Abstract
Anacardic acid (6-pentadecylsalicylic acid) is derived from traditional medicinal plants, such as cashew nuts, and has been linked to anticancer, anti-inflammatory, and radiosensitization activities through a mechanism that is not yet fully understood. Because of the role of nuclear factor-κB (NF-κB) activation in these cellular responses, we postulated that anacardic acid might interfere with this pathway. We found that this salicylic acid potentiated the apoptosis induced by cytokine and chemotherapeutic agents, which correlated with the down-regulation of various gene products that mediate proliferation (cyclin D1 and cyclooxygenase-2), survival (Bcl-2, Bcl-xL, cFLIP, cIAP-1, and survivin), invasion (matrix metalloproteinase-9 and intercellular adhesion molecule-1), and angiogenesis (vascular endothelial growth factor), all known to be regulated by the NF-κB. We found that anacardic acid inhibited both inducible and constitutive NF-κB activation; suppressed the activation of IκBα kinase that led to abrogation of phosphorylation and degradation of IκBα; inhibited acetylation and nuclear translocation of p65; and suppressed NF-κB–dependent reporter gene expression. Down-regulation of the p300 histone acetyltransferase gene by RNA interference abrogated the effect of anacardic acid on NF-κB suppression, suggesting the critical role of this enzyme. Overall, our results demonstrate a novel role for anacardic acid in potentially preventing or treating cancer through modulation of NF-κB signaling pathway.
Introduction
Traditional medicine that has been practiced for thousands of years is generally considered safe, but what the medicine consists of and how it mediates its effects may not be understood. For instance, Amphipterygium adstringens (family Anacardiaceae) is a tree, the bark of which (locally called “cuachalate”) is widely used in Mexico for treatment of gastric ulcers, gastritis, and stomach cancers.1,2 The active and possibly anti-inflammatory component in this plant has been identified as 6-pentadecylsalicylic acid, or anacardic acid (Figure 1A).1 The same compound has also been identified in Ozoroa insignis (an African medicinal plant3), Anacardium occidentale (the cashew nut4), and Ginkgo biloba (Ginkgoaceae; an Asian medicine5). The active principle has been associated with molluscicidal activity6 and antimicrobial activity.7,8 How anacardic acid mediates these effects is not fully understood, but it has been shown to have antioxidant activity9 and to inhibit the activity of numerous enzymes, including tyrosinase,4 xanthine oxidase,10 phosphatidylinositol-specific phospholipase C,11 histone acetyltransferase (HAT),12–14 tissue factor VIIa,15 lipoxygenase, and cyclooxygenase (COX).16–18 This compound has also been shown to be a mitochondrial uncoupler of oxidative phosphorylation.19 Anacardic acid exhibits antitumor activity3,5 and sensitizes tumor cells to ionizing radiation.13 However, it is not fully understood how this salicylic acid mediates antitumor, radiosensitization, and anti-inflammatory activities.
Figure 1.
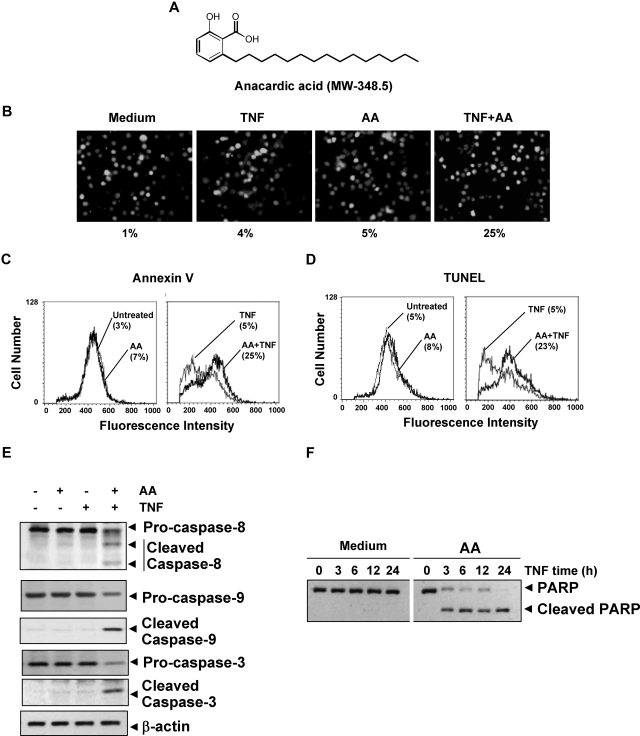
Anacardic acid potentiates apoptosis induced by TNF and chemotherapeutic agents. (A) Structure of anacardic acid (AA). (B) Anacardic acid potentiates TNF-induced apoptosis. KBM-5 cells were pretreated with 25 μmol/L anacardic acid for 4 hours and then incubated with 1 nmol/L TNF for 16 hours. The cells were stained with the Live/Dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope. The results shown are representative of 3 independent experiments. (C) Cells were pretreated with 25 μmol/L anacardic acid for 4 hours and then incubated with 1 nmol/L TNF for 16 hours. Cells were incubated with an anti–annexin V antibody conjugated with FITC and then analyzed by flow cytometry for early apoptotic effects. (D) Cells were pretreated with 25 μmol/L anacardic acid for 4 hours and then incubated with 1 nmol/L TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and then analyzed by flow cytometry for apoptotic effects. (E) KBM-5 cells were incubated with 25 μmol/L anacardic acid for 4 hours and then treated with 1 nmol/L TNF for 24 hours. Whole-cell extracts were prepared and analyzed by Western blotting using the indicated antibodies. (F) Cells were pretreated with 25 μmol/L anacardic acid for 4 hours and then incubated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared and subjected to Western blot analysis using an anti-PARP antibody.
One possible mechanism by which anacardic acid exerts its effects is by modulating the nuclear factor-κB (NF-κB) pathway commonly involved in tumorigenesis, inflammation, and radiosensitization. The transcription factor NF-κB resides in the cytoplasm in its resting stage and then translocates to the nucleus and mediates transcription of various gene products when it is activated. Various inflammatory agents induce NF-κB activation, including cytokines (eg, tumor necrosis factor [TNF]), carcinogens, tumor promoters, cigarette smoke, environmental pollutants, ionizing radiation, and stress. NF-κB activation has been shown to control the expression of more than 400 different gene products that have been linked with inflammation, tumor cell transformation, survival, proliferation, invasion, angiogenesis, metastasis, chemoresistance, and radioresistance. Thus, suitable inhibitors of NF-κB activation are actively being searched.
Because of the critical role of NF-κB in tumorigenesis,20 radiosensitization,21 and inflammation,22 in this study, we tested the hypothesis that anacardic acid may mediate its effects through modulation of the NF-κB pathway. Our results demonstrated that anacardic acid could suppress NF-κB activated by inflammatory cytokines, growth factors, and tumor promoters through the inhibition of inhibitory subunit of NF-κB (IκBα) kinase, leading to suppression of NF-κB–regulated gene products and potentiation of apoptosis.
Methods
Reagents
A 25-mmol/L solution of anacardic acid (Calbiochem, San Diego, CA) was prepared in 100% dimethyl sulfoxide, stored at-20°C, and then diluted as needed in cell culture medium. Bacteria-derived recombinant human TNF-α was kindly provided by Genentech (South San Francisco, CA). Penicillin, streptomycin, Iscove modified Dulbecco medium (IMDM), Dulbecco modified Eagle medium (DMEM), RPMI 1640 medium, and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). Phorbol 12-myristate 13-acetate (PMA), okadaic acid (OA), lipopolysaccharide (LPS), interleukin-1β (IL-1β), epidermal growth factor (EGF), and anti–β-actin antibody were purchased from Sigma-Aldrich (St Louis, MO). HAT p300 siRNA was purchased from Dharmacon (Lafayette, LA). Antibodies against p65, p50, inhibitory subunit of NF-κB (IκBα), cyclin D1, matrix metalloproteinase-9 (MMP-9), poly(ADP-ribose) poly-merase (PARP), inhibitor-of-apoptosis protein 1 (IAP1), Bcl-2, Bcl-xL, c-myc, caspase-3, caspase-8, caspase-9 and intercellular adhesion molecule 1 (ICAM-1), and the annexin V staining kit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-vascular endothelial growth factor (VEGF) was purchased from NeoMarkers (Fremont, CA). Anti-survivin antibody was purchased from R&D Systems (Minneapolis, MN). Anti-COX-2 and anti–X-linked inhibitor of apoptosis (XIAP) antibodies were purchased from BD Biosciences (San Jose, CA). Anti–phospho-IκBα (serine 32/36) and anti–phospho-p65 (serine 536), cleaved caspase-3, cleaved caspase-9, and acetylated-lysine (Ac-K-103) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-p300 antibody was purchased from Millipore (Billerica, MA). Anti-IκBα kinase (IKK)α, anti-IKKβ, and anti-cellular caspase-8 (FLICE)–like inhibitory protein (c-FLIP) antibodies were kindly provided by Imgenex (San Diego, CA).
Cell lines
Human myeloid KBM-5 cells, human T-cell lymphoma Jurkat cells, human lung adenocarcinoma H1299 cells, human embryonic kidney A293 cells, human prostate cancer Du145 cells, and human squamous cell carcinoma SCC4 cells were purchased from the American Type Culture Collection (Manassas, VA). KBM-5 cells were cultured in IMDM supplemented with 15% FBS. H1299, Du145, and Jurkat cells were cultured in RPMI 1640 medium, and A293 cells were cultured in DMEM supplemented with 10% FBS. SCC4 cells were cultured in DMEM containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins. The mouse embryonic fibroblast (MEF) derived from p65−/− C57BL/6J mice and its wild type were kindly provided by Dr David Baltimore (California Institute of Technology, Pasadena, CA). Cells were cultured in DMEM supplemented with 10% FBS. All media were also supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
Electrophoretic mobility shift assay
To assess NF-κB activation by TNF, we performed electrophoretic mobility shift assay (EMSA) essentially as described previously.23
Western blot analysis
To determine the levels of protein expression in the cytoplasm or nucleus, we prepared extracts and fractionated them by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with the relevant antibody, and detected with an electrogenerated chemiluminescence reagent (GE Healthcare, Chalfont St Giles, United Kingdom). Next, to determine the expression of gene products in whole-cell extracts of treated cells (2 × 106 cells in 1 mL medium), 40 μg whole-cell lysate was resolved by SDS-PAGE, electrotransferred to membrane, and then probed with antibodies against various proteins.
IKK assay
To determine the effect of anacardic acid on TNF-induced IKK activation, we analyzed IKK essentially as described previously.24
NF-κB–dependent reporter gene expression assay
The effect of anacardic acid on TNF-induced NF-κB–dependent reporter gene transcription in A293 cells was measured as described previously.24
Down-regulation of p300 histone acetyltransferase by siRNA
A293 cells (2 × 105) were plated in each well of 6-well plates and allowed to adhere for 24 hours. On the day of transfection, 12 μL HiPerFect transfection reagent (Qiagen, Valencia, CA) was added along with 50 and 100 nmol/L siRNA or control scrambled siRNA in 100 μL culture medium. After 48 hours of transfection, cells were recovered and used for appropriate determinations.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay was performed as described previously25 with some modification. KBM-5 (2 × 107 cells) were incubated with 25 μmol/L anacardic acid for 4 hours and then treated with 1 nmol/L TNF for the indicated times. Cells were then cross-linked with formaldehyde, quenched with glycine, resuspended in SDS lysis buffer (1% SDS, 10 mmol/L ethylenediaminetetraacetic acid [EDTA], and 50 mmol/L tris(hydroxymethyl)aminomethane [Tris]-HCl, pH 8.0, with protease inhibitors, pH 8.0), sonicated on ice and centrifuged at 4°C. Supernatants (400 μL) were diluted to a final volume of 4 mL in a mixture of 9 parts dilution buffer (1% Triton X-100, 150 mmol/L NaCl, 2 mmol/L EDTA, and 20 mmol/L Tris-HCl, with protease inhibitors, pH 8.0) and 1 part lysis buffer. Mixtures were incubated with 4 μg anti-p65 antibody per sample with rotation at 4°C overnight and then incubated with 100 μL protein A beads at 4°C for 4 hours. After gentle centrifugation (2000 rpm), beads were resuspended in 1 mL wash buffer (1% Triton X-100, 0.1% SDS, 150 mmol/L NaCl, 2 mmol/L EDTA, and 20 mmol/L Tris-HCl, with protease inhibitors, pH 8.0), washed 3 times. Finally immunocomplexes were washed with buffer (1% Triton X-100, 0.1% SDS, 500 mmol/L NaCl, 2 mmol/L EDTA, and 20 mmol/L Tris-HCl, pH 8.0, with protease inhibitors). The immune complexes were eluted with elution buffer (1% SDS, 100 mmol/L NaHCO3) followed by incubation with proteinase K and RNase A (500 μg/mL each) at 37°C for 30 minutes. Reverse cross-links were performed by placing the tubes at 65°C overnight. Immunoprecipitated DNA was extracted and dissolved in sterile water. PCR analyses were carried out for 39 cycles with primers: 5′-TCTGGCGGAAACCTGTGCGCTGG-3′ (forward) and 5′-AAATTGCGTAAGCCCGGTGGG-3′ (reverse) for human COX-2 (the amplified fragment (−291∼ −120) contains the NF-κB binding sites of −221-GGGACTACCC-−211),26,27 and 5′-CAGTGGAATTCCCCAGCCTTGCCT-3′ (forward, boldface indicates NF-κB binding sites), 5′-CCACACTCCAGGCTCTGTC CTC-3′ (reverse) for the DNA fragment (∼−604∼ −489) of human MMP-9.28 Real-time polymerase chain reaction (PCR) was performed with RT2 Real-time SYBR Green/Rox PCR master mix (SuperArray, Frederick, MD).
Immunocytochemical analysis of NF-κB p65 localization
The effect of anacardic acid on the TNF-induced nuclear translocation of p65 was examined by using an immunocytochemical method with an epifluorescence microscope (Labophot-2; Nikon, Tokyo, Japan) and a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) as described previously.29
Luciferase assay
The effect of anacardic acid on COX-2 promoter activity induced by TNF was analyzed using a luciferase assay. A293 cells (2.5 × 105 cells/well) were seeded in 6-well plates. After overnight culture, the cells in each well were transfected with 0.5 μg of DNA consisting of COX-2 promoter-luciferase reporter by the calcium phosphate method. The COX-2 promoter (−375 ± 59) was provided by Dr Xiao-Chun Xu (The University of Texas M. D. Anderson Cancer Center, Houston, TX). After 24 hours of transfection, the cells were incubated with anacardic acid for 4 hours, then exposed to 1 nmol/L TNF for 20 hours and harvested. Luciferase activity was measured using the luciferase assay system (Promega, Madison, WI) and detected using the Victor3 microplate reader. (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Cytotoxicity assay
Cytotoxicity was assayed by the modified tetrazolium salt 3-(4-5-dimethylthiozol-2-yl)2-5-diphenyl-tetrazolium bromide (MTT) assay as described previously.29
Live/Dead assay
To assess cytotoxicity, we used the Live/Dead assay (Invitrogen), which determines intracellular esterase activity and plasma membrane integrity. In brief, 2 × 105 cells were incubated with 25 μmol/L anacardic acid for 4 hours and then treated with 1 nmol/L TNF for 16 hours at 37°C. Cells were stained with the live and dead reagent (5 μmol/L ethidium homodimer and 5 μmol/L calcein-AM) and incubated at 37°C for 30 minutes. Cells were analyzed under a fluorescence microscope (Labophot-2; Nikon).
Annexin V assay
To detect apoptosis, we used annexin V antibody conjugated with the fluorescent dye fluorescein isothiocyanate (FITC). In brief, 106 cells were pretreated with 25 μmol/L anacardic acid for 12 hours, treated with 1 nmol/L TNF for 24 hours, and then subjected to annexin V staining. Cells were washed, stained with FITC-conjugated anti–annexin V antibody, and then analyzed with a flow cytometer (FACSCalibur; BD Biosciences).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay
We assayed cytotoxicity by the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method using an in situ cell death-detection reagent (Roche Pharmaceuticals, Belleville, NJ). In brief, 106 cells were pretreated with 25 μmol/L anacardic acid for 4 hours and with 1 nmol/L TNF for 24 hours at 37°C. Thereafter, cells were incubated with reaction mixture for 60 minutes at 37°C. Stained cells were quantified by flow cytometry (FACSCalibur; BD Biosciences).
Results
Anacardic acid potentiated apoptosis induced by TNF and chemotherapeutic agents
Because the activation of NF-κB has been shown to inhibit apoptosis induced by TNF and chemotherapeutic agents,30,31 we investigated whether anacardic acid affects TNF- and chemotherapeutic agent–induced apoptosis. As determined by the MTT method, anacardic acid enhanced cytotoxicity induced by TNF, cisplatin, and doxorubicin in variety of human cancer cell lines (Table 1). To determine whether the enhancement of cytotoxicity was due to an increase in apoptosis, we used the Live/Dead assay to detect intracellular esterase activity and plasma membrane integrity. This assay indicated that anacardic acid up-regulated TNF-induced apoptosis from 4% to 25% (Figure 1B). We also used annexin V staining to detect an early step in apoptosis, in which the membrane phospholipid phosphatidylserine moves from the cell cytoplasmic interface to the extracellular surface. These results also indicated enhancement of TNF-induced apoptosis by anacardic acid (Figure 1C). Similar results were obtained with TUNEL staining, which detects DNA strand breaks (Figure 1D). Results from all these assays together suggest that anacardic acid enhances the apoptotic effects of TNF and chemotherapeutic agents.
Table 1.
Anacardic acid potentiates apoptosis induced by TNF and chemotherapeutic agents
| Cell lines | Apoptosis, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| Medium | TNF | Cisplatin | Doxorubicin | |||||
| Anacardic acid, μmol/L | 0 | 25 | 0 | 25 | 0 | 25 | 0 | 25 |
| KBM-5 | 0 ± 0.6 | 3.5 ± 1.9 | 1.7 ± 1.2 | 32.8 ± 1.0 | 12.3 ± 3.4 | 33.8 ± 5.5 | 9.3 ± 2.8 | 31.8 ± 5.4 |
| Jurkat | 0 ± 0.6 | 4.1 ± 1.1 | 3.1 ± 0.8 | 26.7 ± 2.7 | 6.1 ± 1.0 | 24.6 ± 1.8 | 3.2 ± 0.8 | 29.6 ± 2.7 |
| H1299 | 0 ± 0.5 | 4.4 ± 1.2 | 5.4 ± 0.9 | 35.8 ± 5.7 | 8.9 ± 2.1 | 34.9 ± 4.8 | 5.6 ± 1.4 | 29.7 ± 4.7 |
| Du145 | 0 ± 0.9 | 7.6 ± 2.0 | 2.5 ± 1.1 | 29.3 ± 1.1 | 3.2 ± 0.9 | 31.7 ± 2.6 | 7.2 ± 3.1 | 27.3 ± 0.8 |
| SCC4 | 0 ± 0.8 | 3.8 ± 1.7 | 6.7 ± 4.1 | 30.7 ± 5.4 | 7.5 ± 3.1 | 34.6 ± 4.2 | 6.4 ± 1.2 | 33.6 ±4.1 |
Cells (5 × 103/well) were seeded in triplicate in 96-well plates; 12 hours later, cells were pre-exposed to anacardic acid (25 μmol/L) for 4 hours and then to TNF (1 nmol/L), cisplatin (3 μg/mL), or doxorubicin (0.1 μmol/L) for 24 hours. Apoptosis was analyzed by the MTT method.
Anacardic acid potentiated the TNF-induced caspase activation
TNF binds to the TNF receptor (TNFR1), which then sequentially recruits TNFR-associated death domain protein (TRADD), Fas-associated death domain (FADD), and FADD-like IL-1β–converting enzyme (FLICE; also called caspase-8), leading to activation of caspase-9 and caspase-3.32 Whether anacardic acid affects TNF-induced activation of caspases was investigated. We found that TNF alone had a minimal effect on activation of caspase-8, caspase-9, or caspase-3, whereas treatment with anacardic acid potentiated the activation as indicated by the cleaved products (Figure 1E).
The activated caspase-3 is thus known to induce PARP cleavage. Whether anacardic acid activates TNF-induced PARP cleavage was also examined. Results in Figure 1F show that whereas TNF and anacardic acid alone had minimal effect on PARP cleavage; 2 together were very effective in inducing cleavage of PARP. These results suggest that anacardic acid enhances the apoptotic effects of TNF.
Anacardic acid suppressed the expression of antiapoptotic gene products
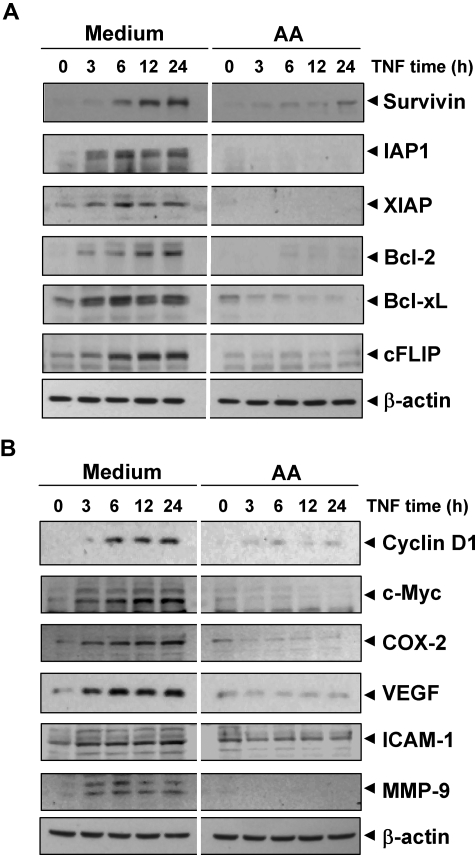
We investigated whether potentiation of TNF-induced apoptosis by anacardic acid is mediated through the down-regulation of cell-survival gene products. Western blot analysis showed that TNF induced these antiapoptotic proteins in a time-dependent manner and that anacardic acid suppressed this increase (Figure 2A). Thus, the enhancement of apoptosis by anacardic acid could be due to down-regulation of these antiapoptotic proteins.
Figure 2.
Anacardic acid represses TNF-induced NF-κB–dependent expression of antiapoptosis-, proliferation-, and metastasis-related gene products. (A) Antiapoptotic proteins. (B) Proliferative and metastatic proteins. KBM-5 cells were incubated with 25 μmol/L anacardic acid for 4 hours and then treated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared and subjected to Western blot analysis using the relevant antibodies.
Anacardic acid suppressed expression of gene products involved in cell proliferation
Various gene products, including cyclin D1, c-myc, and COX-2, are induced by TNF and have been linked with proliferation of tumor cells.20 We investigated whether antitumor effects linked to anacardic acid were mediated through the suppression of these gene products' expression. Western blot analysis showed that TNF induced expression of these proteins and that anacardic acid suppressed the expression (Figure 2B). These results indicate the molecular mechanism by which anacardic acid suppresses tumor cell proliferation.
Anacardic acid suppressed expression of gene products involved in invasion and angiogenesis
Invasion and angiogenesis are critical for tumor metastasis and are induced by TNF. ICAM-1, MMP-9, and VEGF have been implicated in invasion and angiogenesis; therefore, we examined whether this salicylic acid can suppress the expression of these gene products. Western blot analysis showed that TNF induced expression of these proteins and that anacardic acid suppressed their expression (Figure 2B). These results suggest the potential mechanism by which how this compound suppresses invasion and angiogenesis.
Anacardic acid inhibited TNF-dependent NF-κB activation
Because apoptosis and the various gene products noted above are regulated by NF-κB, we investigated whether this salicylate could modulate the NF-κB activation pathway. EMSA showed that anacardic acid suppressed TNF-induced NF-κB activation in both a dose-dependent manner (Figure 3A) and a time-dependent manner (Figure 3B). Anacardic acid alone did not activate NF-κB.
Figure 3.
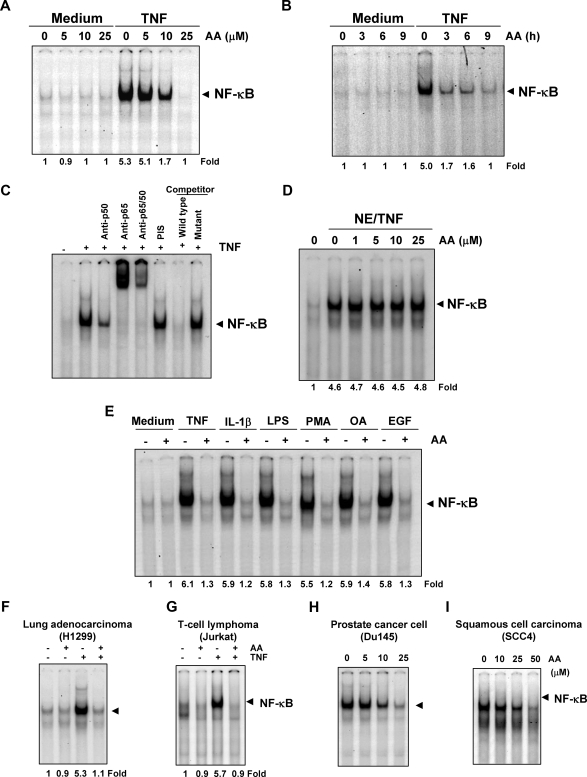
Anacardic acid inhibits TNF-dependent NF-κB activation. (A) Effect of anacardic acid per dose. KBM-5 cells were preincubated with indicated concentrations of anacardic acid for 4 hours, treated with 0.1 nmol/L TNF for 30 minutes, and then subjected to EMSA to test for NF-κB activation. (B) Effect of anacardic acid according to exposure duration. Cells were preincubated with 25 μmol/L anacardic acid for the indicated times, treated with 0.1 nmol/L TNF for 30 minutes, and then subjected to EMSA to test for NF-κB activation. (C) NF-κB induced by TNF is composed of p65 and p50 subunits. Nuclear extracts from untreated or TNF-treated cells were incubated with the indicated antibody, preimmune serum, unlabeled NF-κB oligoprobe, or mutant oligoprobe and then assayed for NF-κB activation by EMSA. (D) The direct effect of anacardic acid on NF-κB complex was investigated. Nuclear extracts were prepared from untreated cells or cells treated with 0.1 nmol/L TNF and incubated for 30 minutes with the indicated concentrations of anacardic acid. They were then assayed for NF-κB activation by EMSA. (E) Anacardic acid blocks NF-κB activation induced by TNF, IL-1β, LPS, PMA, OA, and EGF. KBM-5 cells were preincubated with 25 μmol/L anacardic acid for 4 hours and then treated with 0.1 nmol/L TNF, 100 ng/mL IL-1β, or 10 μg/mL LPS for 30 minutes; 500 nmol/L OA for 4 hours or 25 μg/mL PMA or 100 ng/mL EGF for 2 hours. The cells were then analyzed for NF-κB activation by EMSA. (F-I) Inhibition of NF-κB activation by anacardic acid is not cell type–specific. H1299, Jurkat, Du145, and SCC4 cells were incubated with 25 μmol/L anacardic acid for 4 hours and then incubated with 0.1 nmol/L TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA.
Because NF-κB is a complex of protein in which various combinations of the Rel/NF-κB protein constitute active NF-κB heterodimers that bind specific DNA sequences,33 we decided that it was important to show that the band visualized by EMSA in TNF-treated cells was indeed NF-κB. When nuclear extracts from TNF-stimulated cells were treated with antibodies against the p50 (NF-κB1) or p65 (RelA) subunits of NF-κB, the major band was shifted to a higher molecular mass (Figure 3C), suggesting that the TNF-activated complex consisted of p50 and p65 subunits. Preimmune serum had no effect on this band, excess (100-fold) unlabeled NF-κB caused complete disappearance of the band, and a mutant oligonucleotide of NF-κB did not affect NF-κB binding activity (Figure 3C).
Anacardic acid did not interfere with formation of the TNF-induced NF-κB complex directly
We next sought to determine whether anacardic acid directly modified the binding of NF-κB complex to the DNA. EMSA showed that anacardic acid did not modify the DNA-binding ability of the NF-κB complex (Figure 3D). Therefore, we concluded that anacardic acid inhibits NF-κB activation indirectly rather than directly.
Suppression of NF-κB activation by anacardic acid was not unique to TNF
A wide variety of stimuli have been shown to activate NF-κB, including IL-1β, LPS, PMA, OA, and EGF, through mechanisms that may differ. We investigated whether anacardic acid abrogates NF-κB activation by all these agents. EMSA showed that all these agents activated NF-κB and that anacardic acid suppressed activation (Figure 3E).
Inhibition of NF-κB activation by anacardic acid was not cell type–specific
Because distinct signal transduction pathways can mediate NF-κB induction in different cell types,34 we examined the effect of anacardic acid on TNF-induced NF-κB activation in human lung adenocarcinoma H1299 cells and human T-cell leukemia Jurkat cells. EMSA showed that anacardic acid inhibited TNF-activated NF-κB in both cell types (Figure 3F,G).
Anacardic acid inhibited constitutive NF-κB activation in tumor cells
A wide variety of tumor cells have been shown to express constitutive NF-κB activation through mechanisms that are not fully understood. Using EMSA, we examined whether anacardic acid could suppress constitutive NF-κB activation in human prostate cancer Du145 cells and squamous cell carcinoma SCC4 cells. Treatment with various concentrations of anacardic acid suppressed constitutive NF-κB activation in both cell types (Figure 3H,I).
Anacardic acid inhibited TNF-dependent IκBα phosphorylation and degradation
The translocation of NF-κB to the nucleus is preceded by the proteolytic degradation of IκBα,33 so we next sought to determine whether anacardic acid inhibitory activity was due to inhibition of IκBα degradation. EMSA showed that NF-κB was activated with increasing TNF incubation times and that anacardic acid pretreatment dramatically decreased this activation (Figure 4A). Western blot analysis showed that TNF induced IκBα degradation in control cells within 5 minutes but that anacardic acid inhibited this degradation (Figure 4B). These results indicate that anacardic acid inhibited both TNF-induced NF-κB activation and IκBα degradation.
Figure 4.
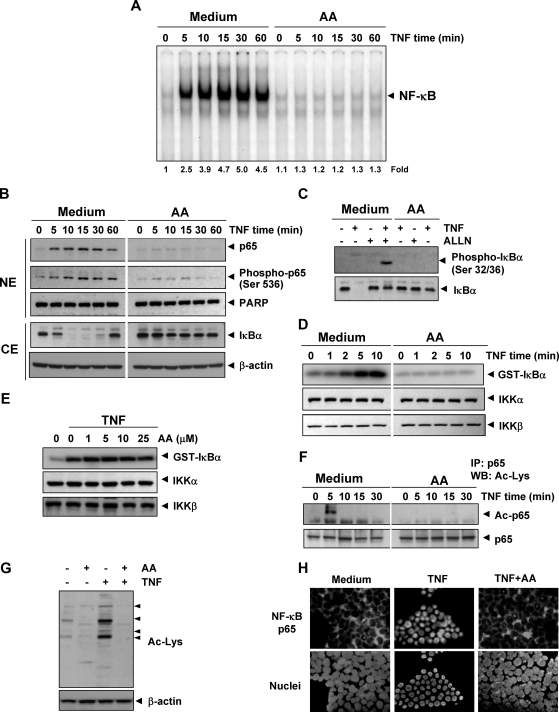
Anacardic acid inhibits TNF-dependent IκBα phosphorylation, IκBα degradation, p65 phosphorylation, and p65 nuclear translocation. (A) Anacardic acid inhibits TNF-induced activation of NF-κB. KBM-5 cells were incubated with 25 μmol/L anacardic acid for 4 hours, treated with 0.1 nmol/L TNF for the indicated times, and then analyzed for NF-κB activation by EMSA. (B) Effect of anacardic acid on TNF-induced IκBα degradation, p65 phosphorylation, and p65 nuclear translocation. Cells were incubated with 25 μmol/L anacardic acid for 4 hours and treated with 0.1 nmol/L TNF for the indicated times. Cytoplasmic extracts (CE) and nuclear extracts (NE) were prepared, fractionated on SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed using the indicated antibody. An anti–β-actin antibody was the loading control. (C) Effect of anacardic acid on the phosphorylation of IκBα by TNF. Cells were preincubated with 25 μmol/L anacardic acid for 4 hours, incubated with 50 μg/mL ALLN for 30 minutes, and then treated with 0.1 nmol/L TNF for 10 minutes. Cytoplasmic extracts were fractionated and then subjected to Western blot analysis using a phospho-specific anti-IκBα antibody. The same membrane was reblotted with anti-IκBα antibody. (D) Anacardic acid inhibits TNF-induced IκBα kinase activity. Whole-cell extracts were immunoprecipitated with antibody against IKKα and analyzed by an immune complex kinase assay. To examine the effect of anacardic acid on the level of expression of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKKα and anti-IKKβ antibodies. (E) Direct effect of anacardic acid on IKK activation induced by TNF. Whole-cell extracts were prepared from KBM-5 cells treated with 1 nmol/L TNF and immunoprecipitated with anti-IKKα antibody. The immunocomplex kinase assay was performed in the absence or presence of the indicated concentration of anacardic acid. (F) Effect of anacardic acid on TNF-induced acetylation of p65. Cells were treated with 25 μmol/L anacardic acid for 4 hours and then exposed to 1 nmol/L TNF. Whole-cell extracts were prepared, immunoprecipitated with an anti-p65 antibody, and subjected to Western blot analysis using an anti–acetyl-lysine antibody. The same blots were reprobed with anti-p65 antibody. (G) Effect of anacardic acid on TNF-induced protein acetylation. Cells were treated with 25 μmol/L anacardic acid for 4 hours and then exposed to 1 nmol/L TNF for 20 minutes. Whole cell extracts were prepared and subjected to Western blot analysis using an anti–acetyl-lysine antibody. (H) Immunocytochemical analysis of p65 localization. Cells were incubated with 25 μmol/L anacardic acid for 4 hours and then treated with 1 nmol/L TNF for 15 minutes. Cells were subjected to immunocytochemical analysis.
To determine whether the inhibition of TNF-induced IκB degradation was due to inhibition of IκBα phosphorylation and ubiquitination, we used the proteasome inhibitor N-acetyl-leucyl-leucyl-norleucinal (ALLN) to block degradation of IκBα.35 Western blot analysis using an antibody that recognizes the serine-phosphorylated form of IκBα showed that TNF induced IκBα phosphorylation and that anacardic acid suppressed phosphorylation (Figure 4C).
Anacardic acid inhibited TNF-induced activation of IKK
Because IKK is required for TNF-induced phosphorylation of IκBα and because anacardic acid inhibited the phosphorylation of IκBα, we determined the effect of anacardic acid on TNF-induced IKK activation. Results from the immune complex kinase assay showed that TNF induced the activation of IKK in a time-dependent manner and that anacardic acid suppressed TNF-activated IKK (Figure 4D). Neither TNF nor anacardic acid affected the expression of IKKα or IKKβ proteins (Figure 4D).
Anacardic acid did not directly inhibit TNF-induced IKK
We and others have previously shown that certain agents suppress NF-κB activation by direct interaction with IKK.29,36 We examined whether anacardic acid suppressed IKK activity directly by binding with the IKK protein. The immune complex kinase assay of whole-cell extracts from untreated and TNF-treated cells showed that anacardic acid did not directly affect the activity of IKK, suggesting that anacardic acid modulated TNF-induced IKK activation indirectly (Figure 4E).
Anacardic acid inhibited nuclear translocation of p65
p65 is a subunit of NF-κB that has nuclear localization signals and retained in the cytoplasm by IκBα. We examined whether the degradation of IκBα leads to nuclear translocation of p65. We found that TNF induced the nuclear translocation of p65 in as little as 5 minutes of incubation and that anacardic acid suppressed p65 translocation (Figure 4B). The immunocytochemical assay also confirmed that anacardic acid suppressed the translocation of p65 from the cytoplasm to the nucleus (Figure 4H).
Anacardic acid inhibited acetylation of p65
Because anacardic acid has been shown to inhibit HAT,13 which has been linked to acetylation of p65,37 we investigated whether anacardic acid could also inhibit acetylation of p65. Western blot analysis showed that TNF induced the acetylation of p65 and that anacardic acid blocked the TNF-induced acetylation (Figure 4F).
Anacardic acid suppressed TNF-induced HAT activity
Whether anacardic acid directly inhibits HAT activity was also investigated. To determine this, whole-cell extracts from cells treated with TNF, anacardic acid, or both were resolved on SDS-PAGE and then analyzed by Western blot using anti-acetyl lysine antibody. As shown Figure 4G, TNF induced acetylation of several proteins, whereas anacardic acid suppressed the acetylation of these proteins.
Anacardic acid repressed TNF-induced NF-κB–dependent reporter gene expression
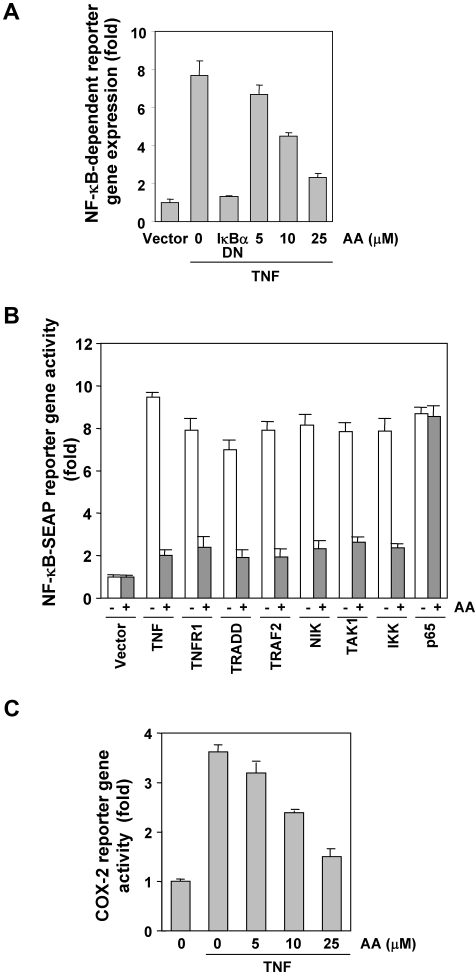
Although EMSA showed that anacardic acid blocked NF-κB activation, DNA binding alone does not always correlate with NF-κB–dependent gene transcription, suggesting that there are additional regulatory steps. We investigated whether anacardic acid could suppress the TNF-induced NF-κB reporter activity. TNF induced a NF-κB–regulated secretory alkaline phosphatase (SEAP) reporter gene's expression in a dose-dependent manner, and anacardic acid suppressed the expression (Figure 5A).
Figure 5.
Anacardic acid represses NF-κB–dependent reporter gene expression induced by TNF and various plasmids. (A) Anacardic acid inhibits the NF-κB–dependent reporter gene expression induced by TNF. A293 cells were transiently transfected with a NF-κB–containing plasmid for 24 hours. After transfection, the cells were incubated with the indicated concentrations of anacardic acid for 4 hours and then treated with 1 nmol/L TNF for an additional 24 hours. The supernatants of the culture media were assayed for SEAP activity. Data are presented as mean (± SD). (B) Anacardic acid inhibits the NF-κB–dependent reporter gene expression induced by TNF, TNFR1, TRADD, TRAF2, NIK, IKK, p65, and TAK1/TAB1. Cells were transiently transfected with a NF-κB–containing plasmid alone or with the indicated plasmids. After transfection, cells were incubated with 25 μmol/L anacardic acid for 4 hours and then incubated with the relevant plasmid for an additional 24 hours. TNF-treated cells were incubated with 25 μmol/L anacardic acid for 4 hours and then treated with 1 nmol/L TNF for an additional 24 hours. The supernatants of the culture media were assayed for SEAP activity. Data are presented as mean (± SD). (C) Anacardic acid inhibits the COX-2 promoter activity induced by TNF. Cells were transiently transfected with a COX-2 promoter linked to the luciferase reporter gene plasmid for 24 hours and treated with the indicated concentrations of anacardic acid for 4 hours. Cells were then treated with 1 nmol/L TNF for an additional 24 hours, lysed, and subjected to a luciferase assay. Data are presented as mean (± SD).
Anacardic acid repressed NF-κB–dependent reporter gene expression induced by TNFR1, TRADD, TRAF2, NIK, and IKK
TNF has been shown to activate NF-κB activation through sequential interaction with the TNF receptor (TNFR), TRADD, TNFR-associated factor 2 (TRAF2), NF-κB–inducing kinase (NIK), and IKK, resulting in phosphorylation of IκBα.38,39 We examined where in this pathway the salicylic acid acts. To determine the effect of anacardic acid on NF-κB–dependent reporter gene expression, cells were transiently transfected with TNFR1-, TRADD-, TRAF2-, NIK-, IKK-, and p65-expressing plasmids and then monitored for NF-κB–dependent SEAP expression. We found that cells transfected with any of these plasmids expressed the NF-κB–regulated reporter gene and that for all except the p65 plasmid, expression was suppressed by anacardic acid (Figure 5B). These results suggest that the anacardic acid effect occurs at a step upstream from p65.
Anacardic acid repressed NF-κB–dependent reporter gene expression induced by TAK1
Tumor growth factor (TGF)-activated kinase 1 (TAK1), a member of the mitogen-activated protein kinase (MAPK) family, was originally identified as a key regulator of MAPK activation in TGF-β–induced signaling pathways. It is activated by various inflammatory stimuli, including TNF, IL-1, and LPS.40 Recent studies indicate that TAK1 plays a major role in TNF-induced NF-κB activation through its interaction with TAK1-binding protein (TAB) 1 and TAB2.41 We examined whether anacardic acid suppressed TNF-induced NF-κB activation through the inhibition of TAK1. As shown in Figure 5B, NF-κB–dependent reporter gene expression was induced in cells transfected with TAK1/TAB1, and anacardic acid inhibited this activation.
Anacardic acid inhibited TNF-induced COX-2 promoter activity
TNF induces COX-2, which has NF-κB binding sites in its promoter.42 Because down-regulation of NF-κB by anacardic acid suppressed the expression of NF-κB–regulated gene products, including COX-2, we examined the effect of anacardic acid on TNF-induced COX-2 promoter activity by using a COX-2 promoter-luciferase reporter plasmid. We found that TNF induced COX-2 promoter activity and that anacardic acid suppressed this activity in a dose-dependent manner (Figure 5C). This result suggests that anacardic acid inhibited NF-κB–regulated gene expression by suppressing NF-κB binding to the COX-2 promoter.
NF-κB was needed for the effects of anacardic acid on suppression of gene products
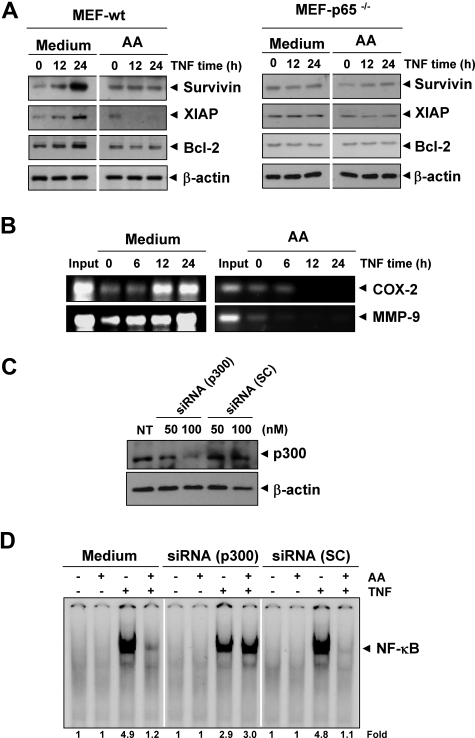
To determine whether presence of NF-κB is essential for the effects of anacardic acid, we used cells in which NF-κB gene (p65) has been deleted. Results showed that TNF induced the expression of antiapoptotic proteins (such as survivin, XIAP, and Bcl-2) in wild-type (Figure 6A left panel), whereas anacardic acid suppressed these gene expression. In p65−/− cells that lack functional NF-κB, TNF failed to induce the expression of these antiapoptotic gene products (Figure 6A right panel). Thus, these results suggest that NF-κB was needed for the expression of these gene products.
Figure 6.
Down-regulation of p300 HAT abrogates the effect of anacardic acid. (A) TNF regulates antiapoptotic gene expression. The wild-type and p65−/− MEF cells were pretreated with 25 μmol/L anacardic acid for 4 hours and then incubated with 1 nmol/L TNF for the indicated times. Whole-cell extracts were prepared and subjected to Western blot analysis using the relevant antibodies. (B) Anacardic acid inhibits binding of NF-κB to the COX-2 and MMP-9 promoter. KBM-5 cells were pretreated with 25 μmol/L anacardic acid for 4 hours and treated with 1 nmol/L TNF for the indicated times, and the proteins were cross-linked with DNA by formaldehyde and then subjected to chromatin immunoprecipitation (ChIP) assay using an anti-p65 antibody with the COX-2 and MMP-9 primers. Reaction products were resolved by electrophoresis. (C) Down-regulation of p300 by RNA interference reverses the effect of anacardic acid. A293 cells were transfected with indicated concentration of p300 siRNA or scrambled (SC) control. After 48 hours, cells were harvested, and whole-cell extracts were prepared and analyzed by Western blotting with an anti-p300 antibody. (D) Transfected cells were preincubated with 25 μmol/L anacardic acid for 4 hours and then treated with 0.1 nmol/L TNF for 30 minutes. The nuclear extracts were prepared and assayed for NF-κB activation by EMSA.
Anacardic acid inhibited TNF-induced COX-2 and MMP-9 through NF-κB
Whether the lack of TNF-induced COX-2 and MMP-9 expression in anacardic acid–treated cells was due to suppression of NF-κB activation in vivo was examined by using chromatin immunoprecipitation assay targeting NF-κB binding in the COX-2 and MMP-9 promoter. For this, KBM-5 cells were pretreated with anacardic acid and then treated with TNF for indicated times. Thereafter, the cross-linked reaction was carried out in situ with DNA-protein complexes, and the chromatin was isolated and sheared. Subsequently, the chromatin was immunoprecipitated with anti-p65 antibody, and the DNA was purified and subjected to PCR using COX-2 or MMP-9 promoter-specific primer. We found that TNF induced NF-κB binding to both COX-2 and MMP-9 promoters in a time-dependent manner and that anacardic acid suppressed it (Figure 6B). Overall, these results suggest that anacardic acid inhibited NF-κB–regulated gene expression by suppressing NF-κB binding to the promoter of these genes.
Down-regulation of p300 HAT abrogates the effect of anacardic acid
Anacardic acid has been shown to inhibit the activity of histone acetyltransferase p300.12 Whether the effect of anacardic acid on NF-κB is mediated by p300 HAT was investigated. The results in Figure 6C show that siRNA but not scrambled control RNA down-regulated the expression of p300 protein. Furthermore, depletion of p300 abrogated the effect of anacardic acid on TNF-induced NF-κB activation (Figure 6D). These results clearly demonstrate that p300 HAT is a target of anacardic acid for suppression of NF-κB activation.
Discussion
The goal of this study was to investigate the effect of anacardic acid on the NF-κB activation pathway and NF-κB–regulated gene products that regulate apoptosis. We found that anacardic acid suppressed NF-κB activated by carcinogens, growth factors, and inflammatory stimuli through inhibition of IKK activation, IκBα phosphorylation, IκBα degradation, p65 phosphorylation, and NF-κB–dependent reporter gene expression. Under identical conditions, anacardic acid alone had no effect. As a result, anacardic acid down-regulates the expression of NF-κB–dependent gene products involved in cell proliferation, antiapoptosis, invasion, and angiogenesis. We also demonstrated that anacardic acid potentiates apoptosis induced by TNF and chemotherapeutic agents and thus may have potential as an anticancer agent.
To our knowledge, this is the first report of an investigation into the effect of anacardic acid on NF-κB activation by a variety of stimuli. Our results show that anacardic acid suppressed NF-κB activated by a variety of stimuli, suggesting that anacardic acid must act at a step common to all these activators. In addition, our results showed that anacardic acid blocked NF-κB activation without directly interfering with its DNA binding. NF-κB activation in response to different stimuli requires IKK activation, which phosphorylates IκBα at serine 32 and 36, leading to degradation of IκBα.33 We found that this inhibition was mediated through the inhibition of IKK by anacardic acid, which led to the suppression of phosphorylation and the degradation of IκBα.
We investigated how anacardic acid suppressed IKK activation by examining its effects on several kinases that function upstream of IKK, such as mitogen-activated protein kinase kinase kinase (MEKK) 1,43 MEKK3,44 protein kinase C,45 glycogen synthase kinase-3β,46 TAK1,47 phosphoinositide-dependent kinase 1,48 and Akt.49 Recent studies indicate that TAK1 plays a major role in the canonical pathway activated by cytokines through its interaction with TAB1 and TAB2.47 TAK1 also has been shown to be recruited by the TNFR1 through TRADD, TRAF2, and receptor-interacting protein. Indeed, our study shows for the first time that TAK1-induced NF-κB activation is inhibited by anacardic acid, thereby suggesting that TAK1 is the main upstream stimulatory kinase modulated by anacardic acid.
Besides inducible NF-κB activation, we found that anacardic acid inhibited constitutive NF-κB activation. Constitutively active NF-κB has been found in a wide variety of leukemic and tumor epithelial cells50 and is needed for these cells' proliferation.51,52 It is not fully understood why tumor cells express constitutively active NF-κB, but IKK has been implicated.51,52 Thus, it is possible that anacardic acid's inhibition of IKK in tumor cells is linked to its ability to suppress constitutive NF-κB activation.
Anacardic acid has been reported to inhibit HAT, p300, and p300/cAMP response element-binding protein–binding protein–associated factor.12,14,53 Anacardic acid has been shown to inhibit HAT-dependent transcription of histone H3 but to have no effect on transcription from naked DNA.12 In addition, acetylation of RelA (p65) at lysine 310, one step in the TNF-induced NF-κB activation pathway, is regulated by prior phosphorylation of serine 276 and 536. Such phosphorylated and acetylated forms of RelA display enhanced transcriptional activity.37 Our data suggest that anacardic acid may suppress TNF-induced p65 acetylation through inhibition of HAT activity.
It is also possible that anacardic acid could suppress TNF-induced NF-κB activation through inhibition of reactive oxygen species (ROS) production. That anacardic acid could exhibit antioxidant role has been well demonstrated.9,10,17 The antioxidant capacity of anacardic acid is more related to inhibition of superoxide generation (IC50 = 0.04 mmol/L) and of xanthine oxidase (IC50 = 0.30 mmol/L) than to scavenging of hydroxyl radicals. Antioxidant effects of anacardic acid could also be due to its activity as mitochondrial uncoupler of oxidative phosphorylation described previously.19 Hayakawa et al, however, reported that production of ROS is unrelated to the NF-κB activation.54 Thus NF-κB inhibitory activity of anacardic acid may not be linked to its ability to quench ROS.
We also found that anacardic acid down-modulated expression of antiapoptotic survivin, XIAP, Bcl-2, Bcl-xL, and FLIP. These gene products are regulated by NF-κB, and their overexpression in numerous tumors has been associated with tumor survival, chemoresistance, and radioresistance. Previous reports show that anacardic acid could sensitize tumor cells to ionizing radiation13; it is possible that these effects are mediated through the mechanism described here. We also observed that anacardic acid potentiates the apoptotic effects of TNF, cisplatin, and doxorubicin, suggesting that anacardic acid could be used to enhance this effect in chemotherapeutic regimens.
Anacardic acid also suppressed gene products that have been implicated in angiogenesis and invasion. We found that the expression of NF-κB–regulated gene products involved in invasion (eg, COX-2, MMP-9, and ICAM-1) and proliferation (eg, cyclin D1 and c-Myc) were abrogated by anacardic acid. Likewise, Paramashivappa et al (2003) showed that anacardic acid inhibits human COX-2 activity18 and Granzzini et al (1991) found this effect on lipoxygenase,16 which also is regulated by NF-κB. These results are in agreement also with results showing that celecoxib also inhibits COX-2 expression through inhibition of NF-κB activation.55
We found that anacardic acid also suppressed the expression of VEGF, a growth factor critical for tumor angiogenesis. Bevacizumab (Avastin), an antibody against VEGF, has been approved for the treatment of cancer and macular degeneration,56 and our results demonstrate the potential of anacardic acid in these diseases.
Whether anacardic acid has any effect on normal cells is not clear. A recent report suggests that anacardic acid and its analogs inhibit the growth of tumor cells but had no effect on nonmalignant cells.57 The pharmacokinetic and pharmacodynamic effects of anacardic acid in animals need to be investigated to fully realize its clinical potential.
Overall, our results demonstrate that anacardic acid is a potent inhibitor of NF-κB activation, which may explain its antiangiogenic, antiproliferative, proapoptotic, antimetastatic, anti-inflammatory, and immunomodulatory effects. Further studies are needed to explore its therapeutic potential against cancer and cardiovascular and neurologic diseases.
Acknowledgments
We thank Alyson Todd for careful comments on the manuscript.
This work was supported financially by the Clayton Foundation (to B.B.A.) and by a National Cancer Institute core grant.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.S., M.K.P., K.S.A., and T.Y. conducted all the experiments. M.M.C. analyzed the data. B.B.A. and M.L. supervised and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Bharat B. Aggarwal, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 143, Houston, TX 77030; e-mail: aggarwal@mdanderson.org.
References
- 1.Olivera Ortega AG, Soto Hernandez M, Martinez Vazquez M, Terrazas Salgado T, Solares Arenas F. Phytochemical study of cuachalalate (Amphiptherygium adstringens, Schiede ex Schlecht). J Ethnopharmacol. 1999;68:109–113. doi: 10.1016/s0378-8741(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 2.Acevedo HR, Rojas MD, Arceo SD, et al. Effect of 6-nonadecyl salicylic acid and its methyl ester on the induction of micronuclei in polychromatic erythrocytes in mouse peripheral blood. Mutat Res. 2006;609:43–46. doi: 10.1016/j.mrgentox.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Rea AI, Schmidt JM, Setzer WN, Sibanda S, Taylor C, Gwebu ET. Cytotoxic activity of Ozoroa insignis from Zimbabwe. Fitoterapia. 2003;74:732–735. doi: 10.1016/j.fitote.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Kubo I, Kinst-Hori I, Yokokawa Y. Tyrosinase inhibitors from Anacardium occidentale fruits. J Nat Prod. 1994;57:545–551. doi: 10.1021/np50106a021. [DOI] [PubMed] [Google Scholar]
- 5.Itokawa H, Totsuka N, Nakahara K, Takeya K, Lepoittevin JP, Asakawa Y. Antitumor principles from Ginkgo biloba L. Chem Pharm Bull. 1987;35:3016–3020. doi: 10.1248/cpb.35.3016. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan JT, Richards CS, Lloyd HA, Krishna G. Anacardic acid: molluscicide in cashew nut shell liquid. Planta Med. 1982;44:175–177. doi: 10.1055/s-2007-971434. [DOI] [PubMed] [Google Scholar]
- 7.Muroi H, Kubo I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J Appl Bacteriol. 1996;80:387–394. doi: 10.1111/j.1365-2672.1996.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 8.Kubo J, Lee JR, Kubo I. Anti-Helicobacter pylori agents from the cashew apple. J Agric Food Chem. 1999;47:533–537. doi: 10.1021/jf9808980. [DOI] [PubMed] [Google Scholar]
- 9.Trevisan MT, Pfundstein B, Haubner R, et al. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem Toxicol. 2006;44:188–197. doi: 10.1016/j.fct.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Masuoka N, Kubo I. Characterization of xanthine oxidase inhibition by anacardic acids. Biochim Biophys Acta. 2004;1688:245–249. doi: 10.1016/j.bbadis.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Cho YS, Park EJ, et al. Phospholipase Cgamma1 inhibitory principles from the sarcotestas of Ginkgo biloba. J Nat Prod. 1998;61:867–871. doi: 10.1021/np970367q. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278:19134–19140. doi: 10.1074/jbc.M301580200. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Jiang X, Chen S, Price BD. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006;580:4353–4356. doi: 10.1016/j.febslet.2006.06.092. [DOI] [PubMed] [Google Scholar]
- 14.Mai A, Rotili D, Tarantino D, et al. Small-molecule inhibitors of histone acetyltransferase activity: identification and biological properties. J Med Chem. 2006;49:6897–6907. doi: 10.1021/jm060601m. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Girard TJ, Kasten TP, LaChance RM, Miller-Wideman MA, Durley RC. Inhibitory activity of unsaturated fatty acids and anacardic acids toward soluble tissue factor-factor VIIa complex. J Nat Prod. 1998;61:1352–1355. doi: 10.1021/np980117p. [DOI] [PubMed] [Google Scholar]
- 16.Grazzini R, Hesk D, Heininger E, et al. Inhibition of lipoxygenase and prostaglandin endoperoxide synthase by anacardic acids. Biochem Biophys Res Commun. 1991;176:775–780. doi: 10.1016/s0006-291x(05)80252-9. [DOI] [PubMed] [Google Scholar]
- 17.Ha TJ, Kubo I. Lipoxygenase inhibitory activity of anacardic acids. J Agric Food Chem. 2005;53:4350–4354. doi: 10.1021/jf048184e. [DOI] [PubMed] [Google Scholar]
- 18.Paramashivappa R, Phani Kumar P, Subba Rao PV, Srinivasa Rao A. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg Med Chem Lett. 2003;13:657–660. doi: 10.1016/s0960-894x(02)01006-5. [DOI] [PubMed] [Google Scholar]
- 19.Toyomizu M, Okamoto K, Ishibashi T, Chen Z, Nakatsu T. Uncoupling effect of anacardic acids from cashew nut shell oil on oxidative phosphorylation of rat liver mitochondria. Life Sci. 2000;66:229–234. doi: 10.1016/s0024-3205(99)00585-8. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 21.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 24.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-κB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IκBα kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 25.Peters AH, Kubicek S, Mechtler K, et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 26.Zabel U, Schreck R, Baeuerle PA. DNA binding of purified transcription factor NF-κB. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J Biol Chem. 1991;266:252–260. [PubMed] [Google Scholar]
- 27.Nakao S, Ogtata Y, Shimizu E, Yamazaki M, Furuyama S, Sugiya H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol Cell Biochem. 2002;238:11–18. doi: 10.1023/a:1019927616000. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa K, Chen F, Kuang C, Chen Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappaB site. Biochem J. 2004;381:413–422. doi: 10.1042/BJ20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J Biol Chem. 2007;282:17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 30.Mayo MW, Wang CY, Cogswell PC, et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 31.Giri DK, Aggarwal BB. Constitutive activation of NF-κB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 32.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 34.Bonizzi G, Piette J, Merville MP, Bours V. Distinct signal transduction pathways mediate nuclear factor-kappaB induction by IL-1beta in epithelial and lymphoid cells. J Immunol. 1997;159:5264–5272. [PubMed] [Google Scholar]
- 35.Vinitsky A, Michaud C, Powers JC, Orlowski M. Inhibition of the chymotrypsin-like activity of the pituitary multicatalytic proteinase complex. Biochemistry. 1992;31:9421–9428. doi: 10.1021/bi00154a014. [DOI] [PubMed] [Google Scholar]
- 36.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 37.Chen LF, Williams SA, Mu Y, et al. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simeonidis S, Stauber D, Chen G, Hendrickson WA, Thanos D. Mechanisms by which IkappaB proteins control NF-kappaB activity. Proc Natl Acad Sci U S A. 1999;96:49–54. doi: 10.1073/pnas.96.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 41.Kanayama A, Seth RB, Sun L, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 43.Lee FS, Peters RT, Dang LC, Maniatis T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc Natl Acad Sci U S A. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 45.Lallena MJ, Diaz-Meco MT, Bren G, Paya CV, Moscat J. Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai H, Miyoshi H, Toriumi W, Sugita T. Functional interactions of transforming growth factor β-activated kinase 1 with IκB kinases to stimulate NF-κB activation. J Biol Chem. 1999;274:10641–10648. doi: 10.1074/jbc.274.15.10641. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Fujita N, Tsuruo T. 3-Phosphoinositide-dependent protein kinase-1-mediated IκB kinase β (IKKB) phosphorylation activates NF-κB signaling. J Biol Chem. 2005;280:40965–40973. doi: 10.1074/jbc.M506235200. [DOI] [PubMed] [Google Scholar]
- 49.Gustin JA, Maehama T, Dixon JE, Donner DB. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor κB activity. J Biol Chem. 2001;276:27740–27744. doi: 10.1074/jbc.M102559200. [DOI] [PubMed] [Google Scholar]
- 50.Jackson-Bernitsas DG, Ichikawa H, Takada Y, et al. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 2007;26:1385–1397. doi: 10.1038/sj.onc.1209945. [DOI] [PubMed] [Google Scholar]
- 51.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 52.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 53.Davidson SM, Townsend PA, Carroll C, et al. The transcriptional coactivator p300 plays a critical role in the hypertrophic and protective pathways induced by phenylephrine in cardiac cells but is specific to the hypertrophic effect of urocortin. Chembiochem. 2005;6:162–170. doi: 10.1002/cbic.200400246. [DOI] [PubMed] [Google Scholar]
- 54.Hayakawa M, Miyashita H, Sakamoto I, et al. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shishodia S, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates activation of cigarette smoke-induced nuclear factor (NF)-kappaB by suppressing activation of IkappaBalpha kinase in human non-small cell lung carcinoma: correlation with suppression of cyclin D1, COX-2, and matrix metalloproteinase-9. Cancer Res. 2004;64:5004–5012. doi: 10.1158/0008-5472.CAN-04-0206. [DOI] [PubMed] [Google Scholar]
- 56.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 57.Eliseeva ED, Valkov V, Jung M, Jung MO. Characterization of novel inhibitors of histone acetyltransferases. Mol Cancer Ther. 2007;6:2391–2398. doi: 10.1158/1535-7163.MCT-07-0159. [DOI] [PubMed] [Google Scholar]