in the emigration of leukocytes into many tissues,1 these studies indicate that hypoxic RBCs have the potential to deliver sparks that can ignite the fire of acute inflammation. The role of endothelial P-selectin in the accumulation of myeloid leukocytes in lung alveoli is unclear, but there is evidence that it can mediate intravascular leukocyte sequestration in models of lung injury.2
In hypoxic rodent lungs, H2O2 is generated in RBCs by auto-oxidation of membrane-bound Hb and is exported to microvascular endothelial cells. This triggers increased cytosolic calcium, translocation of P-selectin to the EC plasma membrane, and leukocyte adhesion in venules and septal capillaries. Illustration by Diana Lim.
Earlier studies of cultured human ECs demonstrate that H2O2 induces endothelium-dependent leukocyte adhesion,3 consistent with results in hypoxic lungs (see figure). Importantly, ROS generation by RBCs did not occur under normoxia. This provides reassuring evidence that pathologic hypoxia (blood pO2 ∼22 mmHg in the current experiments) is required and that these events are unlikely to occur in physiologic breathing or tissue perfusion. The mechanism(s) linking RBC-derived H2O2 to Ca++ entry in lung endothelium under hypoxic conditions was not established, but there is emerging evidence that H2O2 has important signaling roles in addition to cytotoxic effects.4
Where might this model have clinical relevance? The authors suggest that it may be an amplifying event in acute lung injury, which is a common syndrome of lung inflammation complicated by hypoxia. In addition, ROS generation by hypoxic RBCs might contribute to transfusion-related acute lung injury (TRALI),5 and might be a specific risk in transfusion of erythrocytes that have been stored for prolonged periods, resulting in reduced levels of RBC antioxidants. There is also an inflammatory component in some cases of altitude-induced lung dysfunction, especially if the subject has a concomitant lung infection. Finally, hypoxia is an important priming event for vascular dysfunction in a variety of systemic inflammatory syndromes.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
- 1.McIntyre TM, Prescott SM, Weyrich AS, Zimmerman GA. Cell-cell interactions: leukocyte-endothelial interactions. Curr Opin Hematol. 2003;10:150–158. doi: 10.1097/00062752-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan MS, Polley MJ, Bayer RJ, Nunn MF, Paulson JC, Ward PA. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest. 1992;90:1600–1607. doi: 10.1172/JCI116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel KD, Zimmerman GA, Prescott SM, McEver RP, McIntyre TM. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991;112:749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004;126:249–258. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]