Abstract
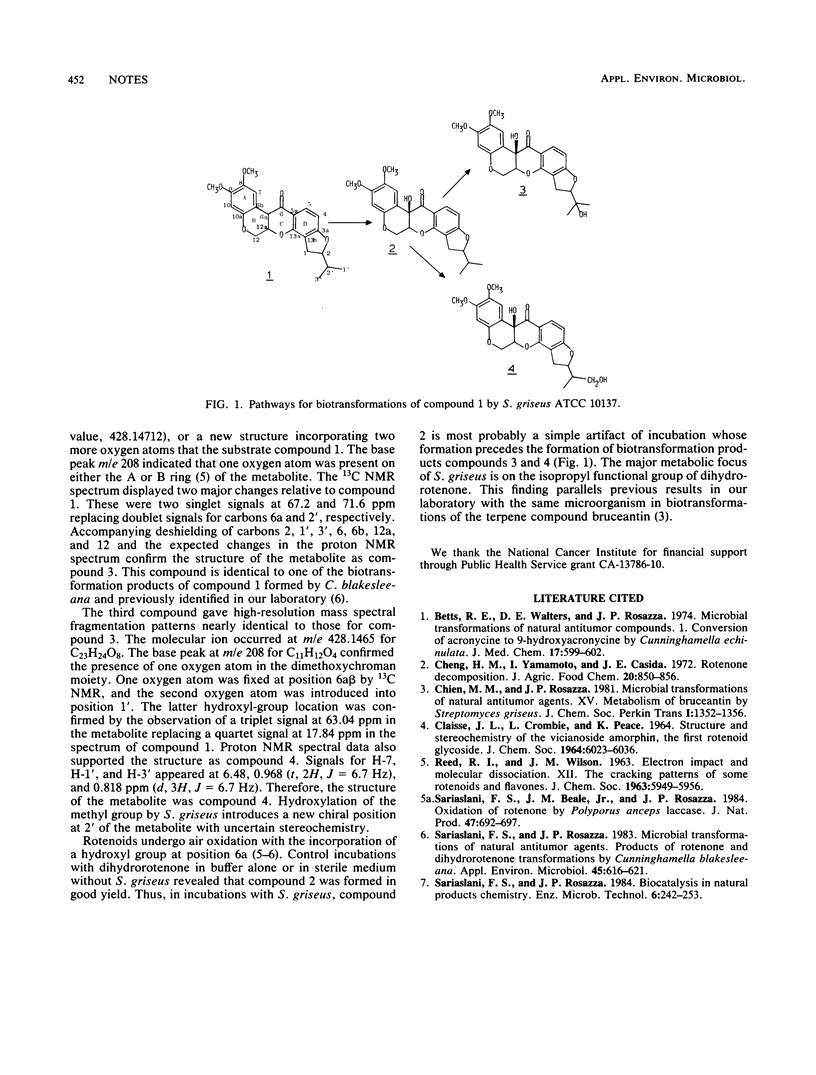
Dihydrorotenone yields three major products when incubated with growing cultures of Streptomyces griseus. These were isolated by solvent extraction and characterized by spectral methods as 1',2'-dihydro-6abeta-hydroxyrotenone, 1',2'-dihydro-2',6abeta-dihydroxyrotenone, and 1',2'-dihydro-1',6abeta-dihydroxyrotenone.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Beale J. M., Jr, Rosazza J. P. Oxidation of rotenone by Polyporus anceps laccase. J Nat Prod. 1984 Jul-Aug;47(4):692–697. doi: 10.1021/np50034a021. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Rosazza J. P. Microbial Transformations of Natural Antitumor Agents: Products of Rotenone and Dihydrorotenone Transformation by Cunninghamella blakesleeana. Appl Environ Microbiol. 1983 Feb;45(2):616–621. doi: 10.1128/aem.45.2.616-621.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


