Abstract
Human plasma fibronectin binds with high affinity to the inflammation-induced secreted protein TSG-6. Fibronectin binds to the CUB_C domain of TSG-6 but not to its Link module. TSG-6 can thus act as a bridging molecule to facilitate fibronectin association with the TSG-6 Link module ligand thrombospondin-1. Fibronectin binding to TSG-6 is divalent cation-independent and is conserved in cellular fibronectins. Based on competition binding studies using recombinant and proteolytic fragments of fibronectin, TSG-6 binding localizes to type III repeats 9–14 of fibronectin. This region of fibronectin contains the Arg-Gly-Asp sequence recognized by α5β1 integrin, but deletion of that sequence does not prevent TSG-6 binding, and TSG-6 does not inhibit cell adhesion on fibronectin substrates mediated by this integrin. This region of fibronectin is also involved in fibronectin matrix assembly, and addition of TSG-6 enhances exogenous and endogenous fibronectin matrix assembly by human fibroblasts. Therefore, TSG-6 is a high affinity ligand that can mediate fibronectin interactions with other matrix components and modulate some interactions of fibronectin with cells.
Keywords: matrix assembly, TSG-6, fibronectin, CUB domains
1. Introduction
Tumor necrosis factor-stimulated gene 6 (TSG-6) is a ~35 kDa secreted protein that is produced during inflammation and related processes. TSG-6 has anti-inflammatory activity and protects joint tissues from destruction in in vivo models (Wisniewski et al., 1996; Milner and Day, 2003; Szanto et al., 2004; Milner et al., 2006). TSG-6 is also expressed in the ovary during ovulation and plays an important role in female fertility by regulating cumulus cell-oocyte complex expansion (Fulop et al., 2003; Ochsner et al., 2003). The protein consists mainly of contiguous Link and CUB (complement component Clr/Cls, Uegf, and bone morphogenetic protein 1) domains. The Link module contains a hyaluronan-binding site and also interacts with heparin/heparan sulfate and thrombospondins-1 and -2 (Blundell et al., 2005; Kuznetsova et al., 2005; Mahoney et al., 2005). Interaction with the Link module of TSG-6 is mediated by the N-modules of thrombospondin-1, which also recognize the Link module-containing domains of versican and aggrecan (Kuznetsova et al., 2006). TSG-6 is a catalyst and cofactor in the covalent transfer of the heavy chains (HC) of inter-α-trypsin inhibitor onto a hyaluronan acceptor (Rugg et al., 2005). Thrombospondin-1 enhances the initial covalent modification of inter-α-trypsin inhibitor by TSG-6 (i.e., HC-TSG-6 complex formation) and transfer of HC to hyaluronan, suggesting a physiological function of thrombospondin-1 binding to TSG-6 in regulation of hyaluronan metabolism at sites of inflammation (Kuznetsova et al., 2005).
To date, no ligands have been identified that bind to the TSG-6 CUB module. Paralogous CUB modules in other proteins have been implicated in protein-protein and protein-carbohydrate interactions (Bork and Beckmann, 1993; Solis et al., 1998; Sieron et al., 2000).
While characterizing TSG-6 interactions with thrombospondin-1, we tested several extracellular matrix (ECM) proteins as controls and unexpectedly found that TSG-6 interacts avidly with fibronectin (FN). FN is a prominent component of ECM and a major circulating protein in plasma that regulates a variety of cellular activities through direct interactions with cell surface integrin and proteoglycan receptors (Mao and Schwarzbauer, 2005). Here we demonstrate that the CUB_C domain of TSG-6 (comprised of the CUB module and C-terminal segment) interacts with the cell- and heparin-binding domains of FN. FN is synthesized by many adherent cells, some of which assemble it into a fibrillar network (Mao and Schwarzbauer, 2005). The assembly process is integrin-dependent, and FN-integrin interactions initiate a step-wise process involving conformational changes that expose FN-binding sites and promote the intermolecular interactions needed for fibril formation (Schwarzbauer and Sechler, 1999). We further show that TSG-6 binding enhances FN fibril assembly by cultured human fibroblasts. These results provide evidence for an additional level of control of FN matrix assembly under conditions where TSG-6 is present.
2. Results
2.1 characterization of recombinant CUB_C domain
Mature human TSG-6 is comprised of a 19-amino acid N-terminal segment followed by contiguous Link and CUB modules of 92 and 122 residues, respectively, and a C-terminal segment of 27 amino acids (residues 18–36, 37–128, 129–250 and 251–277 in the preprotein, respectively) (Milner and Day, 2003). One-dimensional NMR spectroscopy of recombinant human CUB_C (residues 129–277 of the preprotein) demonstrated good dispersion of resonances in both the methyl (−1 to 3 ppm) and amide/aromatic (6–11 ppm) regions of the spectra (Fig. 1A and B, respectively), which is consistent with that of a correctly folded protein. For example, the high-field shifted methyl resonances seen at around 0 ppm are characteristic of the presence of a stable hydrophobic core. These data reveal that the recombinant CUB_C domain is correctly folded in PBS (i.e., at physiological pH and salt concentration). Furthermore, the CUB_C protein used in this study has been crystallized and an X-ray structure determined at 2.3-Å resolution (DC Briggs, T Ali, DJ Mahoney & AJ Day, unpublished data).
Fig. 1. NMR analysis of the CUB_C protein reveals that it is correctly folded in solution.
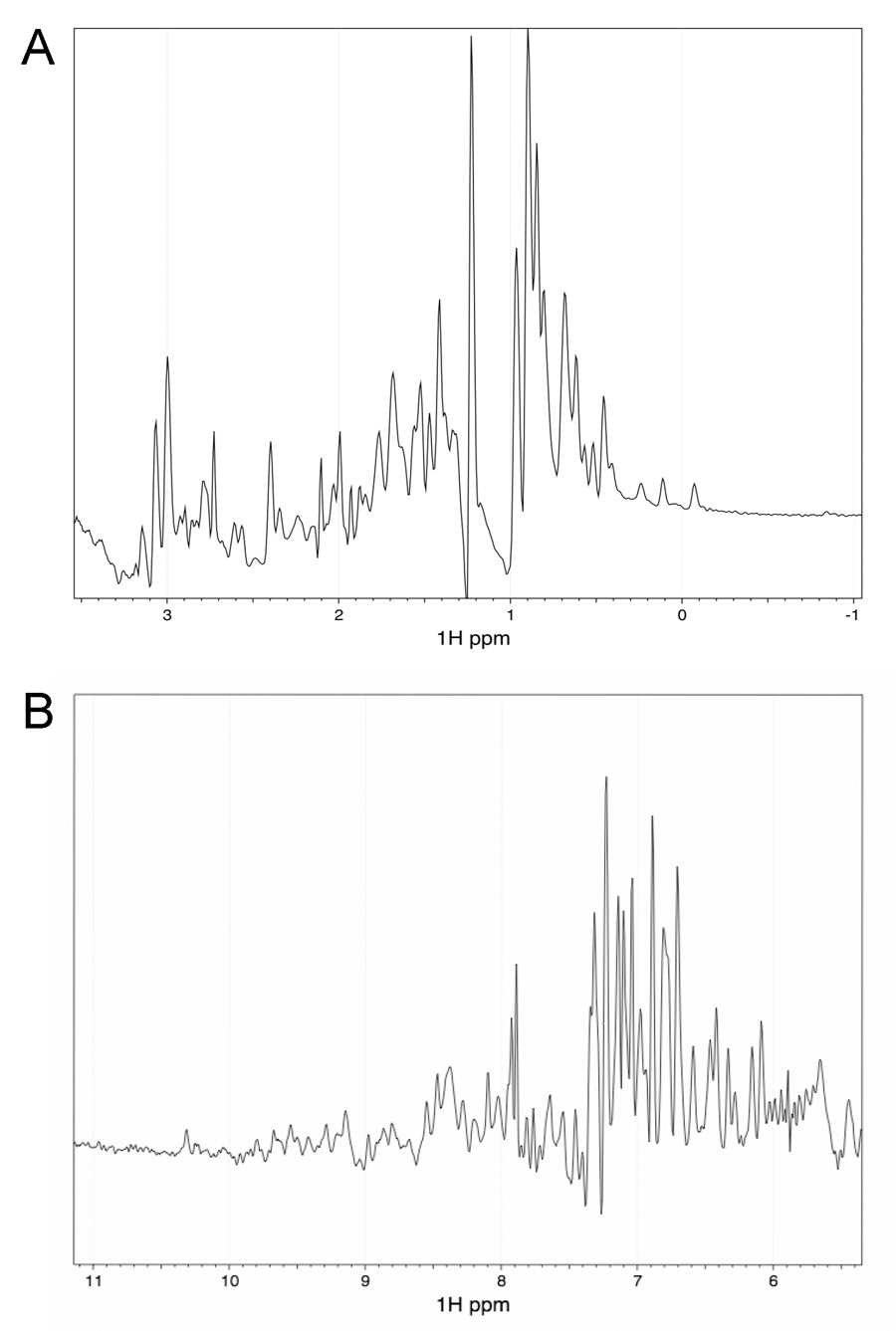
One-dimensional NMR spectrum of the CUB_C domain shows a good dispersion of resonances in both the methyl region (A) and amide/aromatic (B) regions.
2.2 The CUB_C domain of TSG-6 is a high affinity ligand for fibronectins
Full-length recombinant human TSG-6Q, CUB_C domain, and Link module (Link_TSG6) were tested for binding to human plasma FN in a solid phase assay (Fig. 2A). 125I-FN bound to immobilized full-length TSG-6Q and its CUB_C domain in a dose-dependent manner. Weak binding of FN to the Link module was detected only at high coating concentrations. Binding of 125I-FN to full length TSG-6Q was not significantly influenced by divalent cations as binding was maintained in the presence of EDTA in buffer lacking divalent cations (Fig. 2B).
Fig. 2. Fibronectin binding to TSG-6Q.
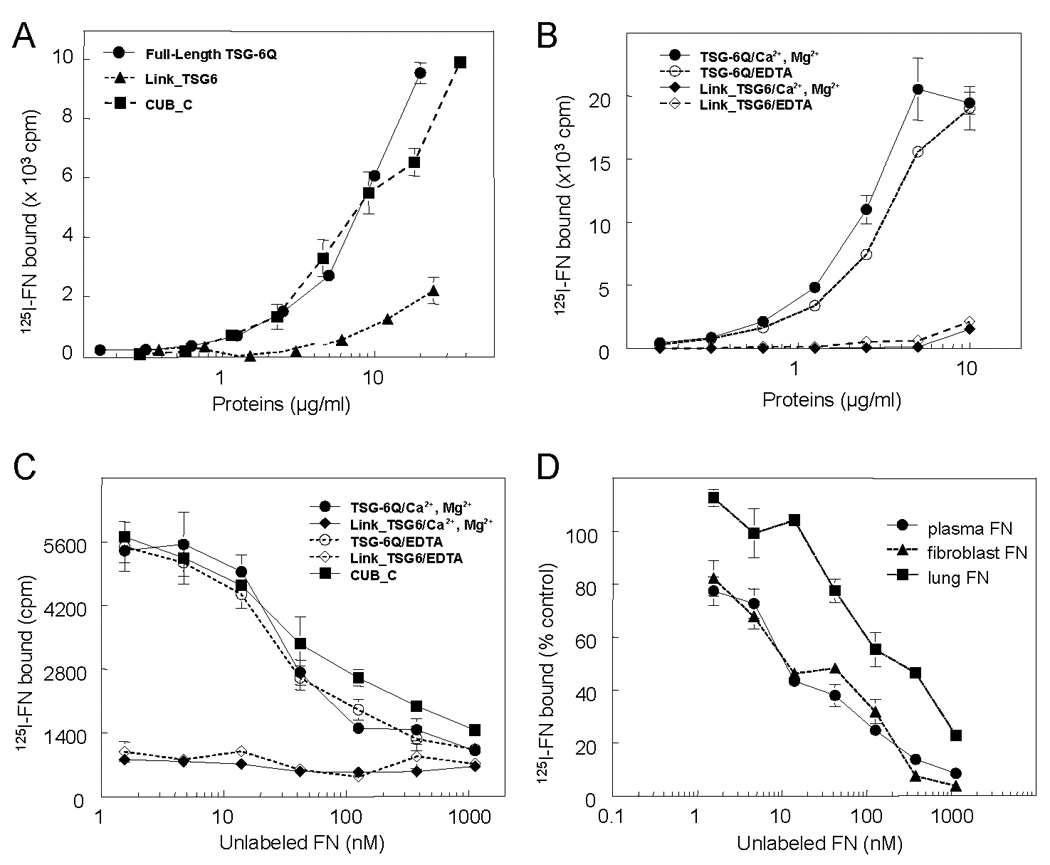
A 125I-labeled plasma FN (0.5 µg/ml or 2.27 nM with respect to 220 kDa subunits) in 50 µl of DPBS, 0.5% BSA, 0.2 mM PMSF was incubated for 3 h at 37°C with recombinant full-length TSG-6Q, recombinant Link_TSG6, or recombinant CUB_C domain immobilized at the indicated concentrations on microtiter plate wells. Solid phase binding assays were performed in the presence of Ca2+, Mg2+ at 37°C. Background nonspecific binding values were subtracted from each data point (133 ±9 cpm). B, 125 I-FN binding to immobilized recombinant full-length TSG-6Q (0–10 µg/ml) or recombinant Link_TSG6 (0–10 µg/ml) was determined in the presence of Ca2+, Mg2+ (solid lines, closed symbols) or without cations in the presence of 5 mM EDTA (dashed lines, open symbols). Background values were subtracted from each data point (110 ± 26 and 92 ± 31 cpm, respectively). The data are the means of quadruplicate determinations ± S.E. C, Self displacement of FN binding to TSG-6Q. Specificity of 125 I-FN binding to immobilized full-length TSG-6Q, Link_TSG6 or CUB_C domain was determined in the presence of indicated concentrations of unlabeled FN. Binding of 125I-FN to immobilized TSG-6Q and CUB_C domain was inhibited by soluble FN, with 50% inhibition at 10 µg/ml (22 nM). D, Comparison of the inhibition of plasma 125I-FN binding to immobilized TSG-6Q (10 µg/ml) by the indicated concentrations of plasma or cellular FNs. Data were normalized so that the binding of 125 I-FN to TSG-6Q in the absence of competing unlabeled FN equals 100%.
Because direct binding to immobilized proteins can be influenced both by the efficiency of protein adsorption on plastic and potential changes in conformation induced by this adsorption, the specificity of FN binding was further examined by inhibition assays (Fig. 2C). Binding of 125I-FN to immobilized TSG-6Q was inhibited by soluble FN, with 50% inhibition at 10 µg/ml (22 nM). In contrast, the weak binding of 125I-FN to immobilized Link_TSG6 was not significantly inhibited by unlabeled soluble FN within the range tested.
Quantitative analysis of the self displacement data yielded an apparent affinity constant of 1.6×108 M−1 for FN binding to immobilized TSG-6 in the presence of divalent cations (Table 1). Consistent with Fig. 2B,C, the binding affinity was only slightly decreased in the presence of EDTA. Binding of FN to immobilized CUB_C domain occurred with an approximately 3-fold lower affinity (apparent Ka = 5.7×107 M−1), whereas the weak binding to immobilized Link_TSG6 was not displaceable by unlabeled FN, and no affinity constant could be determined by this method (Table 1).
Table 1.
Fibronectin binding to immobilized TSG-6 determined by self displacement
| Immobilized ligand | Apparent Ka (M−1) |
|---|---|
| Full length TSG-6Q (Ca2+, Mg2+) | 1.6 ± 0.6 × 108 |
| Full length TSG-6Q (5 mM EDTA) | 1.0 ± 0.2 × 108 |
| CUB_C domain | 5.7 ± 1.3 × 107 |
| Link_TSG6 | -- |
Binding parameters were determined from self displacement of 125I-FN binding to the indicated ligands immobilized on polystyrene. Binding at eight concentrations of FN over the range 0.3 to 250 µg/ml was assessed in quadruplicate and analyzed using Scafit version 3.10 of the LIGAND software. All data were adequately fit using a single site binding model.
Cellular FN differs from plasma FN by the inclusion of alternatively spliced exons (Schwarzbauer, 1991). Cellular FN isolated from fibroblasts inhibited binding of labeled plasma FN with a comparable dose-dependency as unlabeled plasma FN, whereas cellular FN isolated from lung was slightly less active (Fig. 2D). Therefore, both plasma and cellular FNs can interact with TSG-6.
2.3 TSG-6 interacts with the cell binding domain of FN
To localize the TSG-6 binding site in FN, several recombinant and proteolytic fragments of FN were tested as inhibitors (Fig. 3). Of the fragments tested, the r33 kDa and r40-kDa, which contain portions of the cell-binding and heparin-binding domains, were the best inhibitors. A proteolytic 40 kDa fragment (p40), containing heparin-binding domain, and recombinant 28 kDa, containing all but the NH2 terminal 5 kDa of the 33 kDa fragment, were ~10-fold less active. An analog of the recombinant 33 kDa fragment with its integrin binding RGDS sequence deleted (r33ΔRGD) also had less activity. Fragments derived from the N-terminal fibrin and collagen binding domains of FN were inactive. These results demonstrate that TSG-6 binds specifically to type III repeats of FN encompassing the C-terminal region of the cell-binding and part of the heparin-binding domain of FN.
Fig. 3. TSG-6Q binding site on fibronectin.
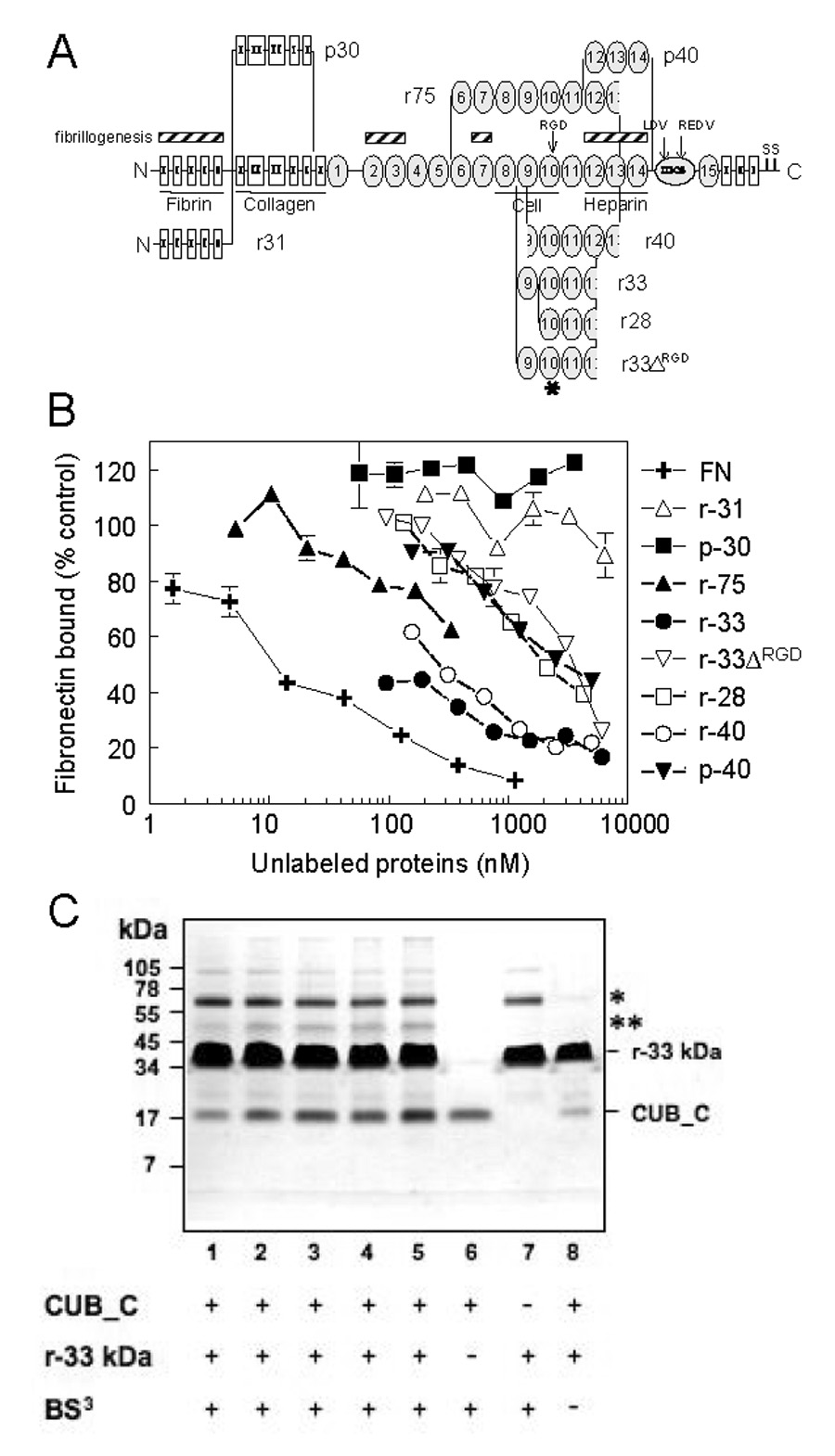
A, Each 220 kDa FN subunit consists of 12 type I modules, 2 type II modules and 15–17 type III modules. Binding domains for fibrin, collagen, cells and heparin are indicated. Arrows indicate the positions of integrin recognition sequences RGD, LDV and REDV. Regions on FN implicated in FN fibrillogenesis are also indicated. * represents the position of the RGDS deletion in the 33-kDa recombinant fragment (r33ΔRGD). The diagram also shows the derivation of the recombinant (r) fragments and proteolytic (p) fragments of FN used in this study. Constructs r28, r33, r40, and r75 terminate within type 3 repeats 12 or 13 as indicated. B, 125I-FN binding to wells coated with TSG-6Q (10 µg/ml) was quantified in the presence of divalent cations and the indicated concentrations of full length FN, recombinant or proteolytic fragments derived from FN. Results are presented as a % of control FN binding determined in the absence of inhibitors. The data are the means of quadruplicate determinations ± S.E. C, Cross-linking of the CUB_C/fibronectin complex. Fibronectin r-33 kDa was incubated with varying concentrations of CUB_C (molar ratios 1:28, 1:14, 1:9, 1:7 and 1:5 of CUB_C:r-33 kDa) in presence of the cross-linker BS3 and analyzed under reducing conditions by SDS-PAGE (lanes 1–5). CUB_C alone (lane 6; at concentration equivalent to lane 3) and r-33 kDa alone (lane 7; concentration equivalent to lanes 1–5) were also incubated in the presence BS3. Lane 8 shows CUB_C and r-33kDa in the absence of BS3 (molar ratio 1:28). In the presence of cross-linking reagent species are seen at ~62 kDa (*; lanes 1–5 and 7) and ~50 kDa (**, lanes 1–5), which are not present in the absence of BS3 (lane 8). The former likely corresponds to a cross-linked dimer of r-33 kDa given that this is present in the absence of CUB_C. The ~50 kDa species (**), which becomes more intense with increasing CUB_C concentrations, is consistent with a 1:1 complex of CUB_C and r-33 kDa (with apparent molecular masses of ~20- and ~35-kDa, respectively).
To further confirm binding between type 3 repeats 9–14 Of FN and the CUB_C domain of TSG-6 and to verify that this binding can occur in solution, we performed chemical cross linking studies (Fig. 3C). In the presence of the cross-linking reagent BS3, species are seen at ~62 kDa (*; lanes 1–5 and 7) and ~50 kDa (**, lanes 1–5) that are not present in the absence of BS3 (lane 8). The former probably corresponds to a cross-linked dimer of r-33 kDa given that this is present in the absence of CUB_C. The ~50 kDa species (**) that becomes more intense with increasing CUB_C concentrations is consistent with a 1:1 complex of CUB_C and r-33 kDa (with apparent molecular masses of ~20- and ~35-kDa, respectively).
To further confirm that this complex contains the CUB_C domain of TSG-6 and the r-33 kDa fragment of fibronectin, the ~50 kDa band (** on Fig. 3C) was excised from the gel and analyzed by MS-MS of tryptic peptides. Both CUB_C and r-33 kDa proteins (5 and 10 peptides, respectively) were identified by this analysis. Thus, in solution the CUB_C domain of TSG-6 forms a 1:1 complex in solution with repeats 9–14 of FN.
2.4 TSG-6 facilitates FN binding to thrombospondin-1
Because most of the other known ligands for TSG-6 interact with its Link module (Milner and Day, 2003; Milner et al., 2006), we considered whether TSG-6 could serve as a bridging molecule between FN and the former ligands. Thrombospondin-1 also binds directly to FN, but the affinity of this interaction in the presence of divalent cations is relatively low (Rodrigues et al., 2001). Therefore, we immobilized thrombospondin-1 and asked whether addition of TSG-6, which interacts with thrombospondin-1 via the Link module with high affinity (Kuznetsova et al., 2005), could enhance the basal binding of 125I-FN to this ligand (Fig. 4). In the absence of TSG-6, approximately 0.3% of input FN bound specifically to the immobilized thrombospondin-1. Addition of 13 nM full-length TSG-6Q increased FN binding to 1%, and maximal binding of 6.5% was achieved at approximately 200 nM TSG-6Q. In contrast, Link_TSG6 only modestly increased FN binding to immobilized thrombospondin-1 over the same concentration range. Addition of CUB_C similarly failed to increase binding, indicating that intact TSG-6 is required to mediate FN binding to thrombospondin-1.
Fig. 4. TSG-6 enhances fibronectin binding to thrombospondin-1.
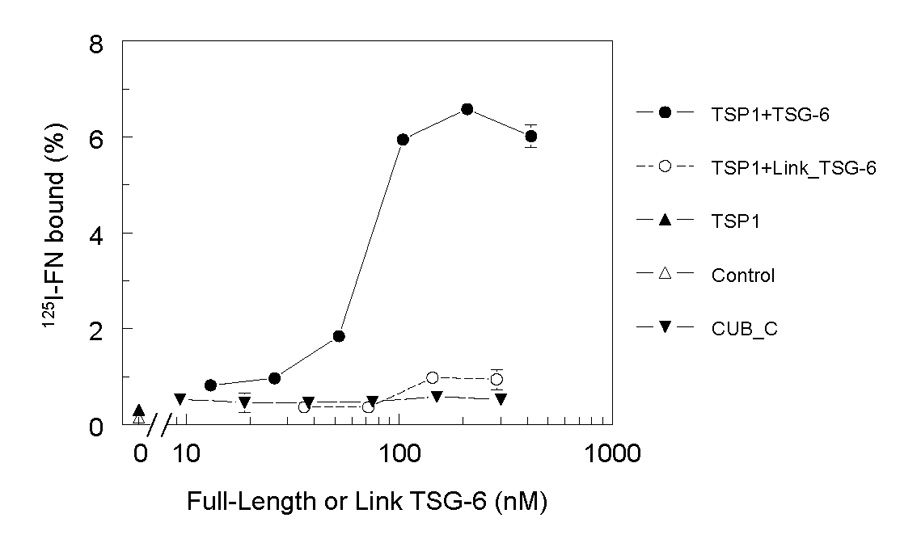
Uncoated wells (Δ) or wells coated using 10 µg/ml thrombospondin-1 (TSP1) were blocked with BSA and incubated with 0.5 µg/ml 125I-FN alone (▲) or in the presence of the indicated concentrations of full length TSG-6Q (●), CUB_C (▼), or Link_TSG6 (○). Results are presented as a percent of the input FN bound for quadruplicate determinations ± SD.
2.5 TSG-6 does not inhibit α5β1 integrin recognition of fibronectin
The domain of FN recognized by TSG-6 contains an RGD sequence that is recognized by α5β1 and several additional integrins (Yamada, 1991). The decreased TSG-6 binding activity of the r33ΔRGD mutant suggested that TSG-6 binding might interfere with α5β1 integrin recognition of FN. To examine this, we assessed adhesion of Jurkat T cells on immobilized FN, which is mediated primarily by α5β1 integrin (Li et al., 2002), in the presence of TSG-6 (Fig. 5). TSG-6Q did not inhibit adhesion of T cells on immobilized FN but instead moderately increased basal T cell adhesion on this substrate (Fig. 5A). This effect was more pronounced in the presence of Link_TSG6, which dose-dependently increased adhesion. The CUB_C domain also increased T cell adhesion on FN at the highest dose, but this was not consistently observed in replicate experiments.
Fig. 5. TSG-6 does not inhibit integrin-mediated cell adhesion on fibronectin.
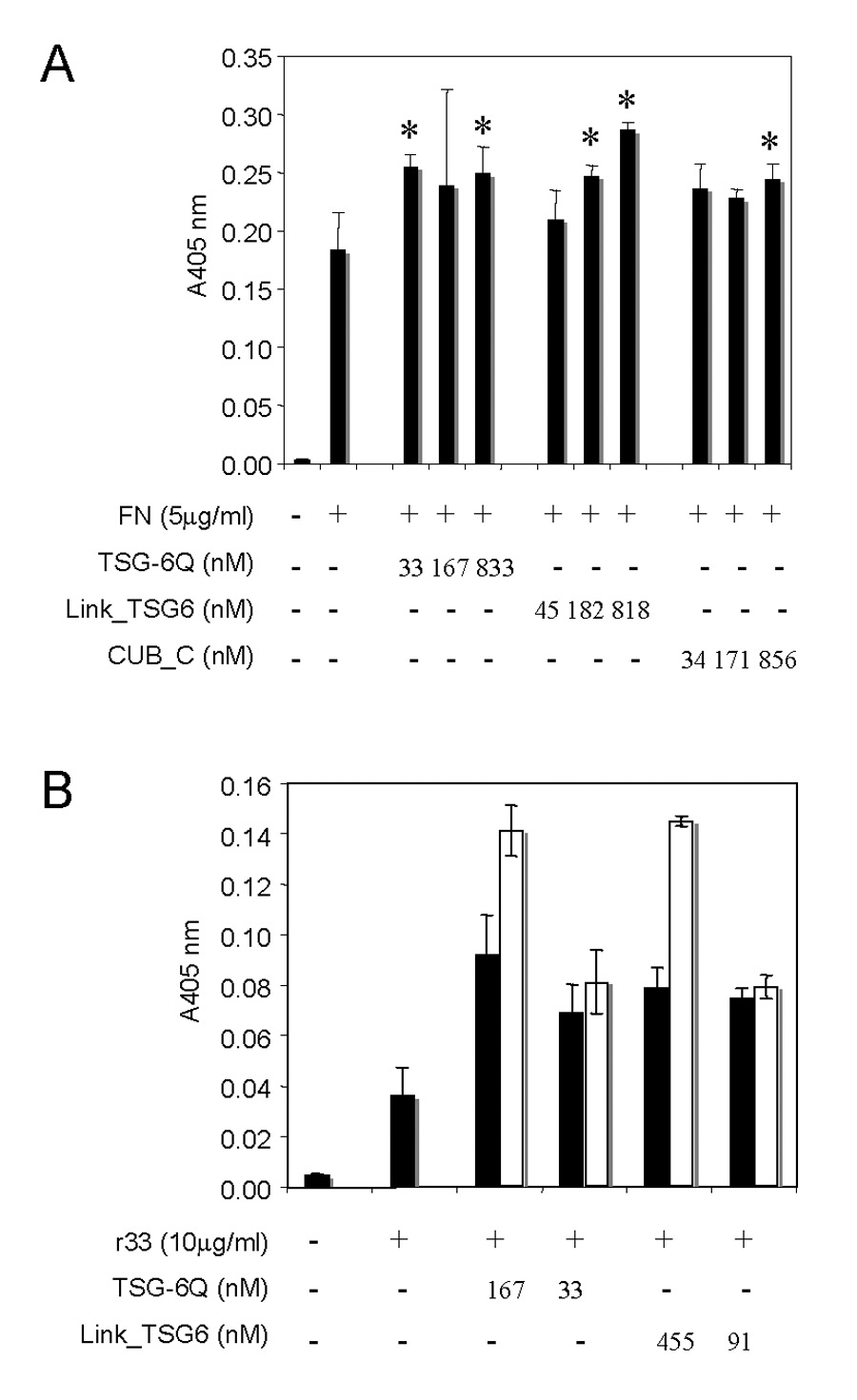
A, Jurkat T cell adhesion on immobilized FN (5 µg/ml) was quantified in the presence of the indicated concentrations of TSG-6Q, Link_TSG6 or CUB_C after 20 min. incubation by colorimetric detection of cell-associated hexosaminidase activity. A significant increase relative to the FN control is indicated by * with p<0.05 by Student’s t-test. B, α5β1-mediated Jurkat T cell adhesion on immobilized recombinant r33 fragment of FN (10 µg/ml, 300 nM), which contains the RGD sequence recognized byα5β1 integrin, was determined by colorimetric detection of hexosaminidase activity and is presented as mean ± SD. Where indicated, the immobilized r33 FN was preincubated with the indicated concentrations of TSG-6Q or with Link_TSG6 for 30 min at 37 °C and then washed to remove unbound TSG-6 prior to adding Jurkat cells (solid bars) or used without washing (open bars).
Because the CUB_C domain but not the Link domain of TSG-6 binds avidly to FN, this suggested that the enhancement of adhesion in the presence of full length TSG-6 and Link_TSG6 might be mediated by direct interaction of the Link module with the cells rather than the matrix. Because serum-free medium was used, indirect binding is unlikely. We tested this hypothesis in the context of α5β1-mediated T cell adhesion by comparing adhesion to immobilized r33 FN that was preincubated with TSG-6Q or Link_TSG6 and then washed or tested without removing the unbound proteins (Fig. 5B). At the higher concentration tested, both TSG-6Q and Link_TSG6 significantly increased T cell adhesion on r33 FN when they remained present after the cells were added but less so when they were washed out before adding the cells. Therefore, the positive effect of TSG-6 and its Link domain on α5β1-mediated adhesion appears to be mediated at least in part by interactions with the cells rather than with FN.
2.6 TSG-6 modulates FN matrix assembly
FN matrix assembly is a cell-dependent process that converts secreted cellular FN and soluble plasma FN into insoluble fibrillar matrices (Mao and Schwarzbauer, 2005). Because the TSG-6 binding site in FN is proximal to regions involved in FN matrix assembly (Fig. 3A), we examined the effect of TSG-6 on assembly of a FN matrix by fibroblasts. We first examined the effect of adding TSG-6 on endogenous FN matrix assembly by freshly plated human Hs68 fibroblasts (Fig. 6). As expected based on the adhesion assays, initial spreading of the fibroblasts was moderately accelerated in the presence of TSG-6Q, but at 16 and 24 h no differences in cell adhesion were observed (Fig. 6 and results not shown). However, endogenous FN matrix deposition was markedly enhanced at both time points in the presence of either full length TSG-6Q or CUB_C (Fig. 6 and results not shown).
Fig. 6. TSG-6Q and CUB_C increase endogenous fibronectin matrix assembly in fibroblasts.
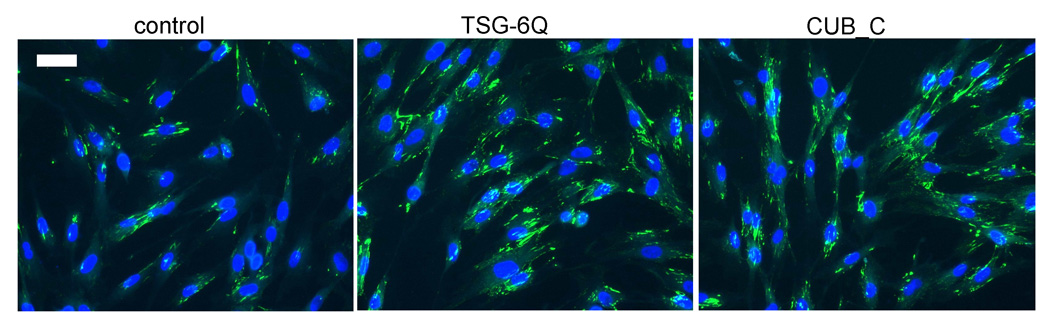
Hs68 human fibroblasts were plated on glass in serum-free medium alone or in the presence of 4 µg/ml TSG-6Q or 2 µg/ml CUB_C and incubated for 24 h. The cells were then fixed and stained with FN antibody to visualize matrix deposition (green) and Hoechst 33258 to visualize nuclei (blue). Representative fields imaged at equal exposure times are shown with the scale bar = 50 µm.
To exclude potential effects of TSG-6 on secretion of endogenous FN, we also examined incorporation of exogenous FN into fibroblast matrix by incubating Hs68 cells for 3 h with exogenous biotinylated-FN (Fig. 7). Incubation in the presence of full-length TSG-6Q markedly increased incorporation of exogenous FN relative to control cells (Fig. 7 lower panel). In contrast, incubation with an equimolar concentration of Link_TSG6 did not enhance FN matrix assembly (center panel).
Fig. 7. TSG-6Q increases exogenous fibronectin matrix assembly.
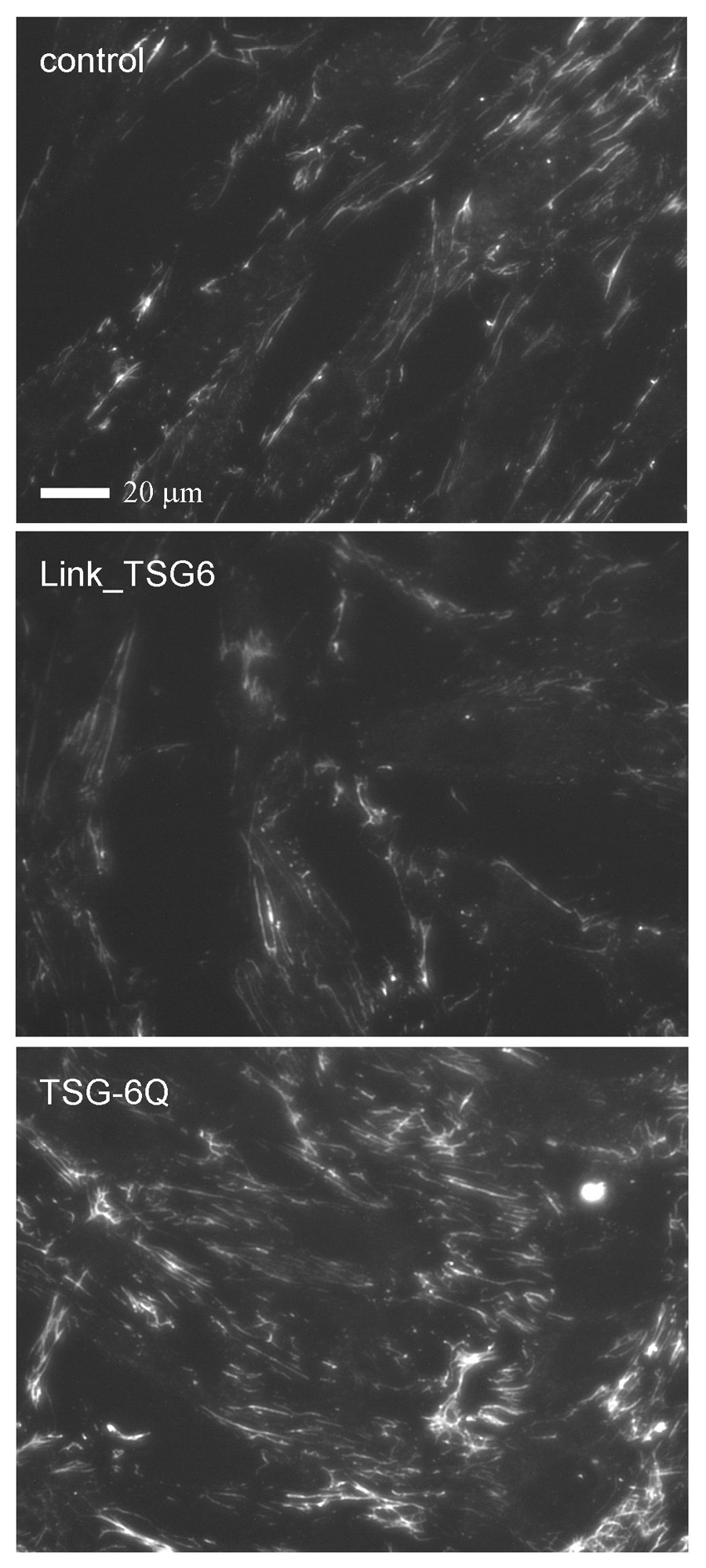
Hs68 fibroblasts were grown on glass slides for 24 h in complete medium and then incubated for 3 h in serum-free medium containing 10 µg/ml of biotinylated FN alone (control) or in the presence of equimolar Link_TSG6 or full length TSG-6Q. The cells were fixed with paraformaldehyde and stained using streptavidin-Alexa Fluor 594. Fluorescence images were captured at equal gain and exposure times, and representative fields are shown. Scale bar = 20 µm.
To confirm the qualitative results from these immunofluorescence studies, we employed a semi-quantitative assay of FN matrix assembly (Pankov and Yamada, 2004). FN that associates with cells is incorporated into two pools that can be distinguished by their solubility in deoxycholate (DOC) detergent. The DOC-soluble pool represents FN bound by cellular receptors and preexisting matrix fibrils, while the DOC-insoluble pool is believed to include bound FN that is incorporated into the matrix through detergent-resistant interactions such as disulfide bonding (Pankov and Yamada, 2004). Addition of full-length TSG-6Q to Hs68 cells increased the amount of biotinylated-FN that became incorporated into the DOC-insoluble matrix fraction (Fig. 8A). In contrast, treatment of the cells with Link_TSG6 did not affect FN matrix assembly. This activity of TSG-6 probably involves its binding to fibrillar FN in the ECM because TSG-6Q but not Link_TSG6 was incorporated into the DOC-insoluble matrix fraction in a dose-dependent manner (Fig. 8B).
Fig. 8. TSG-6 is incorporated into and increases fibronectin incorporation into detergent-insoluble matrix.
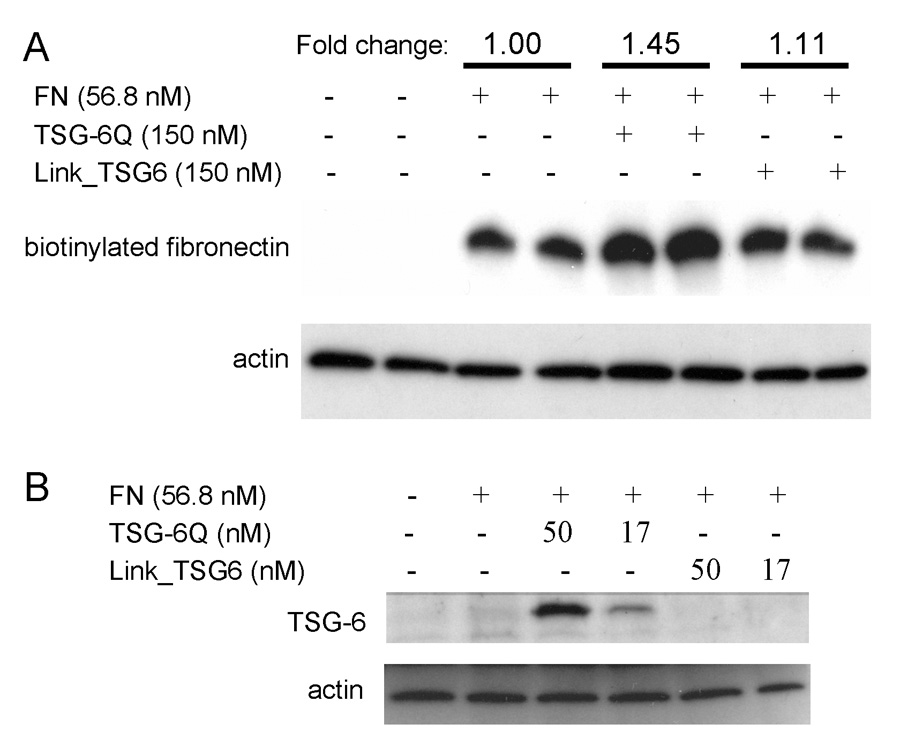
A, Hs68 fibroblasts were cultured overnight in normal medium, washed with medium without serum containing 1% BSA, and incubated in the same medium alone or supplemented with 12.5 µg/ml (57 nM) of biotinylated FN without additional agents or in the presence of a 3-fold molar excess of TSG-6Q or Link_TSG6. Deoxycholate (DOC)-insoluble fractions were resolved on 7.5% polyacrylamide gels, transferred to nitrocellulose membranes, and probed with HRP-conjugated streptavidin to determine the amount of incorporated FN. The same membranes were re-probed with antibodies against actin to assess the efficiency of protein extraction and gel loading. The results shown are representative of three independent experiments and were analyzed by densitometry. The FN signals were normalized to the respective actin signals, and the mean for each duplicate is presented normalized to a value of 1.0 for FN alone. B, Samples from the DOC-insoluble fraction were assayed by immunoblotting with goat polyclonal anti-TSG-6 to assess the incorporation of TSG-6.
3. Discussion
We previously showed that TSG-6 specifically interacts with N-modules of thrombospondin-1 and to a lesser extent with thrombospondin-2, two transient components of ECM (Kuznetsova et al., 2005). In the present study we addressed the possibility that TSG-6 might interact with other components of ECM and thereby modulate ECM-dependent regulation of cell behavior. FN is an abundant and ubiquitous plasma and ECM component that is organized into a fibrillar network by some cell types through direct interactions with cell surface receptors (Mao and Schwarzbauer, 2005; Midwood et al., 2006). This study demonstrates that TSG-6 binds human plasma and cellular FN. FN was found to directly interact with full-length TSG-6 protein and its CUB_C domain with high affinity, whereas it did not bind avidly to the isolated Link domain of TSG-6.
Inhibition studies using recombinant and proteolytic fragments of FN localized TSG-6 binding to the central region of FN in its type 3 repeats, which contains integrin and heparin-binding sites. Since this domain of FN is required for the initial steps of FN matrix assembly (Midwood et al., 2006), effects of TSG-6 on the formation of FN fibrils were investigated. We found that TSG-6 enhances FN fibril formation mediated by human Hs68 fibroblasts. Furthermore, TSG-6 is incorporated into the detergent-insoluble ECM of fibroblasts and co-localizes with FN (data not shown). Therefore, TSG-6 may interact with FN in the ECM surrounding cultured human fibroblasts. Such binding would target TSG-6 deposition into fibrillar FN and appears to enhance FN matrix assembly.
Interactions of FN with both α5β1 integrin and syndecan-4 are important for fibrillogenesis (Midwood et al., 2006). Because the Link module of TSG-6 binds avidly to glycosaminoglycans (e.g., heparan sulfate (Mahoney et al., 2005)), TSG-6 may facilitate FN fibrillogenesis either by directly engaging the glycosaminoglycans of syndecan-4 or by facilitating syndecan-4 binding to FN. Alternatively, the enhanced integrin-dependent adhesion on a FN substrate that we observed in the presence of TSG-6 could enhance FN fibrillogenesis by promoting the integrin function in this process (Clark et al., 2005; Mao and Schwarzbauer, 2005). However, latter is less likely because Link_TSG-6 enhances adhesion on immobilized FN but does not enhance fibrillogenesis.
The Link module of TSG-6 interacts with hyaluronan, chondroitin-4-sulfate, dermatan sulfate, heparin/heparan sulfate, aggrecan, versican, pentraxin-3, thrombospondin-1 and inter-α-trypsin inhibitor (Milner and Day, 2003; Kuznetsova et al., 2005; Kuznetsova et al., 2006; Milner et al., 2006). FN is the first known ligand for TSG-6 that does not bind within the Link module domain, but rather associates with the CUB_C domain, i.e. the C-terminal half of the protein. Our data indicates that at least one of these Link module ligands, thrombospondin-1, can form a ternary complex where TSG-6 acts as bridging molecule between thrombospondin-1 and FN. This resembles the bridging function of versican to mediate thrombospondin-1 association with elastin-containing microfibrils on the surface of poly-I:C-stimulated vascular smooth muscle cells (Kuznetsova et al., 2006). Thus, TSG-6 can serve to increase FN association with at least one ligand of the TSG-6 Link module. Further studies are needed to determine whether this function generalizes to other TSG-6 ligands that interact with the Link module. Additional work is also required to investigate whether binding of TSG-6 alters the conformation of FN or its interactions with other ECM or cell surface receptors other than α5β1 integrin.
TSG-6 is a regulated component of ECM that is induced under specific physiological and pathological conditions (Milner and Day, 2003; Milner et al., 2006). This raises the question of whether the FN binding activities of TSG-6 might play important roles in modulating fibrillar FN assembly or other ECM structures in inflammatory conditions or during ovulation. Considering the biological significance of ECM in regulating the compartmentalization of tissues into functional units, the possibility that TSG-6 modulates additional aspects of ECM structure and function merits further investigation.
4. Experimental Procedures
4.1 Protein Purification
Plasma FN was purified from fresh human plasma (National Institutes of Health Blood Bank) using a nondenaturing method (Akiyama and Yamada, 1985). Recombinant fragments derived from individual domains of FN, expressed in E. coli, were purified and refolded as described previously (Werber et al., 1991; Vogel et al., 1993). A 33 kDa cell-binding domain with the RGDS sequence deleted (r33ΔRGD) was constructed by oligonucleotide-directed mutagenesis of the expression plasmid, pFN 137-2 as described previously (Vogel et al., 1993; Negre et al., 1994). The FN fragments used in this paper and their origin from the FN sequence are summarized in Fig. 3A. Human fibroblast and lung FNs were provided by Dr. Ralph Silverman, Fibrogenex, Inc., Chicago, IL. Other control proteins were obtained from Sigma-Aldrich. FN was labeled with 125I using IODO-GEN (Pierce Biotechnology, Rockford, IL) as described previously (Guo et al., 1992). Biotinylated-FN was prepared using sulfo-N-hydroxysuccinimidyl-biotin (Pierce Biotechnology, Rockford, IL), followed by a dialysis step to remove unconjugated biotin (Pankov and Yamada, 2004). Recombinant full-length TSG-6Q (with a glutamine at residue 144) was expressed in Drosophila S2 cells (Nentwich et al., 2002), and Link_TSG6 was expressed in E. coli as described previously (Day et al., 1996; Kahmann et al., 1997).
4.2 Expression and Purification of TSG-6 CUB_C Domain
The CUB_C domain of human TSG-6 (residues 129–277 in the preprotein (Lee et al., 1992) with a glutamine at residue 144) was expressed in E. coli. Initial attempts to express the CUB module alone (i.e. residues 134–248) did not give rise to folded protein (H.A. Nentwich & A.J. Day, unpublished observations). Briefly, the CUB_C coding sequence was amplified from human osteoblast mRNA, as described previously for the full-length protein (Nentwich et al., 2002), cloned into pRK172 and transfected into Rosetta-gami BL21(DE3)pLysS. Cells were harvested 4h after induction with IPTG and inclusion bodies were purified as described in (Day et al., 1996). These were solubilized in 8M guanidine·HCl, 50mM Tris·HCl, pH 8.0, 100 mM dithiothreitol, incubated for 1h at 37°C and then purified using a 100 × 5-cm S100-HR column equilibrated in 6M guanidine·HCl, 20mM Tris·HCl, pH 8.0. The partially purified CUB_C protein (40 ml) was refolded by dialysis overnight at 4°C into 2L 0.5M L-arginine, 2mM cystine, 1 mM cysteine, 20 mM ethanolamine, 5 mM CaCl2·2H2O, pH 5.0, followed by a 6h dialysis against another 2L of refolding buffer. The protein was then dialyzed overnight at 4°C against 3 L of 0.1M Na-acetate, pH 4.0, and then purified to homogeneity using anion exchange chromatography on a 30 ml SP-Sepharose column, equilibrated in 50 mM Na-acetate, pH 4.0, and eluted with a gradient of 0 to 1M NaCl (in 50 mM Na-acetate, pH 4.0) over 13-column volumes. Finally the protein was dialyzed into PBS and stored at −20°C. Electrospray ionization mass spectrometry showed that the recombinant CUB_C domain had a molecular weight within 2 Da of the expected mass (16,776.6 Da).
4.3 One-dimensional NMR spectroscopy
Following ion exchange chromatography, the CUB_C protein was dialysed extensively against PBS using 3,500 MWCO snakeskin dialysis membrane, followed by concentration on a Vivaspin 3,500 MWCO spin column to a concentration of ~2 mg/ml. D2O (60 µl) was added to 540 µl of the CUB_C preparation to give a final concentration of ~ 0.11 mM. The 1-dimensional NMR spectrum was acquired at 21.7°C on a home-built NMR spectrometer with a 1H operating frequency of 599 MHz at the Department of Biochemistry, University of Oxford and referenced against water (4.805 ppm at 21.7 °C).
4.4 Solid Phase Binding assays
Immulon® 2 HB microtiter strips with breakaway wells (ThermoLabsystems, Franklin, MA) were coated using 50 µl of the indicated concentrations of TSG-6Q, Link_TSG6 or CUB_C domain. For competitive binding studies, wells were coated using 10 µg/ml of full-length TSG-6Q, Link_TSG6 or CUB_C incubated overnight at 4°C in Dulbecco’s PBS without Ca2+ or Mg2+. Nonspecific sites were blocked using 3% (w/v) BSA in Dulbecco’s PBS (DPBS) at room temperature for 1 h. Radioiodinated FN (0.5 µg/ml, 50 µl/well) was added alone or in the presence of increasing concentrations of the indicated unlabeled ligands as competitors in DPBS, containing 0.5% (w/v) BSA, 0.1 mM phenylmethylsulfonyl fluoride with or without Ca2+ and Mg2+, and incubated at 37°C for 3 h. The wells were washed with the same cold buffer, and the bound radioactivity was quantified using a gamma counter (PerkinElmer Life Sciences).
4.5 Cross-linking and mass spectrometry
Cross-linking of CUB_C protein with the recombinant FN fragment r-33kDa was carried out in 150 mM NaCl, 50 mM HEPES, 7.4 with a final concentration of 125 µM of the cross-linking agent Bis(Sulfosuccinimidyl)suberate (BS3) (Pierce) in 200 µl reactions. The cross-linking reactions were quenched by the addition of 10 µl of 1M Tris/HCl, pH 8.0. Protein was recovered using 10 µl of Strataclean resin (Stratagene) and analyzed by SDS-PAGE on 10% (w/v) Tris-Tricine polyacylamide gels after boiling the samples in SDS loading buffer containing β-mercaptoethanol. A Coomassie blue-stained gel slice, corresponding to a ~50 kDa species that had been crossed linked with BS3, was washed in 100% acetonitrile for 5 min, the acetonitrile removed, and the gel slice vacuum dried for 15 min. The gel was hydrated with 10 mM DTT in 25mM NH4HCO3 at 56°C for 1 h, an equal volume of 55 mM iodoacetamide in 25mM NH4CO3 was added, and the gel slice then incubated for 45 min at room temperature in the dark and washed with 25 mM NH4HCO3 for 10 min. This was followed by washes with acetonitrile (5 min), 25 mM NH4HCO3 (5 min) and acetonitrile (5 min) and the gel slice vacuum dried. The gel slices were treated with 12.5-ng/µl trypsin (Promega) in 25 mM NH4CO3, incubated at 4°C for 45 min and then left incubating overnight at 37°C. Peptides were extracted from the gel slice by washing with 20 mM NH4HCO3 for 20 min at room temperature followed by two further washes for 20 min at room temperature with 5% formic acid in 50% acetonitrile. These washes were then combined, the samples concentrated, and mass-spectrometry was performed using MS-MS HCT ion trap mass spectrometer (Brucker).
4.6 Cell culture and treatment
Neonatal human foreskin fibroblasts (Hs68, CRL 1635, American Tissue Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 4 mM glutamine and 4.5 g/l glucose, supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin in an incubator at 37 °C in a humidified atmosphere containing 5% CO2. Non-radioactive quantification of FN matrix assembly was performed as described (Pankov and Yamada, 2004). Briefly, Hs68 cells were cultured in wells of 6-well plates until confluent. Cells were washed with the same medium, 1 ml of medium containing 10 µg/ml biotinylated FN was added to each well and incubated at 37°C in the presence or absence of full-length TSG-6Q or Link_TSG6 for 3 h, except where indicated in figure legend. Medium was removed and cells were washed with DPBS three times. Matrix was solubilized using 500 µl DOC extraction buffer (1% sodium deoxycholate, 20 mM Tris, 2 mM N-ethylmaleimide, 2 mM iodoacetic acid, 2 mM EDTA, 50 µM leupeptin, 50 µM pepstatin, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 50 mM NaF). After centrifugation, the DOC-insoluble pellet was solubilized in 25 µl of 2% SDS, 20 mM Tris·HCl, pH 8.8, 2 mM phenylmethylsulfonyl fluoride, 2 mM iodoacetic acid, 2 mM N-ethylmaleimide and 2 mM EDTA. Equal volumes of DOC-insoluble samples were analyzed by SDS-PAGE using 5% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA). Samples were analyzed by Western blotting with streptavidin peroxidase (Pierce) in parallel with antibodies against β-actin (Sigma-Aldrich, St. Louis, MO) and goat anti-mouse HRP-conjugated antibody (Pierce Biotechnology, Rockford, IL). TSG-6 was detected by blotting with polyclonal goat anti-TSG-6 (sc-21828, Santa Cruz Biotechnology). Immunoblots were developed with SuperSignal West Dura Chemiluminescent substrate (Pierce Biotechnology, Rockford, IL).
To visualize endogenous FN matrix deposition, Hs68 fibroblasts were plated in 8-well glass chamber slides were incubated for the indicated times in serum free medium. To examine exogenous FN matrix deposition, Hs68 cells were plated in chamber slides in complete medium for 24 h and then incubated for 3 h in serum free medium in the absence or presence of 10 µg/ml of biotinylated-FN. Subsequently, the incubation medium was removed, and the cells were fixed with 4% paraformaldehyde in DPBS for 7 min at room temperature and then blocked with DPBS containing 4% BSA for 30 min. For cells treated with biotinylated FN, the slides were incubated with Alexa Fluor 594-streptavidin and Hoechst 33258 (Molecular Probes, Eugene, OR) at a dilution recommended by the manufacturer for 1 h at room temperature. To visualize endogenous FN, the slides were incubated with mouse anti-human FN (clone III, Life Technologies, Inc) followed by Alexa 488 anti-mouse IgG (1:500) and Hoechst 33258. The slides were washed four times with DPBS and then rinsed in water. The cells were imaged using an Olympus IX70 fluorescence microscope and a Spot Insight cooled digital camera (Diagnostic Instruments, Sterling Heights, MI).
4.7 Adhesion assays
Jurkat T cells (provided by Dr. Kevin Gardner, National Cancer Institute) were maintained in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, and penicillin and streptomycin (all culture medium and medium supplements were purchased from GIBCO). Purified FN and recombinants fragments of FN, diluted in Dulbecco’s PBS (without Ca2+, Mg2+), were coated onto 96-well flat-bottom plates (NUNC MaxiSorp) overnight at 4°C. After aspiration of the buffer, nonspecific adherence to plastic was blocked by incubation with Dulbecco’s PBS (DPBS) containing 1% BSA (Sigma) at room temperature for 30 min. Cells were washed in serum-free medium and resuspended at 1×106 cells/ml in RPMI containing 0.1%BSA. Aliquots of cells (50 µl) were added to each well containing 50 µl of the indicated concentrations of full-length recombinant human TSG-6Q, Link_TSG6 or CUB_C domain diluted in RPMI/0.1% BSA. The plate was incubated at 37°C for 20 min. Nonadherent cells were removed by washing. The number of adherent cells was quantified using the previously described colorimetric hexosaminidase assay (Wilson et al., 1999).
4.8 Data Analysis
All experiments were reproduced at least three times. Self-displacement binding experiments were analyzed using Scapre and Scafit version 3.10 of the LIGAND program (Munson and Rodbard, 1980).
Acknowledgments
We thank Dr. Ralph Silverman and BioTechnology General for providing reagents, Victoria Higman for performing the NMR analysis, and Iain Campbell for access to the NMR facilities. We would like to thank Emma-Jayne Keevil from the Biomolecular Analysis Core Facility, Faculty of Life Sciences, University of Manchester for carrying out mass spectrometry; this facility is supported by the Wellcome Trust. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (DDR) and the Arthritis Research Campaign (grant 16539, AJD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnotes: The abbreviations used are: DOC, deoxycholate; DPBS, Dulbecco’s phosphate buffered saline; ECM, extracellular matrix; FN, fibronectin; TSG-6, tumor necrosis factor-stimulated gene-6; CUB, complement component Clr/Cls, Uegf, and bone morphogenic protein 1
References
- Akiyama SK, Yamada KM. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985;260:4492–4500. [PubMed] [Google Scholar]
- Blundell CD, Almond A, Mahoney DJ, DeAngelis PL, Campbell ID, Day AJ. Towards a structure for a TSG-6.hyaluronan complex by modeling and NMR spectroscopy: insights into other members of the link module superfamily. J Biol Chem. 2005;280:18189–18201. doi: 10.1074/jbc.M414343200. [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, Newham P, Yamada KM, Humphries MJ. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci. 2005;118:291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, Aplin RT, Willis AC. Overexpression, purification, and refolding of link module from human TSG-6 in Escherichia coli: effect of temperature, media, and mutagenesis on lysine misincorporation at arginine AGA codons. Protein Expr Purif. 1996;8:1–16. doi: 10.1006/prep.1996.0068. [DOI] [PubMed] [Google Scholar]
- Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- Guo NH, Krutzsch HC, Nègre E, Zabrenetzky VS, Roberts DD. Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J Biol Chem. 1992;267:19349–19355. [PubMed] [Google Scholar]
- Kahmann JD, Koruth R, Day AJ. Method for quantitative refolding of the link module from human TSG-6. Protein Expr Purif. 1997;9:315–318. doi: 10.1006/prep.1996.0694. [DOI] [PubMed] [Google Scholar]
- Kuznetsova SA, Day AJ, Mahoney DJ, Rugg MS, Mosher DF, Roberts DD. The N-terminal module of thrombospondin-1 interacts with the link domain of TSG-6 and enhances its covalent association with the heavy chains of inter-alpha -trypsin inhibitor. J Biol Chem. 2005;280:30899–30908. doi: 10.1074/jbc.M500701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova SA, Issa P, Perruccio EM, Zeng B, Sipes JM, Ward Y, Seyfried NT, Fielder HL, Day AJ, Wight TN, Roberts DD. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J Cell Sci. 2006;119:4499–4509. doi: 10.1242/jcs.03171. [DOI] [PubMed] [Google Scholar]
- Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Calzada MJ, Sipes JM, Cashel JA, Krutzsch HC, Annis D, Mosher DF, Roberts DD. Interactions of thrombospondins with α4β1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157:509–519. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Mulloy B, Forster MJ, Blundell CD, Fries E, Milner CM, Day AJ. Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparin: Implications for the inhibition of plasmin in extracellular matrix microenvironments. J Biol Chem. 2005 doi: 10.1074/jbc.M502068200. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of Cell-Fibronectin Matrix Interactions during Tissue Repair. J Invest Dermatol. 2006;(126 Suppl):73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochemical Society transactions. 2006;34:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Negre E, Vogel T, Levanon A, Guy R, Walsh TJ, Roberts DD. The collagen binding domain of fibronectin contains a high affinity binding site for Candida albicans. J Biol Chem. 1994;269:22039–22045. [PubMed] [Google Scholar]
- Nentwich HA, Mustafa Z, Rugg MS, Marsden BD, Cordell MR, Mahoney DJ, Jenkins SC, Dowling B, Fries E, Milner CM, Loughlin J, Day AJ. A novel allelic variant of the human TSG-6 gene encoding an amino acid difference in the CUB module. Chromosomal localization, frequency analysis, modeling, and expression. J Biol Chem. 2002;277:15354–15362. doi: 10.1074/jbc.M110765200. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003;144:4376–4384. doi: 10.1210/en.2003-0487. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Non-Radioactive Quantification of Fibronectin Matrix Assembly. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current Protocols in Cell Biology. Wiley InterScience; 2004. pp. 10.13.11–10.13.19. [DOI] [PubMed] [Google Scholar]
- Rodrigues RG, Guo N, Zhou L, Sipes JM, Williams SB, Templeton NS, Gralnick HR, Roberts DD. Conformational regulation of the fibronectin binding and α3β1 integrin-mediated adhesive activities of thrombospondin-1. J Biol Chem. 2001;276:27913–27922. doi: 10.1074/jbc.M009518200. [DOI] [PubMed] [Google Scholar]
- Rugg MS, Willis AC, Mukhopadhyay D, Hascall VC, Fries E, Fulop C, Milner CM, Day AJ. Characterization of complexes formed between TSG-6 and inter-alpha -inhibitor that act as intermediates in the covalent transfer of heavy chains on to hyaluronan. J Biol Chem. 2005;280:25674–25686. doi: 10.1074/jbc.M501332200. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991;13:527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, Sechler JL. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol. 1999;11:622–627. doi: 10.1016/s0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- Sieron AL, Tretiakova A, Jameson BA, Segall ML, Lund-Katz S, Khan MT, Li S, Stocker W. Structure and function of procollagen C-proteinase (mTolloid) domains determined by protease digestion, circular dichroism, binding to procollagen type I, and computer modeling. Biochemistry. 2000;39:3231–3239. doi: 10.1021/bi992312o. [DOI] [PubMed] [Google Scholar]
- Solis D, Romero A, Jimenez M, Diaz-Maurino T, Calvete JJ. Binding of mannose-6-phosphate and heparin by boar seminal plasma PSP-II, a member of the spermadhesin protein family. FEBS Lett. 1998;431:273–278. doi: 10.1016/s0014-5793(98)00772-8. [DOI] [PubMed] [Google Scholar]
- Szanto S, Bardos T, Gal I, Glant TT, Mikecz K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004;50:3012–3022. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
- Vogel T, Werber MM, Guy R, Levanon A, Nimrod A, Legrand C, Gorecki M, Eldor A, Panet A. Studies on fibronectin and its domains. I. Novel recombinant cell-binding domain of fibronectin--a modulator of human platelet functions. Arch Biochem Biophys. 1993;300:501–509. doi: 10.1006/abbi.1993.1068. [DOI] [PubMed] [Google Scholar]
- Werber MM, Vogel T, Kook M, Greenstein LA, Levanon A, Zelig Y, Havron A, Gorecki M, Panet A. Biologicals from recombinant microorganisms and animal cells: production and recovery. New York: VCH and Balaban Publishers; 1991. pp. 369–382. [Google Scholar]
- Wilson KE, Li Z, Kara M, Gardner KL, Roberts DD. β1 integrin- and proteoglycan-mediated stimulation of T lymphoma cell adhesion and mitogen-activated protein kinase signaling by thrombospondin-1 and thrombospondin-1 peptides. J Immunol. 1999;163:3621–3628. [PubMed] [Google Scholar]
- Wisniewski HG, Naime D, Hua JC, Vilcek J, Cronstein BN. TSG-6, a glycoprotein associated with arthritis, and its ligand hyaluronan exert opposite effects in a murine model of inflammation. Pflugers Arch. 1996;431:R225–R226. doi: 10.1007/BF02346350. [DOI] [PubMed] [Google Scholar]
- Yamada KM. Adhesive recognition sequences. J. Biol. Chem. 1991;266:12809–12812. [PubMed] [Google Scholar]


