Abstract
Protein Z (PZ) is a plasma vitamin K–dependent protein that functions as a cofactor to dramatically enhance the inhibition of coagulation factor Xa by the serpin, protein Z–dependent protease inhibitor (ZPI). In vitro, ZPI not only inhibits factor Xa in a calcium ion–, phospholipid-, and PZ-dependent fashion, but also directly inhibits coagulation factor XIa. In murine gene-deletion models, PZ and ZPI deficiency enhances thrombosis following arterial injury and increases mortality from pulmonary thromboembolism following collagen/epinephrine infusion. On a factor VLeiden genetic background, ZPI deficiency produces a significantly more severe phenotype than PZ deficiency, implying that factor XIa inhibition by ZPI is physiologically relevant. The studies in mice suggest that human PZ and ZPI deficiency would be associated with a modest thrombotic risk with ZPI deficiency producing a more severe phenotype.
Introduction
Protein Z (PZ) is a vitamin K–dependent plasma protein with structural homology to the coagulation factors VII, IX, X, and protein C.1–3 In contrast to other coagulation factors that are serine proteinase zymogens, in PZ the region around the typical “activation site” is absent and the histidine and serine residues of the canonical catalytic triad have been replaced with lysine and aspartic acid residues, respectively. Thus, like protein S, PZ does not possess a proteolytic function. Instead, PZ serves as a cofactor for the inhibition of factor Xa by another plasma protein called protein Z–dependent protease inhibitor (ZPI),4 a member of the serpin superfamily of protease inhibitors.5
In healthy individuals, the range of plasma levels for both PZ and ZPI are unusually broad, apparently related to heritable factors.6–8 PZ and ZPI form a complex and in pooled normal plasma, which contains excess ZPI, all the PZ appears to be bound to ZPI.9 The plasma levels of PZ and ZPI are interrelated: oral contraceptive use raises both PZ (∼35%) and ZPI (∼17%) levels, whereas warfarin treatment reduces PZ (∼92%) and ZPI (∼53%) levels.7
In the presence of phospholipids and Ca2+, the rate of factor Xa inhibition by ZPI is enhanced several hundred-fold by preincubation of factor Xa with PZ.4 Indirect evidence strongly suggests that the inhibitory process involves the formation of a stoichiometric complex of factor Xa-ZPI-PZ at the phospholipid surface. The combination of PZ and ZPI dramatically delays the initiation and reduces the ultimate rate of thrombin generation in mixtures containing prothrombin, factor V, phospholipids, and Ca2+.10 In similar mixtures containing activated factor V (FVa), however, PZ and ZPI do not inhibit thrombin generation. Thus, the anti–factor Xa action of PZ and ZPI presumably occurs early in coagulation, before the activation of factor V and the formation of the prothrombinase complex. ZPI also inhibits factor XIa in a reaction that does not require the presence of PZ, phospholipids or Ca2+, and that is not affected by the presence of high-molecular-weight kininogen.10 An apparent interaction between factor XIa and ZPI can be detected in the plasma milieu, suggesting that ZPI competes effectively with other XIa inhibitors (eg, α1-antirypsin, C1 esterase inhibitor, antithrombin) and the substrate factor IX in plasma for the active site of factor XIa.10 Consistent with this notion, kinetic studies have shown that ZPI inhibits factor XIa 100-fold more potently than the other known factor XIa inhibitors in plasma.11
Unchallenged, PZ knockout mice do not express an obvious phenotype. When combined with the homozygous factor VLeiden [FV(λ/λ)] genotype, however, the PZ(−/−) genotype causes perinatal thrombosis and an apparent consumptive coagulopathy that leads to near absolute mortality.12 The genetic combinations FV(λ/λ)/PZ(+/−) and FV(λ/+)/PZ(−/−) also reduce the survival of mice. To better define the physiologic role of ZPI, ZPI gene-deleted mice were produced and their phenotype compared with that of PZ gene-deleted animals. The results demonstrate a more severe thrombotic phenotype in ZPI-null mice than in PZ-null mice, suggesting that the inhibition of factor XIa by ZPI is physiologically relevant.
Methods
Materials
Restriction enzymes, HighPrime, DIG-High Prime Labeling and Detection Starter Kit II, Lumi-Light Western blotting substrate, and positively charged nylon membranes were from Roche (Indianapolis, IN). StrataPrep DNA Gel Extraction Kit was from Stratagene (La Jolla, CA). The Protein Chemistry Laboratory at Washington University School of Medicine synthesized all oligonucleotides. Protran pure nitrocellulose transfer and immobilization membranes were from Schleicher and Schuell (Keene, NH) and Kaleidoscope prestained standards from Bio-Rad (Hercules, CA). Taq DNA polymerase, lambda DNA/HindIII markers and PCR markers were from Promega (Madison, WI). KB-100 Magnum Plasmid Purification Kits, GSI embryonic stem (ES) cells and RW4 and MC50 ES cells were from Genome Systems (St Louis, MO) and the Washington University Embryonic Stem Cell Core facility, respectively. Mouse embryonic fibroblasts were from Genome Systems. Tris, EDTA, and horseradish peroxidase–labeled goat anti–rabbit IgG antibodies were from Sigma Chemical (St Louis, MO). QIAprep Spin Miniprep Kit, Qiagen plasmid Maxi Kit, and DNeasy Tissue Kit were from Qiagen (Valencia, CA). Collagen type 1 from equine tendons was from Chrono-Log (Havertown, PA) and epinephrine from Sigma Chemical.
Construction of ZPI gene-targeting vector
Based on the murine ZPI cDNA sequence,13 3 pairs of primers were designed to screen a BAC II genomic DNA library made from the 129/Sv mouse strain (Genome Systems). A positive clone was sequenced and subcloned for restriction enzyme mapping and partial DNA sequencing. The mouse ZPI gene consists of 5 exons spanning more than 14 kb. An 8.5 kb EcoR1 fragment of the ZPI gene, spanning intron A to intron D, was subcloned for construction of the targeting vector. The 1286 bp fragment between the PstI site in intron A and an EcoRV site in exon 2 was replaced with a 1.7 kb PGK-neomycin phosphotransferase cassette (Neo), thereby removing the ZPI DNA that encodes the signal peptide and N-terminus of ZPI and inducing a frame-shift mutation. A 1.8 kb HSV-thymidine kinase cassette was added at the 3′ end of the construct to permit negative selection. The structure of the targeting construct was confirmed by PCR, restriction mapping, and sequencing of ligation junctions.
Targeted ZPI gene disruption in embryonic stem cells
The targeting vector was linearized with Not I and introduced into 129/Sv-derived embryonic stem cells by electroporation. The transfected ES cells were grown on irradiated neomycin-resistant mouse embryonic fibroblasts and selected with G418 (300 μg/mL; Gibco/BRL, Grand Island, NY) and gancyclovir (2 μM; Syntex, Palo Alto, CA). After 7 days, surviving clones were tested for homologous recombination at a ZPI allele by PCR and confirmed by Southern analysis.
The PCR assay to screen ES cells used 3 primers derived from (1) intron A just 5′ of the EcoR 1 site (5′-GAG CGC TTA ATT TAC AAA GCA GAG-3′); (2) a portion of intron A deleted in the mutant allele (5′AGA TCC TTG AGC AAT GTG TGG TCC-3′); and (3) the Neo cassette (5′CGA GGC CAG AGG CCA CTT GTG TAG CGC CAA GAG-3′). The amplicon derived from the wild-type ZPI allele is 1443 bp; that derived from the mutant ZPI allele is 1563 bp.
Generation of ZPI gene-disrupted mice
Three GSI, 3 RW4, and 1 MC-50 clones contained the disrupted ZPI allele by PCR and Southern analysis. The GSI stem cell clones were injected into C57Bl/6 blastocysts, but failed to produce highly chimeric mice based on coat color or to transmit the targeted gene to subsequent progeny. The injection of a RW4 stem clone into blastocysts led to the production of highly chimeric mice and a chimeric male was bred to C57Bl/6 females to generate initial F1 ZPI(+/−) offspring.
To produce mice that were heterozygous for factor VLeiden and ZPI [FV(λ/+)/ZPI(+/−)], ZPI(−/−) mice derived from F1 intercrosses were mated with homozygous FV(λ/λ) mice in a mixed C57Bl/6 × 129/Sv genetic background.14 Animals from subsequent FV(λ/+)/ZPI(+/−) crosses were then used in various mating strategies to determine the effect of the ZPI genotype on the survival of FVLeiden mice. Mice heterozygous for factor VLeiden and PZ were produced in the same fashion.
Genotyping of the mice
DNA prepared from tail biopsies or blood was used for Southern and PCR analysis. For Southern analysis of ZPI mice, genomic DNA (20 μg) was treated with XbaI, agarose electrophoresed, and transferred to a positively charged nylon membrane. The hybridization probe was a 673 bp XbaI–EcoRI fragment of ZPI genomic DNA 5′ of the region of homologous recombination.
The PCR assay for the ZPI gene used 3 primers derived from (1) intron A (5′-GGC ACA ACT CAG AGC CAG GTT TCG GAT CTG-3′); (2) a portion of intron A deleted in the mutant allele (5′CAG AGT TCC CGA TAG GTC TTC TTT CAG TCC-3′); and (3) the NEO cassette (5′-CCA TCT GCA CGA GAC TAG TGA GAC GTG CTA-3′). The amplicon derived from the wild-type ZPI allele is 256 bp; that derived from the mutant allele is 376 bp.
Two PCR reactions were used for PZ genotyping: (1) the primer pair AAA CAA CGT TCT GCG GAG GTG GA and AAC GAA CTA GTT AGT CCT GAG ACA produced an amplicon of 200 bp that identified the wild-type allele; and (2) the primer pair TTC CTG AXT AGG GGA GGA GTA GAA G and TGC TCA CAC TGT TCT GCC TCT CTA C produced an amplicon of 300 bp that identified the mutant allele.
The PCR assay for the FVLeiden genotype used 2 primers: (1) intron 10, upstream of an inserted lox P sequence (5′-CCT CTG GAC TCXT GAC TGC AG-3′); and (2) intron 10, downstream of the lox P (5′-TAT TCT GGA CTA CAA GAG TGA-3′). The amplicon derived from the wild-type FV allele is 400 bp; that derived from the FVLeiden allele is 544 bp.
Western blotting
Western blotting was used to determine the levels of ZPI and PZ in mouse plasma. For ZPI, 5 μL of 1:10 diluted mouse plasma was run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The blot was developed using rabbit polyclonal antihuman ZPI antibodies and chemiluminescence. For PZ, 12.5 μL sodium citrate (80 mM) was added to 25 μL heparinized plasma followed by 12.5 μL BaCl2 (400 mM). The mixture was incubated on ice, with occasional mixing, for 10 minutes and then centrifuged 2 minutes at 14 000g. The supernatant was aspirated and discarded and the pellet was washed by suspending it in 100 μL BaCl2 (25 mM) and centrifuging 2 minutes at 14 000g. After discarding the supernatant, the pellet was suspended in 12.5 μL ammonium sulfate (1.0 M), and the mixture was then mixed and centrifuged 2 minutes at 14 000g. A portion of the supernatant containing the vitamin K–dependent coagulation factors, including PZ, was subsequently analyzed by 10% SDS-PAGE and Western blotting using rabbit polyclonal antihuman PZ antibodies and chemiluminescence. The relative amounts of ZPI and PZ were determined by densitometry using a Kodak Image Station 440 (Eastman Kodak, Rochester, NY) and standards produced using mixtures of plasma from ZPI and PZ wild-type and gene-deleted mice.
Murine thrombosis models
In the FeCl3 carotid artery occlusion model the right carotid artery is isolated from surrounding tissues, a distal flow probe is placed beneath the artery, a 1.5 × 2 mm piece of filter paper soaked in 5% freshly prepared FeCl3 is placed on the anterior surface of the artery for 3 minutes, and the area is then flushed with normal saline. Blood flow is recorded on a flow meter (Transonic Systems, Ithaca, NY) and monitored for 40 minutes for vascular occlusion. In the collagen/epinephrine-induced pulmonary thromboembolism model a combination of collagen (270 μg/kg) and epinephrine (27 μg/kg) is injected into the left jugular vein and death due to respiratory arrest is recorded over the following 5 minutes. All animal studies were approved by the institutional Animal Studies Committee of Washington University.
Histology
Embryos were fixed in buffered 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. Hematoxylin-eosin was used for histologic staining and the RTU Vectastain Universal Elite ABC Kit (Vector Laboratories, Burlingame, CA) was used for fibrin/fibrinogen immunohistochemistry after sections had been blocked with 3% hydrogen peroxide. Sections were incubated with the primary antibody, rabbit antihuman fibrin/fibrinogen IgG (1:500) (Dako, Carpenteria, CA) or control nonimmune rabbit IgG, for 1 hour. The peroxidase substrate 3-amino-9-ethyl-carbazole (AEC; Vector Laboratories) was used for color development, the sections were counterstained with hematoxylin, and the slides were mounted using VectaMount AQ Aqueous Mounting Medium (Vector Laboratories). Slides were examined with a Leica DMLS microscope and digital images were imported into Adobe Photoshop.
Statistics
The chi-square test for goodness of fit was used to assess Mendelian genetics and the one-sided Fisher exact test was used to evaluate the effect of PZ and ZPI deficiency in the murine thrombosis models. A P value less than .05 was considered significant.
Results
ZPI gene disruption in mice
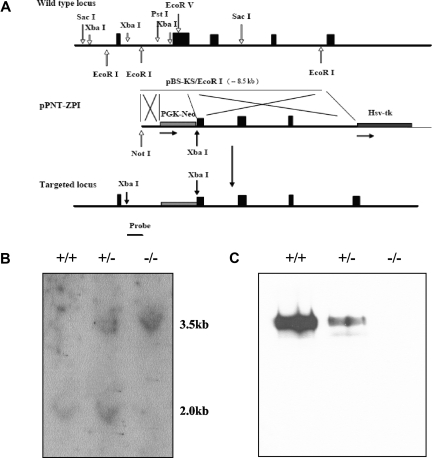
To investigate the in vivo consequences of ZPI deficiency, the ZPI gene was disrupted in mice. Successful targeting resulted in replacement of a 1.3 kb genomic fragment from intron A and exon 2, encoding the signal peptide and the N-terminus of ZPI, by a Neo cassette with the induction of a frame-shift mutation (Figure 1A). The expected structure of the targeted ZPI locus was confirmed by Southern blot analysis and ZPI was not detectable in the plasma of homozygous gene-disrupted mice by Western blotting (Figure 1B,C).
Figure 1.
ZPI gene disruption in mice. (A) The targeting construct (middle) contains a PGK-Neo cassette that replaces DNA fragment between the Pst 1 site in intron A and the EcoRV site in exon 2, thereby removing the ZPI DNA that encodes the signal peptide and N-terminus of ZPI, and inducing a frame-shift mutation. An HSV-TK cassette was added at the 3′-end of the construct to permit negative selection. The targeting vector was linearized with Not 1 and introduced into 129/Sv-derived RW4 ES cells by electroporation and stable transfectants were selected using G418 and gancyclovir. The predicted product of homologous recombination is shown at the bottom. The position of the 673 bp hybridization probe used to detect successful gene targeting is also depicted. (B) Southern blot analysis. Genomic DNA prepared from tail biopsies was analyzed by restriction digestion with Xba1 and hybridization with the probe. (C) Western blot analysis of ZPI in mouse plasma. SDS-PAGE (10%) and Western blotting of mouse plasma (5 μL of a 1:10 dilution) with rabbit antihuman ZPI polyclonal antibodies.
Phenotype of ZPI(−/−) mice
DNA analysis of 385 progeny derived from ZPI(+/−) intercrosses in the C57Bl/6 × 129/Sv genetic background showed: 116 (30%) ZPI (+/+), 194 (50%) ZPI(+/−), and 75 (20%) ZPI(−/−) (Table 1). These results are not consistent with Mendelian inheritance (P = .013) and suggest that some ZPI(−/−) mice are lost during gestation or the perinatal period since the growth, development, and survival of ZPI-null mice following birth were indistinguishable from that of their heterozygotic and wild-type littermates. Although the loss of ZPI(−/−) mice was statistically significant, this effect was modest, precluding an extensive analysis of the timing of the loss of ZPI-null mice during gestation.
Table 1.
Survival of ZPI and PZ gene-deleted mice
| Mating pairs | ZPI, N (%) | PZ, N (%) | ZPI vs PZ |
|---|---|---|---|
| Het × Het | |||
| 6 weeks | |||
| WT | 116 (30) | 157 (27) | |
| Het | 194 (50) | 309 (52) | |
| KO | 75 (20) | 126 (21) | |
| P | .013 | .111 | .448 |
| FV/(λ/λ)/Het × FV(λ/λ)/Het | |||
| 6 weeks | |||
| FV(λ/λ)/WT | 23 (77) | 24 (67) | |
| FV(λ/λ)/Het | 7 (23) | 12 (33) | |
| FV(λ/λ)/KO | 0 (0) | 0 (0) | |
| P | < .001 | < .001 | .372 |
| E18.5 | |||
| FV(λ/λ)/WT | 13 (57) | 7 (21) | |
| FV(λ/λ)/Het | 9 (39) | 18 (53) | |
| FV(λ/λ)/KO | 1 (4) | 9 (26) | |
| P | .001 | .838 | .009 |
| E13.5 | |||
| FV(λ/λ)/WT | 4 (24) | ||
| FV(λ/λ)/Het | 8 (47) | ND | |
| FV(λ/λ)/KO | 5 (29) | ||
| P | .916 | ||
| FV/(λ/λ)/Het × FV(+/+)/Het | |||
| 6 weeks | |||
| FV(λ/+)/WT | 39 (40) | 40 (26) | |
| FV(λ/+)/Het | 41 (42) | 86 (55) | |
| FV(λ/+)/KO | 17 (18) | 30 (19) | |
| P | .002 | .232 | .047 |
Numbers in italics are derived from Yin et al.12
E indicates embryonic day; and ND, not determined.
ZPI(−/−) mice were able to produce and sustain multiple litters without detectable fetal wastage (data not shown) and suffered no untoward effects from tail transection used to obtain tissue for genotyping. Blood counts did not differ between WT (n = 10) and null (n = 8) ZPI animals: hematocrit (Hct) 46.9 ± 4.2 and 48.4 ± 4.0; WBC 6.7 ± 2.3 and 7.3 ± 2.8; platelets 1081 ± 203 and 1018 ± 307.
In humans, PZ and ZPI circulate as a complex and the level of one protein affects the level of the other in plasma.7,9 In ZPI(−/−) mice (n = 12), the plasma concentration of PZ was 57% ± 12% compared with 100% ± 12% in ZPI(+/+) mice (n = 12) (P < .001). In PZ(−/−) mice (n = 8), the plasma concentration of ZPI was 76% ± 12% compared with 100% ± 15% in PZ(+/+) mice (P = .003). Thus, in both PZ and ZPI gene-deleted mice, a complete deficiency of one protein is associated with a modest reduction in the circulating level of the partner protein.
Effect of PZ and ZPI deficiency in vivo
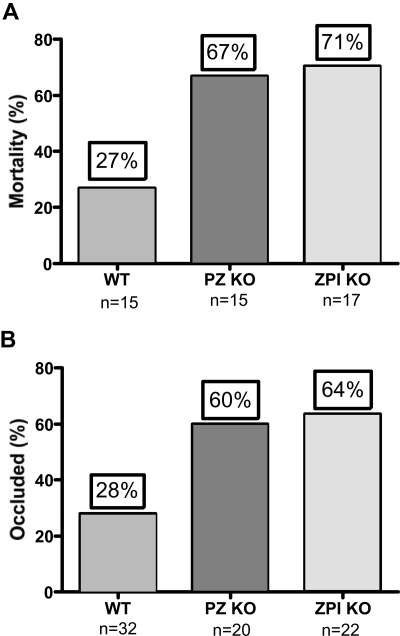
The murine collagen/epinephrine-induced thromboembolism and ferric chloride–induced carotid artery thrombosis models were used to demonstrate an effect of PZ and ZPI on in vivo thrombosis. The collagen/epinephrine model has been used extensively in the evaluation of antiplatelet agents,15 but has recently also been shown to be dependent on factor XII and factor XI and, thus, coagulation as well.16 Deficiency of either PZ (P = .03) or ZPI (P = .01) reduced the survival of mice in the model, but the difference in survival between the PZ- and ZPI-null mice was not statistically significant given the limited number of mice tested (Figure 2A). PZ- (P = .02) and ZPI-null (P = .01) mice also developed enhanced thrombosis in the FeCl3 arterial injury model, but again the difference in occlusion rates between the PZ and ZPI mice was not significant (Figure 2B).
Figure 2.
PZ and ZPI mice in thrombosis models. (A) Collagen/epinephrine-induced (270 μg/27 μg/kg) mortality from pulmonary thromboembolism with respiratory arrest by 5 minutes. (B) FeCl3-induced (5%) carotid artery occlusion at 40 minutes.
Comparison of PZ- and ZPI-null mice
To more carefully compare the PZ- and ZPI-deficient phenotypes, the effect of PZ and ZPI deficiency in mice carrying the FVLeiden gene mutation was determined using a variety of mating strategies. In Table 1, numbers in italics for PZ genotyping are taken from our previous publication.12 All mice were in the C57Bl/6 × 129/Sv mixed genetic background.
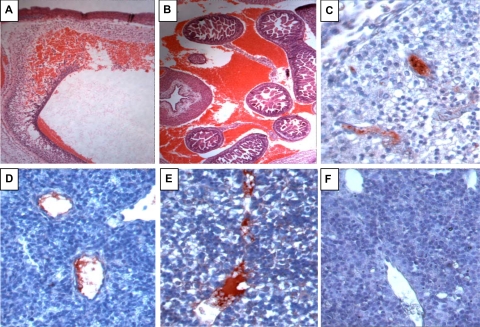
Heterozygotic and homozygotic PZ and ZPI deficiency both dramatically increase the mortality of homozygotic factor VLeiden mice (6 weeks; Table 1). In the case of PZ deficiency, this has been shown to be due to a consumptive coagulopathy that induces perinatal death.12 FV(λ/λ)/PZ(−/−) mice are present near the end of gestation, embryonic day 18.5 (E18.5), in expected numbers, albeit some with obvious signs of hemorrhage and vascular thrombosis.12 In contrast, FV(λ/λ) embryos with ZPI deficiency are reduced near the end of gestation (E18.5) (Table 1 middle). FV(λ/λ)/ZPI(−/−) embryos are present, however, in expected numbers at E13.5, but frequently show signs of intravascular thrombosis and intracranial and abdominal hemorrhage, consistent with a consumptive coagulopathy (Figure 3). The consequences of PZ and ZPI deficiency were directly compared using a mating strategy producing a FV(λ/+) genetic background (Table 1 bottom). In contrast to PZ-null mice, which are produced in near appropriate numbers from heterozygote matings, ZPI-deficient mice are produced in statistically reduced numbers. The difference between surviving offspring from PZ and ZPI heterozygous matings on the FV(+/−) genetic background is statistically significant (P = .047).
Figure 3.
Hemorrhage and intravascular coagulation in E13.5 FV(λ/λ)/ZPI(−/−) embryos. A-E, FV(λ/λ)/ZPI(−/−) embryos; F, FV(λ/λ)/ZPI(+/+) embryo. A,B, hematoxylin-eosin staining; C-F, antifibrinogen/fibrin staining (red) developed using AEC with hematoxylin counterstaining. (A) Intracranial hemorrhage (×5). (B) Intra-abdominal hemorrhage (×5). (C-E) Intravascular fibrin in the vessels of the brain (C, ×40), lung (D, ×40), and liver (E, ×40). In addition to the intravascular thrombosis, note disturbed cellular architecture and parenchymal fibrinogen/fibrin staining in the liver of the FV(λ/λ)/ZPI(−/−) embryo (E) compared with the liver of the FV(λ/λ)/ZPI(+/+) embryo (F).
Discussion
A deficiency of either PZ or ZPI was associated with increased thrombosis in the 2 murine models tested, collagen/epinephrine-induced pulmonary thromboembolism and FeCl3-induced carotid artery injury. Both models have been shown to be factor XI–dependent,16 but, although end-point thrombosis was reduced somewhat in PZ-null versus ZPI-null mice in each of these models (nonsignificantly), the results suggest that the enhanced thrombosis was predominantly related to the loss of PZ-dependent ZPI inhibition of factor Xa. In combination with the FVLeiden genotype, ZPI deficiency produces a considerably more severe phenotype than PZ deficiency. PZ-deficient mice die perinatally, whereas ZPI-deficient embryos die during gestation as well as the perinatal period. In both cases, the manner of death involves intravascular coagulation and hemorrhage due to an apparent consumptive coagulopathy.
ZPI not only inhibits factor Xa in a PZ-dependent fashion, but also directly inhibits factor XIa.10 Indeed, ZPI appears to be the most potent inhibitor of factor XIa in plasma in the absence of heparin.11 Therefore, the more severe phenotype of murine ZPI deficiency, in comparison to PZ deficiency, is most likely due to the loss of regulation of both factor Xa and factor XIa in ZPI deficiency, but only regulation of factor Xa in PZ deficiency. Alternatively, it is conceivable that an additional, PZ-independent, antithrombotic action of ZPI has yet to be discovered. In this regard, Heeb and colleagues17 have recently reported that ZPI modestly inhibits factor IXa and the intrinsic Xase complex consisting of factor IXa, factor VIIIa, phospholipids, and Ca2+ in the absence of PZ. We, however, have not been able to confirm these observations.
Some, but not all, clinical studies have found an association between reduced PZ plasma levels and thromboembolic disease.18–26 In contrast with other known thrombophilic traits, for example factor V Leiden, the prothrombin gene mutation, protein C deficiency, and antithrombin deficiency, which are associated with venous thrombosis, several studies have suggested that PZ deficiency is also a risk factor for arterial disease, including acute coronary syndrome and stroke.18–21 Studies of a potential association of ZPI levels with human thrombosis are limited. A relationship between heterozygous nonsense mutations in the ZPI gene and venous thrombosis was found in 2 reports,27,28 but could not be confirmed in 2 additional studies.29,30 Further, an association was not detected between reduced plasma levels of antigenic ZPI and venous thrombosis in the Leiden Thrombophilia Study (LETS) or arterial thrombosis in the prospective Atherosclerosis Risk in Communities (ARIC) study.7,31 The wide range of ZPI plasma levels in healthy individuals is perhaps consistent with the inability to demonstrate a thrombotic risk with isolated modest reductions in ZPI, including that associated with heterozygous non–sense mutations. The only reported individual with homozygous non–sense mutations in the ZPI gene, however, had recurrent thrombosis.28 Similar to the studies in mice, reduced levels of PZ are reportedly associated with an increased clinical severity of the FVLeiden(λ/+) genotype in people.25,32 The potential effect of modest reductions in ZPI on the human heterozygous FVLeiden phenotype has not yet been reported.
With an expanded number of offspring from heterozygous matings since our initial report12 on PZ-deficient mice, there is a trend toward a reduction in PZ(−/−) pups (Table 1). Indeed, concentrating on the ratio of PZ-null offspring versus the PZ(+/−) and PZ(+/+) offspring (1:3 ratio expected), a chi-square test shows that the PZ-null pups are underrepresented (P = .037). As equal numbers of null and wild-type offspring are anticipated from these heterozygous matings, we estimate that approximately 35% of the ZPI- and approximately 20% of the PZ-null mice are lost. For comparison, about 50% of FV(λ/λ) offspring are lost from heterozygote matings in the C57Bl/6 × 129/Sv genetic background,14 whereas protein C, antithrombin, and tissue factor pathway inhibitor null mice do not survive to the neonatal period.33–35 Based on these murine studies, one would anticipate that PZ or ZPI deficiency would be a modest risk factor for human thrombosis with complete deficiencies of PZ or ZPI producing a human phenotype less severe than that of homozygous factor VLeiden and for the ZPI-null phenotype to be more severe than that of the PZ-null phenotype.
Acknowledgments
We thank David Ginsburg (University of Michigan, Ann Arbor, MI) for providing the factor VLeiden mice. This work was supported by a National Institutes of Health National Heart, Lung and Blood Institute grant RO1 HL060782 (G.J.B.Jr).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.Z., Y.T., L.L., N.L., and G.J.B. performed the research. G.J.B. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George J. Broze Jr, Division of Hematology, Washington University St Louis, Campus Box 8125, 660 S Euclid Ave, St Louis, MO 63110; e-mail: gbroze@im.wustl.edu.
References
- 1.Broze G, Jr, Miletich J. Human protein Z. J Clin Invest. 1984;73:933–938. doi: 10.1172/JCI111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sejima H, Hayashi T, Deyashiki Y, Nishioka J, Suzuki K. Primary structure of vitamin K-dependent human protein Z. Biochem Biophys Res Commun. 1990;171:661–668. doi: 10.1016/0006-291x(90)91197-z. [DOI] [PubMed] [Google Scholar]
- 3.Ichinose A, Takeya H, Espling E, Iwanaga S, Kisiel W, Davie E. Amino acid sequence of human protein Z, a vitamin K-dependent plasma glycoprotein. Biochem Biophys Res Commun. 1990;172:1139–1144. doi: 10.1016/0006-291x(90)91566-b. [DOI] [PubMed] [Google Scholar]
- 4.Han X, Fiehler R, Broze G., Jr Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci U S A. 1998;97:6734–6738. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han X, Huang Z-F, Fiehler R, Broze G., Jr The protein Z-dependent protease inhibitor is a serpin. Biochemistry. 1999;38:11073–11078. doi: 10.1021/bi990641a. [DOI] [PubMed] [Google Scholar]
- 6.Miletich J, Broze G., Jr Human plasma protein Z antigen: range in normal subjects and effect of warfarin therapy. Blood. 1987;69:1580–1586. [PubMed] [Google Scholar]
- 7.Al-Shanqeeti A, van Hycklama A, Berntorp E, Rosendaal FR, Broze GJ., Jr Protein Z and protein Z-dependent protease inhibitor: determinants of levels and risk of venous thrombosis. Thromb Haemost. 2006;93:411–413. doi: 10.1160/TH04-11-0715. [DOI] [PubMed] [Google Scholar]
- 8.Vossen CY, Hasstedt SJ, Rosendaal FR, et al. Heritability of plasma concentrations of clotting factors and measures of a prothrombotic state in a protein C-deficient family. J Thromb Haemost. 2004;2:242–247. doi: 10.1111/j.1538-7933.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 9.Tabatabai A, Fiehler R, Broze G., Jr Protein Z circulates in plasma in a complex with protein Z-dependent protease inhibitor. Thromb Haemost. 2001;85:655–660. [PubMed] [Google Scholar]
- 10.Han X, Fiehler R, Broze G., Jr Characterization of protein Z-dependent protease inhibitor. Blood. 2000;96:3049–3055. [PubMed] [Google Scholar]
- 11.Rezaie AR, Sun M-F, Gailani D. Contributions of basic amino acids in the autolysis loop of factor XIa to serpin specificity. Biochemistry. 2006;45:9427–9433. doi: 10.1021/bi060820+. [DOI] [PubMed] [Google Scholar]
- 12.Yin Z-F, Huang Z-F, Cui J, Lasky N, Ginsburg D, Broze GJ., Jr Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci U S A. 2000;97:6734–6738. doi: 10.1073/pnas.120081897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Broze GJ., Jr Mouse protein Z-dependent protease inhibitor cDNA. Thromb Haemost. 2001;85:861–865. [PubMed] [Google Scholar]
- 14.Cui J, Eitzman DT, Westrick RJ, et al. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96:4222–4226. [PubMed] [Google Scholar]
- 15.DiMinno G, Silver MJ. Mouse antithrombotic assay: A simple method for the evaluation of antithromotic agents in vivo: potentiation of antithrombotic activity by ethyl alcohol. J Pharmacol Exp Ther. 1983;225:57–60. [PubMed] [Google Scholar]
- 16.Renne T, Nieswandt B, Gailani D. The intrinsic pathway of coagulation is essential for thrombus stability in mice. Blood Cells Mol Dis. 2006;36:148–151. doi: 10.1016/j.bcmd.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Heeb MJ, Cabral KM, Ruan L. Down-regulation of factor IXa in the factor Xase complex by protein Z-dependent protease inhibitor. J Biol Chem. 2005;280:33819–33825. doi: 10.1074/jbc.M506502200. [DOI] [PubMed] [Google Scholar]
- 18.Vasse M, Guegan-Massardier E, Borg J-Y, Woimant F, Soria C. High frequency of protein Z deficiency in patients with ischemic stroke. Lancet. 2001;357:933–934. doi: 10.1016/S0140-6736(00)04218-5. [DOI] [PubMed] [Google Scholar]
- 19.Heeb M, Paganini-Hill A, Griffin J, Fisher M. Low protein Z levels and risk of ischemic stroke; differences by diabetic status and gender. Blood Cells Mol Dis. 2002;29:139–144. doi: 10.1006/bcmd.2002.0549. [DOI] [PubMed] [Google Scholar]
- 20.Fedi S, Sofi F, Brogi D, et al. Low protein Z plasma levels are independently associated with acute coronary syndromes. Thromb Haemost. 2003;90:1173–1178. doi: 10.1160/TH03-04-0237. [DOI] [PubMed] [Google Scholar]
- 21.Sofi F, Cesari F, Vigiani S, et al. Protein Z plasma levels in different phases of activity of coronary atherosclerosis. J Thromb Haemost. 2005;3:2254–2258. doi: 10.1111/j.1538-7836.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 22.Morange PE, Juhan-Vague I the PRIME study group. Protein Z plasma levels are not associated with the risk of coronary heart disease: The PRIME study. J Thromb Haemost. 2004;2:2050–2051. doi: 10.1111/j.1538-7836.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 23.McQuillan A, Eikelboom J, Hankey G, et al. Protein Z in ischemic stroke and its etiologic subtypes. Stroke. 2003;34:2415–2419. doi: 10.1161/01.STR.0000092124.52084.4B. [DOI] [PubMed] [Google Scholar]
- 24.Santacroce R, Sarno M, Cappucci F, et al. Protein Z low levels and risk of occurrence of deep venous thrombosis. J Thromb Haemost. 2006;4:2417–2422. doi: 10.1111/j.1538-7836.2006.02186.x. [DOI] [PubMed] [Google Scholar]
- 25.Martenilli I, Razzari E, Bucciarelli P, Mannucci PM. Low levels of protein Z and the risk of venous thromboembolism. J Thromb Haemost. 2005;3:2817–2819. doi: 10.1111/j.1538-7836.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- 26.Gris J-C, Quere I, Dechaud H, et al. High frequency of protein Z deficiency in patients with unexplained early fetal loss. Blood. 2002;99:2606–2608. doi: 10.1182/blood.v99.7.2606. [DOI] [PubMed] [Google Scholar]
- 27.Van de Water N, Tan T, Ashton F, et al. Mutations within the protein Z- dependent protease inhibitor gene are associated with venous thromboembolic disease: a new form of thrombophilia. Brit J Haemat. 2004;127:190–194. doi: 10.1111/j.1365-2141.2004.05189.x. [DOI] [PubMed] [Google Scholar]
- 28.Corral J, Gonzalez-Conejero R, Soria JM, et al. A nonsense polymorphism in the protein Z-dependent protease inhibitor increases the risk for venous thrombosis. Blood. 2006;108:177–183. doi: 10.1182/blood-2005-08-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razzari C, Marinelli I, Bucciarelli P, Viscardi Y, Biguzzi E. Polymorphisms of the protein Z-dependent protease inhibitor (ZPI) gene and the risk of venous thromboembolism. Thromb Haemost. 2006;95:909–910. [PubMed] [Google Scholar]
- 30.Folsom AR, Cushman M, Rasmussen-Torvik LJ, Heckbert SR, Tsai MY. Prospective study of polymorphisms of the protein Z-dependent protease inhibitor and risk of venous thromboembolism. Thromb Haemost. 2007;97:493–494. [PubMed] [Google Scholar]
- 31.Refaai MA, Ahn C, Lu L, Wu K, Broze GJ., Jr Protein Z and ZPI levels and cardiovascular events. J Thromb Haemost. 2006;4:1628–1629. doi: 10.1111/j.1538-7836.2006.02012.x. [DOI] [PubMed] [Google Scholar]
- 32.Kemkes-Matthes B, Matzdorff A, Matthes K. Protein Z influences the prothrombotic phenotype of factor V Leiden in humans. Thromb Res. 2002;106:183–185. doi: 10.1016/s0049-3848(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 33.Jalert LR, Rosen ED, Moons L, et al. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102:1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishiguro K, Kojima T, Kadomatsu K, et al. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest. 2000;106:873–878. doi: 10.1172/JCI10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]