Abstract
The transcription factor Gli3 is important for brain and limb development. Mice homozygous for a mutation in Gli3 (Gli3Xt/Xt) have severe abnormalities of telencephalic development and previous studies have suggested that aberrant cell death may contribute to the Gli3Xt/Xt phenotype. We demonstrate that telencephalic cells from embryonic Gli3Xt/Xt embryos survive better and are more resistant to death induced by cytosine arabinoside, a nucleoside analogue that induces death in neuronal progenitors and neurons, than are control counterparts in vitro. Culture medium conditioned by Gli3Xt/Xt cells is more effective at enhancing the viability of control telencephalic cells than medium conditioned by control cells, indicating that Gli3Xt/Xt cells release a factor or factors which enhance telencephalic cell viability. Gli3Xt/Xt cells are also more sensitive to released factors present in conditioned media. These data suggest that Gli3 plays both cell-autonomous and cell-nonautonomous roles in mediating telencephalic cell viability.
Keywords: cell death, mouse, primary culture
Introduction
Gli genes encode zinc-finger transcription factors homologous to the Drosophila segment polarity gene cubitus interruptus (Ingham & McMahon, 2001). Gli3Xt/Xt mice die perinatally with many brain abnormalities (Johnson, 1967; Hui & Joyner, 1993). Structures in the dorsal telencephalon are particularly affected: cortical lamination is disrupted and midline structures, such as the hippocampus, are absent (Hui & Joyner, 1993; Franz, 1994; Grove et al., 1998; Theil et al., 1999; Tole et al., 2000). Little is known about the mechanisms underlying this severe telencephalic phenotype but one process that may be disrupted is programmed cell death (PCD). There is a reduced frequency of apoptotic cells in the Gli3Xt/Xt forebrain (Aoto et al., 2002) and mice with mutations in the genes encoding essential components in the mammalian PCD pathway display similar CNS abnormalities to those seen in Gli3Xt/Xt embryos. These similarities include cerebral cortical heterotopias and partially penetrant exencephaly (Grove et al., 1998; Hakem et al., 1998; Kuida et al., 1998; Theil et al., 1999; Tole et al., 2000). It is probable therefore that the defects in PCD in the Gli3Xt/Xt telencephalon contribute significantly to its structural defects. Our aims were to test whether increased viability is an intrinsic property of Gli3Xt/Xt telencephalic cells and whether this is achieved though changes in cell-cell signalling.
Materials and methods
Gli3 expression
We examined the expression of Gli3 using in situ hybridization (Nieto et al., 1996). A 449-bp fragment (nucleotides 4585-5033 of the mouse Gli3 cDNA) was PCR-amplified, subcloned and used to generate digoxigenin-labelled RNA antisense probes. For Western blot analysis, protein lysates were prepared from embryonic day (E)14.5 dorsal and ventral telencephalon and run on NuPAGE 3-8% Tris-Acetate gels (Invitrogen, UK). Blots were incubated with a rabbit polyclonal anti-Gli3 antibody (Santa Cruz Biotechnology, USA; 1: 100).
Cultures
Gli3Xt/+ mice (the mutant allele is XtJ; Hui & Joyner, 1993) on a CBA background were interbred. All animals were treated in accordance with the UK Animal Scientific Procedures Act (1986). Animals were killed by cervical dislocation. The morning of vaginal plug was designated E0.5. Gli3Xt/Xt embryos were easily identified by their severe polydactyly and abnormal head morphology (Hui & Joyner, 1993); exencephalic embryos were excluded. Previous studies found no differences in forebrain morphology nor in patterns of gene expression between wild-type and Gli3Xt/+ E12.5-15.5 brains (Tole et al., 2000; our unpublished observations). We found no differences in cell viability or proportions of different cell types in telencephalic cultures from wild-type and Gli3Xt/+ E15.5 brains. At E14.5 therefore, where the presence of an extra digit in heterozygotes could not be assessed reliably, embryos that were not Gli3Xt/Xt were pooled and used as the source of ‘control’ cells. Dorsal and ventral telencephalon were isolated: ventral tissue included the lateral and medial ganglionic eminences. Cells were dissociated by incubation for 45 min at 37 °C with papain (20 units/mL; Papain Dissociation System, Worthington Biochemical, UK). Cells in suspension were either plated for cell death experiments and immunocytochemistry or fixed for flow cytometry.
Cell death experiments
Dissociated cells were plated on poly l-lysine at 1000 cells/mm2 in a volume of 200 μL and cultured for 24 h at 37 °C in defined serum-free medium. In some experiments, cells were treated with 100 μm cytosine arabinoside (AraC) (Sigma, UK) for the final 6 h of the 24-h culture period. For conditioned medium experiments, cells were plated at 1000 cells/mm2 and allowed to settle for 2 h; 150 μL of medium was then replaced with medium from wells in which cells had been grown for 24 h at 1000-16 000 cells/mm2. Experiments were terminated after either 24 or 48 h.
Cells were fixed with 4% paraformaldehyde containing Hoescht 33342 (Sigma). Healthy and dead cells were distinguished by nuclear morphology (Kerr et al., 1972). Dying and dead cells showed dense chomatin condensation, some of which had fragmented into apoptotic bodies, and were immunoreactive for active caspase-3 (our unpublished observations). Data from wild-type and Gli3Xt/+ cells were pooled and presented as ‘control’. At least three fields of view were counted from two or three wells of each condition. Significant differences in viability were established with Student’s t-test or an anova by Univariate General Linear Model.
BrdU labelling
Dissociated cells were treated with 10 μm bromodeoxyuridine (BrdU) for the 24-h culture period. BrdU was detected using mouse anti-BrdU (Becton Dickenson, USA; 1 : 200) and goat antimouse Alexa 488 (Molecular Probes, USA; 1 : 200).
Flow cytometry
Dissociated telencephalic cells were fixed in ice-cold 70% ethanol and 5-10 × 105 cells were incubated with primary antibodies: mouse antimicrotubule-associated protein 2 (MAP2; Sigma; 1 : 800) and antiproliferating cell nuclear antigen (PCNA; Dako, UK; 1 : 200). Primary antibodies were detected with goat antimouse Alexa 488 (Molecular Probes; 1 : 400). Cellular DNA was then stoichiometrically stained by incubation with propidium iodide (PI, Molecular Probes) at 50 μg/mL with RNAse A at 125 μg/mL at 37°C for 30 min. Cells were analysed on a Beckman-Coulter XL flow cytometer. 10 000-20 000 cells were analysed per sample. Single cells were gated away from clumps by pulse height-width analysis of the DNA signal generated by the fluorescence of DNA-bound PI, measured in the FL3 channel. Fluorescence intensity of bound secondary antibody was measured in the FL1 channel.
Results
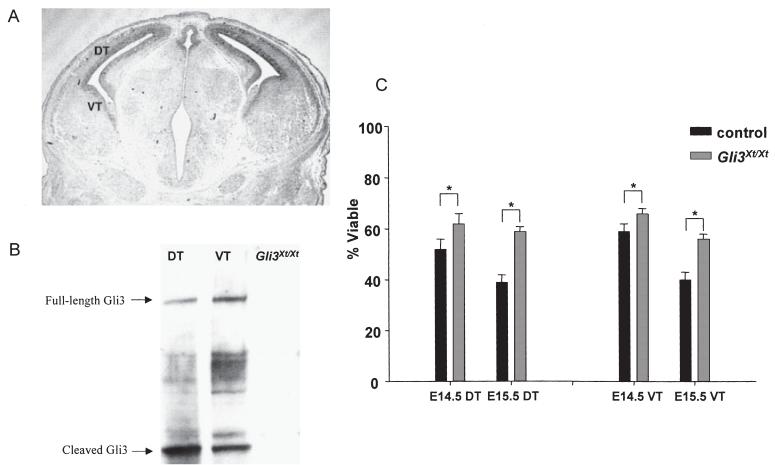
In situ hybridization showed that Gli3 is normally expressed in both dorsal and ventral telencephalon at E14.5 (Fig. 1A; Hui et al., 1994). Western blots confirmed that Gli3 protein is produced in both these regions (Fig. 1B). The viabilities of cultured E14.5-15.5 Gli3Xt/Xt dorsal and ventral telencephalic cells were significantly higher than the viabilities of corresponding control cells (Fig. 1C).
Fig. 1.
(A) In situ hybridization for Gli3 at E14.5: DT, dorsal telencephalon; VT, ventral telencephalon. (B) Both full-length and cleaved forms of the Gli3 protein are present in E14.5 DT and VT. Bands corresponding to these isoforms are absent from Gli3Xt/Xt tissue. (C) Mean + SEM viabilities of cells from DT and VT of control and Gli3Xt/Xt embryos: significant differences are marked (*P< 0.01; Student’s paired t-tests, n = 7-14).
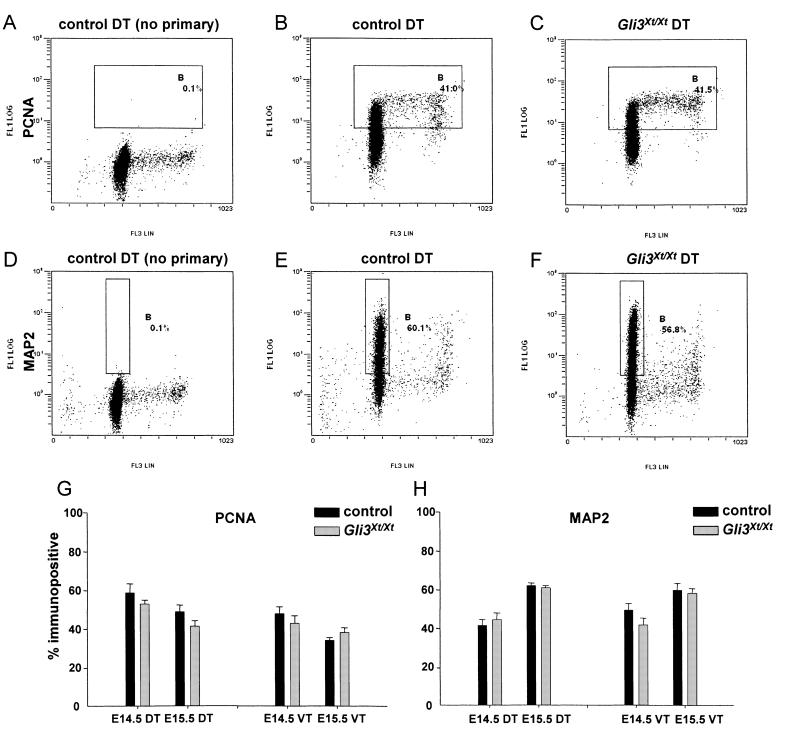
An important consideration in interpreting this increased viability was evidence that rates of PCD in normally developing cortex are higher in the proliferating population than among differentiating cells (Thomaidou et al., 1997). If Gli3Xt/Xt telencephalon has an abnormally low proportion of proliferating cells, this might account for its enhanced viability in culture. To examine this possibility, dissociated cells were prepared for flow cytometry using antibodies for PCNA, a marker of cycling cells (Fig. 2A-C), and MAP2, a marker of postmitotic neurons (Fig. 2D-F). A threshold that included only 0.1% of cells during control runs (Fig. 2A and D) was maintained in experimental runs (Fig. 2B, C, E and F). The proportions of cells whose fluorescence intensities exceeded this threshold were counted. For MAP2 (Fig. 2D-F), the gate was set to exclude a small fraction with a higher-than-diploid DNA content (i.e. those undergoing replication) that expressed a low level of MAP2. No expression of MAP2 was detected among cells from non-neural tissue, whether they had a diploid or a higher DNA content (data not shown), indicating that enhanced fluorescence of telencephalic cells stained with MAP2, even those that were proliferating, was due to specific staining.
Fig. 2.
Representative examples of flow cytometry: (A-C) PCNA and (D-F) MAP2 staining on E15.5 control and Gli3Xt/Xt dorsal telencephalon (DT). Antibody staining intensity (FL1LOG on a log scale) is plotted against DNA content (FL3LIN on a linear scale). Gate B was set to include (A-C) cells expressing a high level of PCNA or (D-F) cells expressing a high level of MAP2 with a diploid DNA content. (G,H) There were no differences between proportions of PCNA- and MAP2-immunopositive cells in samples from control and Gli3Xt/Xt embryos in either the DT or ventral telencephalon (VT) (means of 2-4 experiments are shown).
There were no significant differences between control and Gli3Xt/Xt dorsal or ventral telencephalon in the proportions of cells labelled with PCNA or MAP2 at either E14.5 or E15.5 (Fig. 2G and H). Similar results were obtained when dissociated cells were plated, stained with antibodies recognizing MAP2 and the neural progenitor markers RC2 and nestin, and quantified (our unpublished observations).
In some experiments on E14.5 telencephalic cells, BrdU was included in the medium for the entire culture period. The proportions of BrdU-labelled cells were similar in all cultures (mean ± SEM, 12.8 ± 1.0% for control dorsal telencephalon, 10.4 ± 2.4% for Gli3Xt/Xt dorsal telencephalon, 9.4 ± 2.3% for control ventral telencephalon and 12.6 ± 9.8% for Gli3Xt/Xt ventral telencephalon). From these and the preceding experiments, we concluded that there were no major differences in the proportions of proliferating and differentiated cells between control and Gli3Xt/Xt cultures.
Further evidence that increased viability in Gli3Xt/Xt cultures most probably reflects a difference in the properties of the mutant cells came from an analysis of the effect of the nucleoside analogue AraC, which induces apoptosis in neural progenitors and neurons (D’Sa-Eipper & Roth, 2000; Geller et al., 2001). The viability of E15.5 dorsal and ventral telencephalic control cells was significantly reduced by treatment with AraC to 73 ± 3% (dorsal cells; P = 0.035, Student’s t-test, n = 4) and 88 ± 6% (ventral cells; P = 0.045, n = 4) of the survival of untreated cells. AraC treatment did not significantly decrease the viability of Gli3Xt/Xt ventral telencephalic cells (95 ± 3% of the survival of untreated cells, n = 4). Although the viability of Gli3Xt/Xt dorsal telencephalic cells was decreased to 89 ± 2% of the survival in untreated control cultures after AraC treatment (P = 0.025, n = 4), this decrease was significantly smaller than that induced in control dorsal telencephalic cells (P = 0.002, n = 4). We conclude that Gli3Xt/Xt dorsal and ventral telencephalic cells are more resistant to AraC than are corresponding control cells.
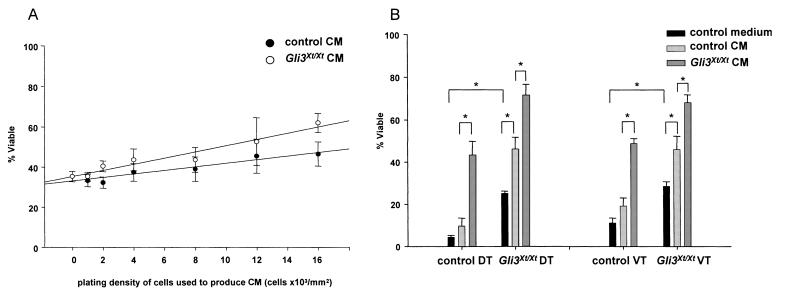
To test the possibility that Gli3Xt/Xt telencephalic cells release more and/or more potent survival-promoting factors than control cells, we carried out conditioned medium experiments. Medium from cultures of control or Gli3Xt/Xt dorsal telencephalic cells at densities from 1000 to 16 000 cells/mm2 was added to control dorsal telencephalic cells. The viability of the test cells after 24 h was related to the density of cells used to condition the media, regardless of the genotype of the conditioning cells (Fig. 3A; P = 0.013, anova by Univariate General Linear Model, n = 11). In addition, Gli3Xt/Xt-conditioned medium had a significantly greater effect on viability than control-conditioned medium (Fig. 3A; P = 0.029, anova by Univariate General Linear Model, n = 11). This was clearer when cultures containing conditioned medium were compared after 48 h (Fig. 3B). In these experiments, conditioned medium was from control or Gli3Xt/Xt dorsal or ventral telencephalic cells (at 12 000 cells/mm2). Similar to cultures grown for 24 h, viabilities of Gli3Xt/Xt dorsal and ventral telencephalic cells grown for 48 h (without any conditioned medium added) were significantly higher than those of corresponding control cells (solid bars in Fig. 3B). The viabilities of control and Gli3Xt/Xt dorsal and ventral telencephalic cells treated with Gli3Xt/Xt-conditioned medium were significantly higher than the viabilities of these cells treated with control-conditioned medium (Fig. 3B). Additionally, dorsal and ventral telencephalic Gli3Xt/Xt cells, unlike control cells, had their viability enhanced by treatment with conditioned medium from control cells (Fig. 3B). This suggests that Gli3Xt/Xt cells (i) release more and/or more potent survival-promoting factors than control telencephalic cells, and (ii) Gli3Xt/Xt telencephalic cells are more sensitive than control cells to such factors.
Fig. 3.
(A) Viability of control dorsal telencephalic cells cultured for 24 h with conditioned medium (CM) plotted against the density of the cells used to produce the CM (1,000-16 000 cells/mm2). Viability was affected significantly by the density of the conditioning cells and by their genotype. (B) Mean viabilities (+ SEM) of Gli3Xt/Xt dorsal telencephalic (DT) and ventral telencephalic (VT) cells grown for 48 h in control medium or CM. Significant differences are marked (*P< 0.05, Student’s t-test, n = 4-7).
Discussion
Our results indicate that the increased viability of Gli3Xt/Xt telencephalic cells is an intrinsic property of these cells. This property results from changes in both the production of viability-enhancing factor(s) by the mutant cells (a cell-nonautonomous mechanism) and the responsiveness of mutant cells to such factors (a cell-autonomous mechanism). The viability of cells in both the dorsal and the ventral telencephalon are affected to a similar degree. Although Gli3 is expressed both dorsally and ventrally, previous work has shown that the morphological defects in the telencephalon of Gli3Xt/Xt embryos are more severe dorsally (Theil et al., 1999; Tole et al., 2000). Interestingly, decreased PCD in mice mutant for caspase-3 or caspase-9 also results in more severe defects in the dorsal telencephalon (Kuida et al., 1996, 1998; Hakem et al., 1998). The organization of the dorsal telencephalon might be more sensitive to changes in PCD than other areas of the telencephalon, although additional cellular defects probably contribute to the phenotype of Gli3Xt/Xt embryos.
Similarities between the consequences of Gli3 deficiency and reduced PCD in embryos extend to other Gli3-expressing structures. Like Gli3Xt/Xt embryos (Theil et al., 1999; Franz & Besecke, 1991; Hui & Joyner, 1993; Tole et al., 2000), mice mutant in caspase-9 (Kuida et al., 1998) or apoptotic protease-activating factor (Cecconi et al., 1998; Yoshida et al., 1998; Honarpour et al., 2000) display persistence of interdigital webbing and defects in neural tube closure and in eye and lens development. The molecular mechanisms by which Gli3 influences PCD are unknown; our results suggest that altered secretion of and sensitivity to molecules involved in controlling PCD are likely to be involved.
Acknowledgements
We thank Duncan McNeil for excellent technical help. This work was funded by the National Institutes of Health (NEI individual NRSA postdoctoral fellowship, P.A.Z.), the Wellcome Trust (B.M., D.J.P.), Medical Research Council (JTD-B; D.J.P.) and Biotechnology and Biological Sciences Research Council (V.F., D.J.P.).
Abbreviations
- AraC
cytosine arabinoside
- BrdU
bromodeoxyuridine
- E
embryonic day
- MAP2
microtubule-associated protein 2
- PCD
programmed cell death
- PCNA
proliferating cell nuclear antigen
- PI
propidium iodide.
References
- Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev. Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- D’Sa-Eipper C, Roth KA. Caspase regulation of neuronal progenitor cell apoptosis. Dev. Neurosci. 2000;22:116–124. doi: 10.1159/000017433. [DOI] [PubMed] [Google Scholar]
- Franz T. Extra-toes (Xt) homozygous mutant mice demonstrate a role for the Gli-3 gene in the development of the forebrain. Acta Anat. (Basel) 1994;150:38–44. doi: 10.1159/000147600. [DOI] [PubMed] [Google Scholar]
- Franz T, Besecke A. The development of the eye in homozygotes of the mouse mutant Extra-toes. Anat. Embryol (Berl.) 1991;184:355–361. doi: 10.1007/BF00957897. [DOI] [PubMed] [Google Scholar]
- Geller HM, Cheng KY, Goldsmith NK, Romero AA, Zhang AL, Morris EJ, Grandison L. Oxidative stress mediates neuronal DNA damage and apoptosis in response to cytosine arabinoside. J. Neurochem. 2001;78:265–275. doi: 10.1046/j.1471-4159.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Dev. Biol. 2000;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyn-dactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Johnson DR. Extra-toes: a new mutant gene causing multiple abnormalities in the mouse. J. Embryol. Exp. Morph. 1967;17:543–581. [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Meth. Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Theil T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J. Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes (J) Dev. Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]