Abstract
Introduction
Colorectal cancer is the second leading cause of cancer-related death in western countries. The objective of this systematic review was to show that laparoscopic-assisted colon resection for cancer is not inferior to open colectomy with respect to cancer survival and perioperative outcomes.
Method
We performed a comprehensive literature review. Inclusion criteria were adults aged over 16 years with a colon resection for documented colon cancer and randomized controlled trials with laparoscopic-assisted or open resections. We excluded studies that did not document colon cancer recurrence in their article. We assessed data extraction and study quality and performed a quantitative data analysis.
Results
Six published and 4 unpublished studies fulfilled our inclusion criteria, with a total of 1262 patients. All primary and secondary outcomes showed good homogeneity, except for morbidity, which was described heterogeneously between the studies. There was no disadvantage to laparoscopic colon resection in any of these primary and secondary outcomes, compared with the conventional open technique.
Conclusion
The results of this study suggest that, although there is no definitive answer, present evidence indicates that laparoscopic colon cancer resection is as safe and efficacious as the conventional open technique.
Abstract
Introduction
Le cancer colorectal est la deuxième cause en importance de mortalité reliée au cancer dans les pays occidentaux. Cette étude systématique visait à montrer que la résection du côlon par laparoscopie dans un cas de cancer ne donne pas des résultats moins bons que la colostomie sanglante en ce qui concerne la survie au cancer et les résultats périopératoires.
Méthode
Nous avons procédé à une recension détaillée des écrits. Les critères d'inclusion étaient les suivants : adultes âgés de plus de 16 ans ayant subi une résection du côlon pour un cancer du côlon documenté et essais contrôlés randomisés avec résection par laparoscopie ou sanglante. Nous avons exclu les études qui n'ont pas documenté une réapparition du cancer du côlon. Nous avons évalué l'extraction des données et la qualité de l'étude et procédé à une analyse de données quantitatives.
Résultats
Six études publiées et quatre études non publiées ont satisfait à nos critères d'inclusion et portaient au total sur 1262 patients. Tous les résultats primaires et secondaires ont montré une bonne homogénéité, sauf dans le cas de la morbidité, que l'on a décrite de façon hétérogène entre les études. La résection du côlon par laparoscopie n'a présenté aucun désavantage dans aucun de ces résultats primaires et secondaires par rapport à la technique sanglante classique.
Conclusion
Les résultats de cette étude indiquent que même s'il n'y a pas de réponse définitive, les données actuelles indiquent que la résection d'un cancer du côlon par laparoscopie est aussi sécuritaire et efficace que la technique sanglante classique.
The era of modern laparoscopic surgery began in the late 1980s with cholecystectomies. Jacobs1 reported the technical feasibility of laparoscopic colectomy in 1991. The innovative application of laparoscopic procedures has reinvigorated general abdominal surgery in recent years. Technical approaches range from intracorporeal procedure, where no additional incisions are required,2 to extracorporeal or laparoscopic-assisted techniques, where a small abdominal incision facilitates the resection and anastomosis of the bowel outside of the abdominal cavity.2
Colorectal cancer is the second leading cause of cancer-related death in western countries.3 Adequate surgical resection is the only curative treatment, with overall survival rates of just under 50% at 5 years. The surgical technique is critical, with respect to both cure and local recurrence. Rates of complications and death from standard colon cancer surgery have been reported to range from 8% to 15% and 1% to 2%, respectively.4–11
Laparoscopic colorectal surgery is being used increasingly for benign disease. Recent published series have proven that morbidity from laparoscopic procedures is less than that seen after traditional open procedures. These include diminished postoperative pain, quick return of gastrointestinal function, shorter hospital stay, more rapid convalescence and less immunosuppression.4–13 However, although the technical feasibility of laparoscopic bowel resection has been confirmed, the oncological advisability has not. Others have raised legitimate concerns regarding difficult learning curves, longer operative times, increased complication rates and excessive costs.4,9–11,14 Surgeons have been reluctant to embrace laparoscopic techniques in colorectal cancer because of concerns about whether an adequate intro-abdominal exploration for cancer can be undertaken, and reports of unusual early metastases to the port sites of the abdominal wall after laparoscopic colectomy for cancer.8,15,16 There is also the uncertainty regarding long-term survival, recurrence, extent of resection, staging limitations and possible altered tumor spread.4–6,9,14 As a consequence, some surgeons have abandoned or not adopted the laparoscopic approach in patients with colorectal malignancy. To date, the pathogenesis of port-site recurrences has not been clarified, and with growing data on reduced morbidity after laparoscopic resection of colorectal cancer, controversy persists.
A combination of the development of expensive new treatments for patients with diseases such as cancer, the limited budgets available for healthcare and increasing demands on the health system have led to an increase in the number of clinical trials that evaluate the cost of implementing new treatments into health service. There are multiple on going and recently published randomized prospective trials looking at the issue of recurrence postlaparoscopic colectomy for colon cancer, compared with conventional treatment.
The primary aim of these trials is to test the hypothesis that disease-free survival and overall survival are equivalent, regardless of whether patients receive laparoscopic-assisted or open colectomy. The secondary aim of the trials is to assess the safety of a laparoscopic-assisted colectomy, compared with open colectomy. Finally, the tertiary aim is to assess the cost effectiveness of laparoscopic-assisted colectomy and patients' quality of life, compared with those who undergo open colectomy. The objective of this systematic review is to show that laparoscopic-assisted colorectal resection for cancer is not inferior to open colectomy with respect to cancer survival and perioperative outcomes. The primary outcome of concern is cancer-related mortality. Secondary outcomes include all-cause mortality, all recurrence, local recurrence, port-site recurrence and morbidity.
Method
Search strategy
We searched Ovid MEDLINE (1966–2004), Ovid MEDLINE in-process and other nonindexed citations in Ovid MEDLINE; EMBASE (1988–week 3 of 2004); the Cochrane Library (1981–2004), including the Cochrane Database of Systematic Reviews, the American College of Physicians Journal Club, the Database of Abstracts of Reviews of Effectiveness, the Cochrane Controlled Trials Register, Cancerlit (1975–October 2002) and First Search for conference proceedings.
We used the following search terms: Rect-or Col-or Bowel or Intestin-and Neoplas-or Tumo-or Mass or Cancer or Carcino-or Adenocarcinoma or Malignan. We combined these with (Surg-or Laparo-or Video Assisted Surg-or Operat-or Resect-or Hemicolectomy or Subtotal or Colect-or Anterior or Abdominoperineal or Panprocto-or Minimal. Finally, we combined the results of all above combinations with Random-or Clinical Trial or Controlled Trial.
We searched the related article feature of PubMed and manually searched all references. We also manually searched 5 high-impact journals, chosen on the basis of the frequency of articles found and on expert opinion from 1998 to 2004 (Disease of Colon and Rectum, Surgical Endoscopy, Archives of Surgery, British Journal of Surgery and Journal of American College of Surgery). We wrote to experts and corresponding authors to identify other published or unpublished studies. There were no language restrictions. We then cross-referenced SCISEARCH in the Web of Science for other articles.
We reveiwed the titles and abstracts and photocopied any article that the reviewer thought might meet our inclusion criteria. For abstracts, the corresponding author was contacted, and a complete copy of the text and data were requested.
Study selection criteria
We retrieved articles considered potentially relevant by either one or both reviewers. We were concerned that, in our desire to be comprehensive, we would collect many articles that might not have been appropriate for inclusion in the overview. Thus we used the following inclusion criteria for the overview:
1. Adults aged over 16 years who underwent colorectal resection for documented colorectal cancer
2. Randomized controlled trials
3. Laparoscopic-assisted colorectal resection versus open or conventional colorectal resection.
We excluded studies that did not have the outcome of recurrence of colorectal cancer documented in their articles.
In duplicate and independently, 2 authors applied these criteria to the full articles. If there were any disagreements, we decided a priori to resolve them by consensus.
The level of agreement between reviewers was measured and reported with both kappa and percentage agreement if it was less than 100%. A minimum a priori criterion was that agreement as measured by kappa should be greater than 0.65. A kappa of greater than 0.65 was selected for good consistency. When disagreement occurred, 2 reviewers met. Our experience with other overviews suggested that the cause of the disagreement is often a simple oversight on the part of one of the reviewers. When this was not the case, the issue was resolved first by consensus between 2 of the reviewers and, if still unresolved, by consensus of the overview team.
Data extraction and study quality
Independently and in duplicate, 2 authors abstracted data on the study method, patient population, interventions and outcomes. The primary outcome was cancer-related mortality. The secondary outcomes were all-cause mortality, all recurrence, local recurrence, port-site recurrence and morbidity. Differences were resolved by consensus, as previously described.
We developed a quality assessment form to critically appraise these studies. We contacted authors when key data were unclear or not reported, and we deleted unsuitable and duplicate studies from the database.
We assessed validity by systematically considering each of the potential sources of error and bias in relation to the specific questions being addressed. We developed the following criteria to evaluate the validity of the studies included in the overview. We calculated N of 500 on the basis of a 10% difference being considered equal.
Simply adding the scores for each of the individual criteria to form a single score for each study would be hazardous, because the study that fulfills all but 1 of the criteria might receive a high total score but produce erroneous results if the remaining criteria are violated in a way that creates bias. Conversely, a study with a relatively low score might still be valid if the criteria that were not met were not critically important for the particular study. Nonetheless, it is desirable to have an explicit approach for summarizing the quality of each study. Our approach to ranking the quality of studies is to present them in their transparency for the reader to decide.
The same reviewers who judged eligibility conducted a blinded review of papers meeting the eligibility criteria; this time, the methodological quality of the primary research was rated. Quality assessment was calculated, and disagreements were resolved in the same fashion that relevance was assessed. If the missing information was important, the investigator was contacted by letter, e-mail or telephone and was asked to provide what was missing.
Some of the information needed to write the report was provided by abstracting—one component of the assessment of methodological quality. However, a separate review of papers was required to abstract the study findings. This was also conducted in duplicate by the same 2 reviewers to avoid any errors of abstraction.
Given the extent of insufficient reporting in medical literature, we obtained missing information from investigators when possible. It is otherwise impossible to distinguish between what was done but not reported and what was not done. To avoid introducing bias, unpublished information was obtained in writing and was coded in the same fashion as published information, with equal regard for intercoder agreement.
In addition to using blinding and multiple codes to ensure the reproducibility of the overview, sensitivity analysis around important or questionable judgments regarding the inclusion or exclusion of studies, we also performed validity assessments and data abstraction.
Data analysis
We measured crude agreement between reviewers regarding study selection, data abstraction and quality assessment. We reported proportions and percentages, means and standard deviation or standard error, medians and ranges, odds ratios (ORs) and 95% confidence intervals (CIs).
To evaluate our primary and secondary outcomes, we summarized data with a random effect model,17 and we explored publication bias with a funnel plot. With a funnel plot, a trial effect (OR, relative risk) is plotted against a measure of its precision. Precision may be defined in different ways. Commonly used definitions are the number of subjects in a trial or a function of the standard error. If the plot is symmetric, like an inverted V, there is probably no publication bias. We tested for statistical heterogeneity, using the chi-square test.18 Statistical calculations and graphical analyses were performed with the Comprehensive Meta Analysis, version I, by Biostat. Tests of significance were 2-tailed, and a p value of less than 0.05 was considered significant.
A priori, we specified that randomization, staging inequality and colon versus rectum factors might influence the outcomes and explain heterogeneous results among the studies. We performed a sensitivity analysis, comparing studies of the colon with those of the rectum.
The analysis begins with a visual plot of the study results summarized as ORs, including CIs around the point estimate.
The estimates will be combined to estimate the typical effect size across studies that are not heterogeneous, and 95% CIs will be calculated around the point of estimate(s). ORs were used as they are widely used, exact distributions, and available in our computer program by Biostat.
The power of the test for heterogeneity was calculated and reported, and a p value of less than 0.10 was considered significant for heterogeneity. Conclusions regarding both the typical effect size and the effect size relative to the specific study characteristics were interpreted cautiously if there was a significant heterogeneity. Conclusions based on between-study differences must, in any case, be interpreted cautiously. In general, conclusions based on between-study differences will be considered hypothesis-generating analyses rather than hypothesis testing.
Results
Study selection
We identified 127 citations. We excluded 119, because since they did not fulfill inclusion and exclusion criteria. This is well demonstrated in Figure 1 with the subheadings “not randomized,” “wrong outcome,” “duplicates,” “letter,” “unrelated” and ‚nonhuman,” as the reasons for exclusion. In addition to 5 studies that were included, we identified 5 unpublished studies that will most likely be published within the next 2 years, one of which was published during the analysis of the study and therefore included as the 6th study.19 Agreement for selecting abstracts was 95% and for selecting full articles was 100%.

FIG. 1. Study flow diagram.
Study characteristics
We report study details in Table 1 and study characteristics in Table 2. One study was conducted in Spain,21 2 in United States,8,20 1 in Denmark,4 1 in Brazil22 and 1 in North America.19
Table 1

Table 2

The funding source was reported in 3 studies.8,21,23 Two studies observed the patients for 2 years or less.4,8 Four studies solely involved colon cancer,4,19–21 1 study involved rectal cancer22 and the rest had a combination of both.8 Four studies did not have equal demographics at baseline, specifically in consideration of their preoperative staging of cancer.4,20–22
As seen in Table 2, one multicentre study was identified.19 Most of these studies were from tertiary care centres. Only 2 reported the number of patients screened.8,21 The study dates ranged from March 1992 to May 2004, and there were 1262 patients between the studies. Only one study mentioned the number of surgeons involved, or whether any form of blinding was attempted.19
Unfortunately, due to the nonuniformity of study characteristics presented in each study, such as means, medians, standard deviations (SDs), and ranges, we were not able to pool some of the data, and we presented these in their transparency in Table 2. In these tables, the age of patients in both study arms are presented, as well as the percentage of men and the various ASA classifications, which were reported by 2 studies.8,19 All but 2 studies4,22 excluded patients who required emergency surgery. The Duke's staging, as shown in Table 3 was mentioned in 3 of the 6 studies.4,22,24–26 One study used TNM classification.19 The territory for resection was mentioned in all studies. Evidence for preoperative metastases was mentioned in all studies and was higher in the open group in one study.4 Only 2 studies19,21 mentioned past history of colorectal cancer. Similarly, only 3 studies mentioned prior abdominal surgery.21–23
Table 3

Methodological quality
Because we designed the quality assessment, which was not previously validated, we did not give a quality score to the studies. We believe that our quality assessment represents what most experts in this field would regard as factors that increase the internal validity of each study. We present these points in Table 4.
Table 4

Most of the studies are average-to-poor quality RCTs. However, we reviewed the abstracts of the 5 unpublished studies, and they would receive a high validity score.23,27,28 One of them is now published and included in our analyses.19 Thus, in a future analysis of the data from the 4 unpublished studies, we would do a sensitivity analysis, keeping in mind the quality of the studies.
Patient outcomes
The tertiary outcomes are presented in Table 3. Unfortunately, due to above-noted reasons, we were not able to pool the data and thus present them in their transparency. These include length of stay, follow-up months, operating room time, number of days to a full diet, morbidity rates and reoperation rates. The conversion rates in the studies were reported to be as low as 0% to as high as 28%, with a weighted average of about 18%.
We performed a sensitivity analysis, excluding studies that were mixed colorectal or only rectal disease. These sensitivity analyses did not change any of the results. Morbidity was the only outcome that showed heterogeneity. This is easily explained, because the definition of the morbidity varied among studies. All other outcomes showed good homogeneity (p = 0.36–0.98).
The funnel plot for all-cause mortality is presented in Figure 2. The funnel plots for other outcomes were very similar and not included, owing to limited space. The graphical representations of each primary and secondary outcome are presented in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8, including the sensitivity analyses. We did not do the sensitivity analysis for morbidity (equal) because there was only one study in this pool.
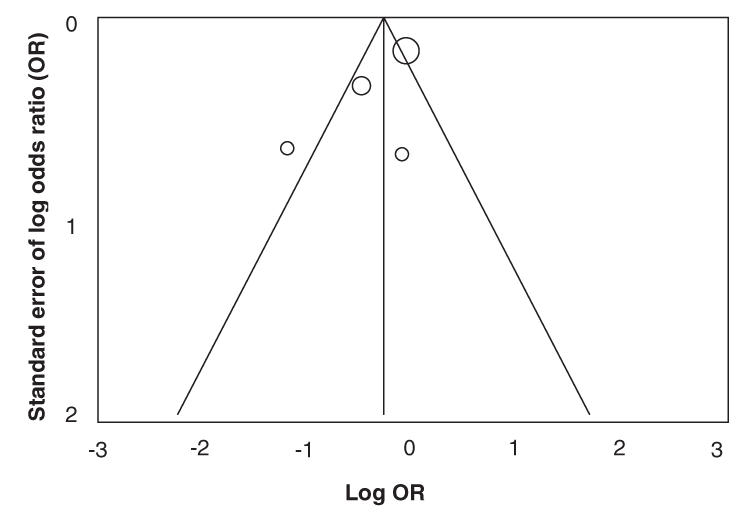
FIG. 2. Funnel plot of all-cause mortality.

FIG. 3. Cancer-related mortality.

FIG. 4. All-cause mortality.

FIG. 5. All recurrence.

FIG. 6. Local recurrence: we present odds ratio as point estimates with 95% confidence intervals.

FIG. 7. Port-site recurrence.
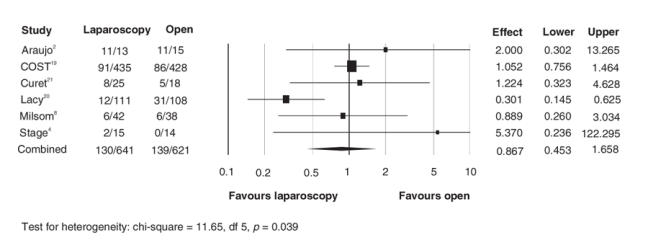
FIG. 8. Morbidity.
There was no disadvantage to having laparoscopic colon resection in any of these primary and secondary outcomes, compared with the conventional open technique. Interestingly, the results favour the laparoscopic side in all outcomes, except port-site recurrence and morbidity, where there was no difference. The number needed to treat for cancer-related mortality was 23 and after sensitivity analysis was 21.
Discussion
In this systematic review, we summarized studies that assessed the effect of laparoscopic colon resection for colon cancer, compared with the conventional open technique. We identified possible sources of variability among studies, including unequal preoperative staging of cancer, adjuvant and neoadjuvant treatment differences, differing morbidity definitions and variability in follow-up or randomization. Except for the COST study,19 most were of poor-to-moderate quality. However, we identified 4 unpublished, ongoing studies. We found that most studies have similar age and sex distributions between each arm. Similarly, the American Society of Anesthesiologists classifications were similar. However, we identified some preoperative cancer staging differences between the laparoscopic and open arms that could bias the results. We found that the length of stay was shorter and the OR time was longer on the laparoscopic side. It is difficult to comment on the morbidity rates, because they are so varied and so poorly defined in the studies that the results are quite heterogeneous, which limits our conclusion. The conversion rate varied from 0% to 28% between studies, a difference that might be explained by the learning curve for the laparoscopic technique and skill variation among surgeons. It is difficult to comment on the potential effect of conversion rate on morbidities, because no clear pattern is evident.
It is not surprising that none of the primary and secondary outcomes were significantly different between the 2 groups. The early studies citing port-site recurrence were strongly criticized for their poor technique.
Possible sources of variability among studies are unequal preoperative staging and cancer location, adjuvant and neoadjuvant treatment differences, definition differences of morbidity and variability in follow-up, and randomization.
The strengths of this review include rigorous methods and transparent reporting. We searched multiple data sources and identified translated studies in English, Russian, Japanese and Italian. We evaluated the validity of the primary studies and conducted a quantitative synthesis when appropriate. We presented a quantitative summary of the studies, in addition to a sensitivity analysis. Although the studies show no heterogeneity, due to the moderate-to-poor quality of the studies in the review, the results should be interpreted with caution and reviewed again once the 4 ongoing RCTs are published.
Conclusion
The results of this review suggest that, although there is no definitive answer, overwhelming evidence presently indicates that laparoscopic colorectal cancer resection is as safe and efficacious as the conventional open technique. However, the precision or the magnitude of the difference, safety, and effectiveness would be better understood once the 4 large multicentre RCTs are included in this review.
Acknowledgments
We acknowledge the substantial contribution of Ms. Jennifer Bailey and Ms. Anna Garnett in reviewing the manuscript and retrieving the references.
Accepted for publication Jul. 19, 2005
Competing interests: None declared.
Correspondence to: Dr. Kamyar Kahnamoui, Hamilton General Hospital, 6 North, Room 607, 237 Barton St. E, Hamilton ON L8L 2X2; fax 905 577-1405; kahnam@mcmaster.ca
References
- 1.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection “laparoscopic colectomy”. Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- 2.Nelson H, Weeks JC, Wieand HS. Proposed phase III trial comparing laparoscopic-assisted colectomy versus open colectomy for colon cancer. J Natl Cancer Inst Monogr 1995;(19):51-6. [PubMed]
- 3.Canadian Cancer Society. Media backgrounder: Colorectal Cancer Statistics. Toronto: the Society; 2002. Elec tronic Citation; http://www.cancer.ca.
- 4.Stage JG, Schulze S, Moller P, et al. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg 1997;84:391-6. [PubMed]
- 5.Gellman L, Salky B, Edye M. Laparoscopic assisted colectomy. Surg Endosc 1996;10:1041-4. [DOI] [PubMed]
- 6.Liberman MA, Phillips EH, Carroll BJ, et al. Laparoscopic colectomy vs traditional colectomy for diverticulitis. Outcome and costs. Surg Endosc 1996;10:15-8. [DOI] [PubMed]
- 7.Lord SA, Larach SW, Ferrara A, et al. Laparoscopic resections for colorectal carcinoma. A three-year experience. Dis Colon Rectum 1996;39:148-54. [DOI] [PubMed]
- 8.Milsom JW, Bohm B, Hammerhofer KA, et al. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg 1998;187: 46-54. [DOI] [PubMed]
- 9.Fleshman JW, Nelson H, Peters WR, et al. Early results of laparoscopic surgery for colorectal cancer. Retrospective analysis of 372 patients treated by Clinical Outcomes of Surgical Therapy (COST) Study Group. Dis Colon Rectum 1996;39:S53-8. [DOI] [PubMed]
- 10.Franklin ME Jr, Rosenthal D, Abrego-Medina D, et al. Prospective comparison of open vs. laparoscopic colon surgery for carcinoma. Five-year results. Dis Colon Rectum 1996;39:S35-46. [DOI] [PubMed]
- 11.Kwok SP, Lau WY, Carey PD, et al. Prospective evaluation of laparoscopic-assisted large bowel excision for cancer. Ann Surg 1996;223:170-6. [DOI] [PMC free article] [PubMed]
- 12.Basse L, Jakobsen DH, Bardram L, et al. Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg 2005;241:416-23. [DOI] [PMC free article] [PubMed]
- 13.Hjort Jakobsen D, Sonne E, Basse L, et al. Convalescence after colonic resection with fast-track versus conventional care. Scand J Surg 2004;93:24-8. [DOI] [PubMed]
- 14.Lacy AM, Garcia-Valdecasas JC, Pique JM, et al. Short-term outcome analysis of a randomized study comparing laparoscopic vs open colectomy for colon cancer. Surg Endosc 1995;9:1101-5. [DOI] [PubMed]
- 15.Berends FJ, Kazemier G, Bonjer HJ, et al. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994;344:58. [DOI] [PubMed]
- 16.Vukasin P, Ortega AE, Greene FL, et al. Wound recurrence following laparoscopic colon cancer resection. Results of the American Society of Colon and Rectal Surgeons Laparoscopic Registry. Dis Colon Rectum 1996;39:S20-3. [DOI] [PubMed]
- 17.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993;2:121-45. [DOI] [PubMed]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7: 177-88. [DOI] [PubMed]
- 19.The Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350: 2050-9. [DOI] [PubMed]
- 20.Curet MJ, Putrakul K, Pitcher DE, et al. Laparoscopically assisted colon resection for colon carcinoma: perioperative results and long-term outcome. Surg Endosc 2000;14:1062-6. [DOI] [PubMed]
- 21.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224-9. [DOI] [PubMed]
- 22.Araujo SE, da Silva eSousa AH Jr, de Campos FG, et al. Conventional approach x laparoscopic abdominoperineal resection for rectal cancer treatment after neoadjuvant chemoradiation: results of a prospective randomized trial. Rev Hosp Clin Fac Med Sao Paulo 2003;58:133-40. [DOI] [PubMed]
- 23.Stocchi L, Nelson H. Laparoscopic colectomy for colon cancer: trial update. J Surg Oncol 1998;68:255-67. [DOI] [PubMed]
- 24.Lezoche E, Feliciotti F, Paganini AM, et al. Laparoscopic vs open hemicolectomy for colon cancer. Surg Endosc 2002;16:596-602. [DOI] [PubMed]
- 25.Feliciotti F, Paganini AM, Guerrieri M, et al. Results of laparoscopic vs open resections for colon cancer in patients with a minimum follow-up of 3 years. Surg Endosc 2002;16:1158-61. [DOI] [PubMed]
- 26.Lezoche E, Feliciotti F, Paganini AM, et al. Results of laparoscopic versus open resections for non-early rectal cancer in patients with a minimum follow-up of four years. Hepatogastroenterology 2002;49:1185-90. [PubMed]
- 27.The COLOR Study Group. COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Dig Surg 2000;17:617-22. [DOI] [PubMed]
- 28.Stead ML, Brown JM, Bosanquet N, et al. Assessing the relative costs of standard open surgery and laparoscopic surgery in colorectal cancer in a randomised controlled trial in the United Kingdom. Crit Rev Oncol Hematol 2000;33:99-103. [DOI] [PubMed]


