Abstract
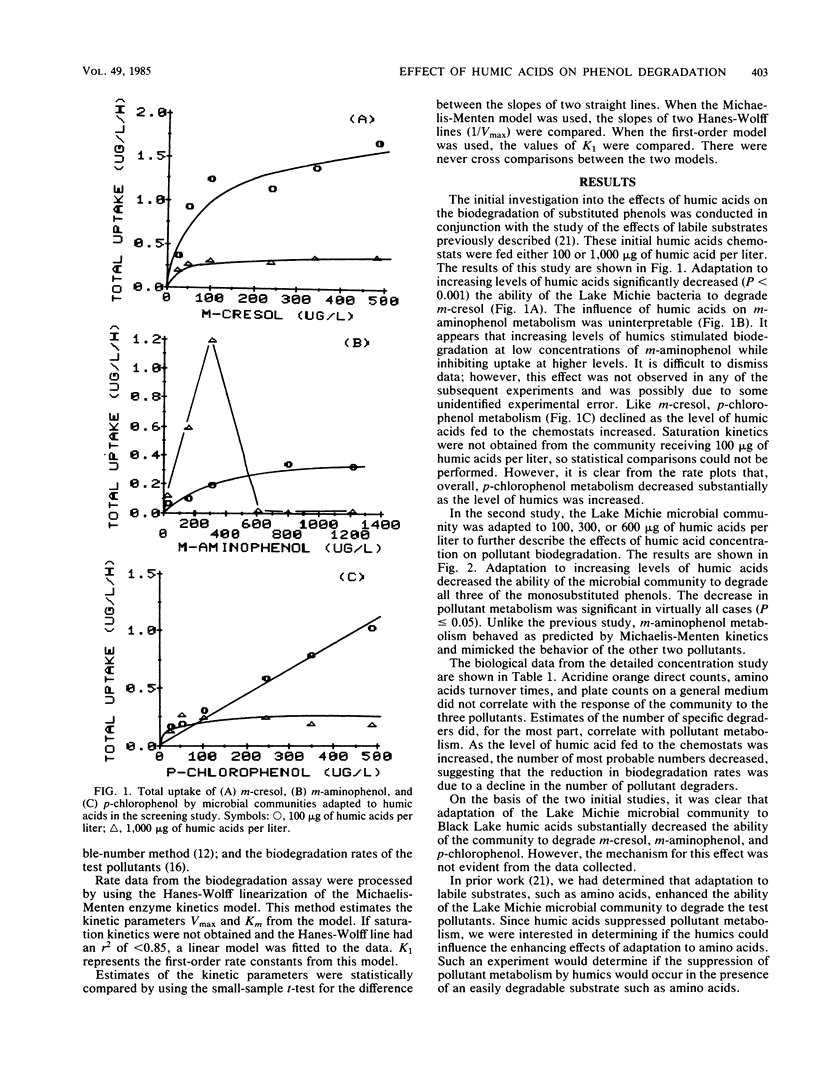
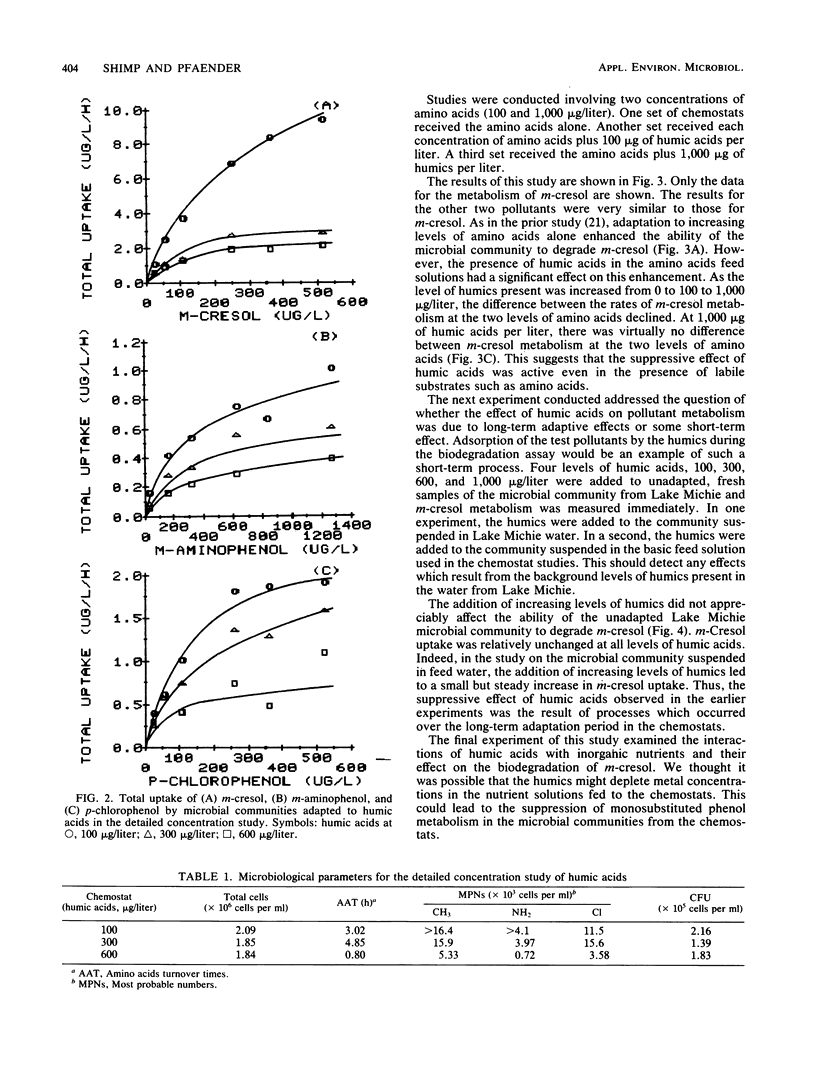
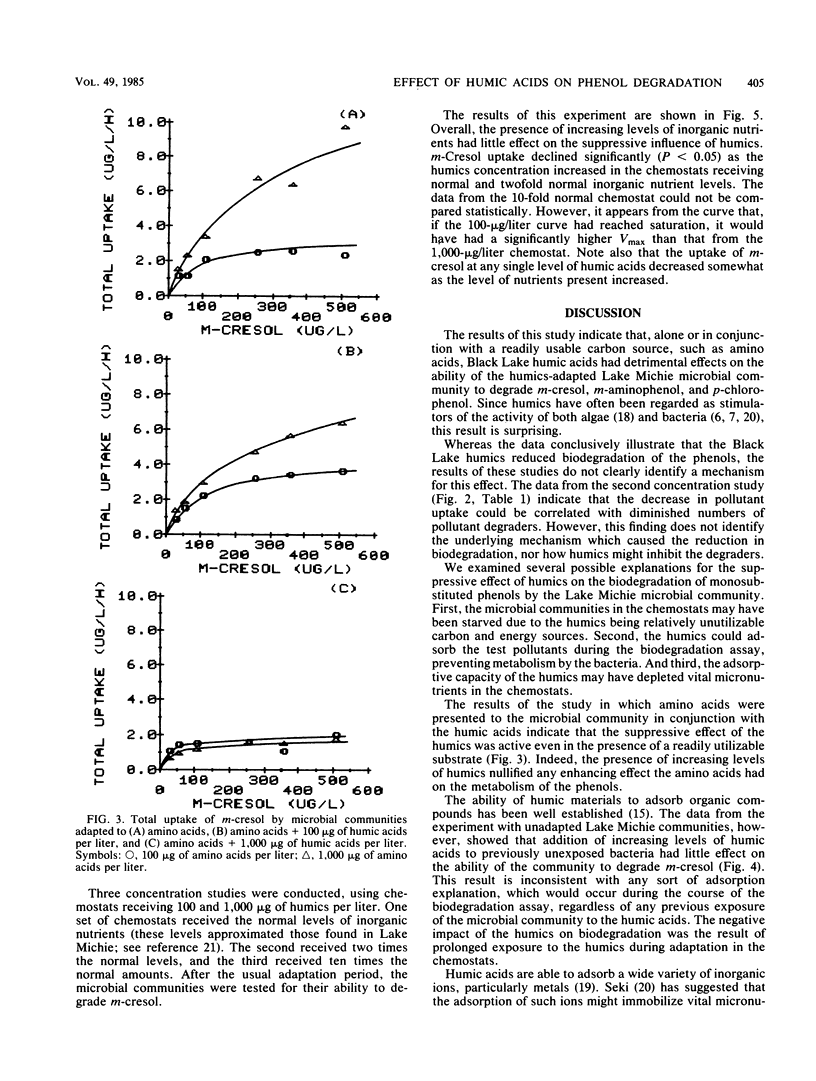
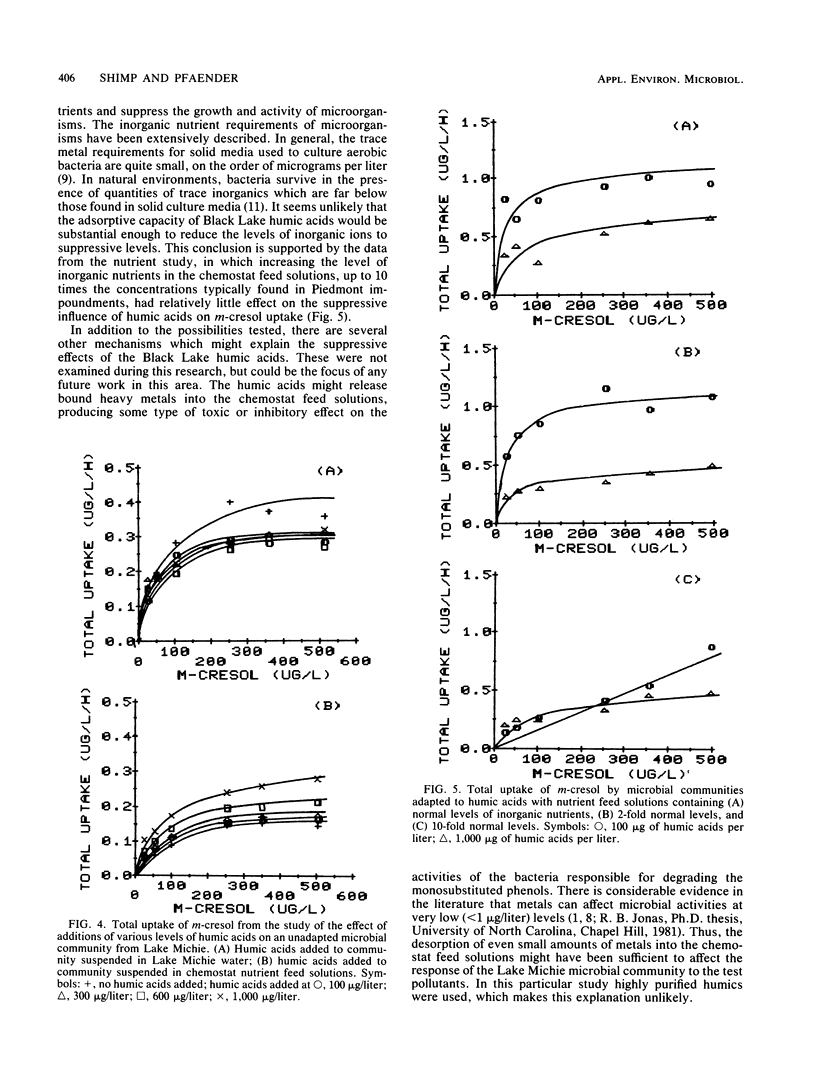
Samples of the microbial community from Lake Michie, a mesotrophic reservoir in central North Carolina, were adapted to various levels (100 to 1,000 μg/liter) of natural humic acids in chemostats. The humic acids were extracted from water samples from Black Lake, a highly colored lake in the coastal plain of North Carolina. After adaptation, the microbial community was tested for its ability to degrade the monosubstituted phenols m-cresol, m-aminophenol, and p-chlorophenol. Adaptation to increasing levels of humic acids significantly reduced the ability of the microbial communities to degrade all three phenols. The decline in biodegradation was accompanied by a decrease in the number of specific compound degraders in the adapted communities. Short-term exposure of the community to increasing levels of humic acids had no significant effect on the ability of the community to degrade m-cresol. Thus the suppressive effect of humic acids on monosubstituted phenol metabolism was the result of long-term exposure to the humic materials. Increasing the levels of inorganic nutrients fed to the chemostats during the humic acid adaptation had little effect on the suppressive influence of the humic acids, indicating that nutrient limitation was probably not responsible for the metabolic suppression. The results of the study suggest that long-term exposure to humic acids can reduce the ability of microbial communities to respond to monosubstituted phenols.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation: problems of molecular recalcitrance and microbial fallibility. Adv Appl Microbiol. 1965;7:35–80. doi: 10.1016/s0065-2164(08)70383-6. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmicke L. G., Williams R. T., Crawford R. L. 14C-most-probable-number method for enumeration of active heterotrophic microorganisms in natural waters. Appl Environ Microbiol. 1979 Oct;38(4):644–649. doi: 10.1128/aem.38.4.644-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Bartholomew G. W. Measurement of aquatic biodegradation rates by determining heterotrophic uptake of radiolabeled pollutants. Appl Environ Microbiol. 1982 Jul;44(1):159–164. doi: 10.1128/aem.44.1.159-164.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R. J., Pfaender F. K. Influence of easily degradable naturally occurring carbon substrates on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):394–401. doi: 10.1128/aem.49.2.394-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


