Abstract
Background
Complications of the residual postoperative pleural space (RPPS) after partial pulmonary resections increase hospital stay, cost and morbidity. The objectives of this study were to define and identify the long-term outcome of RPPS.
Methods
A total of 140 partial pulmonary resections were performed in a 3-year period. Fifty-eight (41.4%) patients who had RPPS on the first postoperative day were followed up. We examined the chest x-rays of these patients on postoperative day 1 and 7 and week 4 and 12, and we documented any complications and reoperations.
Results
RPPS persisted in 6 patients (10.4%) and was reabsorbed in 44 patients (75.8%) in the 12th week. Residual spaces were complicated in 8 patients (13.7%), of whom 4 (6.8%) had reoperation and 4 (6.8%) were redrained. Reoperated patients had a mean of 13 (standard deviation [SD] 2.4, range 11–16) days of postoperative hospitalization, whereas redrained patients had a mean of 58.5 (SD 21.7, range 36–88) days of additional hospitalization.
Conclusions
We determined air leakage and space infection to be the major complications of the RPPS. Infectious complications were noticed in the postoperative third and fourth weeks (14–30 d), and reoperated patients had a shorter duration of postoperative hospitalization. Early operation is recommended in complicated pleural space patients. The space that is not complicated until the end of the first month can be defined as benign. This study demonstrated that follow-up of a benign space after the first postoperative month is not necessary.
Abstract
Contexte
Les complications de l'espace pleural postopératoire résiduel (EPPR) après une résection partielle d'un poumon prolongent l'hospitalisation, augmentent les coûts et font grimper les taux de morbidité. Cette étude visait à définir et déterminer l'issue à long terme de l'EPPR.
Méthodes
On a pratiqué au total 140 résections partielles d'un poumon au cours d'une période de trois ans et on a suivi 58 (41,4 %) patients qui avaient un EPPR le lendemain de l'intervention. Nous avons étudié les radiographies pulmonaires de ces patients les jours 1 et 7, ainsi que les semaines 4 et 12 après l'intervention, et nous avons documenté toute complication et toute nouvelle intervention.
Résultats
L'EPPR a persisté chez 6 patients (10,4 %) et a été réabsorbé chez 44 (75,8 %) patients au cours de la 12e semaine. Des complications de l'espace résiduel sont survenues chez 8 patients (13,7 %), dont 4 (6,8 %) ont été réopérés et 4 (6,8 %) ont subi un nouveau drainage. Chez les patients réopérés, la durée moyenne de l'hospitalisation qui a suivi l'intervention s'est établie à 13 (écart type [ET] 2,4; intervalle de 11 à 16) jours tandis que les patients qui ont subi un nouveau drainage sont demeurés hospitalisés en moyenne 58,5 (ET 21,7; intervalle de 36 à 88) jours de plus.
Conclusions
Nous avons déterminé que la fuite d'air et l'infection de l'espace sont les principales complications de l'EPPR. On a remarqué des complications infectieuses au cours des troisième et quatre semaines qui ont suivi l'intervention (14–30 j) et les patients réopérés ont été hospitalisés moins longtemps après l'intervention. On recommande l'intervention précoce chez les patients qui ont un espace pleural à complications. L'espace qui ne présente pas de complications avant la fin du premier mois peut être considéré comme bénin. Cette étude a démontré qu'il n'est pas nécessaire de suivre un espace bénin après le premier mois suivant l'intervention.
The complication of residual postoperative pleural space (RPPS) after pulmonary resections is a common problem resulting in prolonged hospital stay and increased cost. The factors that cause RPPS are inefficient pleural drainage, restrictive lung disease, bilobectomy operations, prolonged air leak and mediastinal fixation owing to radiotherapy.1 Pleural space was reported to occur in 20% of patients who underwent lobectomy operation, 40% of those who underwent bilobectomy or lobectomy and wedge resection operation, and 5%–10% of those who underwent segmentectomy or wedge resection operation.1–3 There is no well-documented definition of RPPS; thus we aimed to define and investigate the complications of RPPS.
Methods
We analyzed 140 consecutive patients who were admitted to our hospital in a 3-year period. They underwent a lung resection lesser than pneumonectomy. We performed lobectomy in 80 patients, bullectomy in 36 patients, wedge resection in 16 patients, and bilobectomy or lobectomy combined with segmentectomy in 8 patients.
Patients with chest wall resection or patients who demonstrated bronchopleural fistula in the postoperative period were excluded from the study. RPPS was defined as radiological pneumothorax of more than 20% according to a nomogram described by Rhea4 in a posteroanterior chest x-ray. Patients with bronchopleural fistula, infection and atelectasis of the underlying lung were excluded. The study comprised 58 patients with RPPS on postoperative day 1. Patients had posterolateral or axillary thoracotomy and standard perioperative and postoperative techniques. All attempts were made to repair pulmonary paranchym to prevent air leaks before closing the thoracotomy. Operations were supervised by a single surgeon. All patients had a straight 28F apical and a 32F tube placed along the diaphragm. Chest radiograms were made daily, until the tubes were taken out. Negative suction was applied to chest tubes on 20 cmH20 if the patient had an air leak and pleural space. If the air leak stopped and the drainage was less than 100 mL per 24 hours, we stopped pleural drainage in spite of the pleural space. Chest x-rays of the 1st, 4th and 12th weeks were examined by 2 chest surgeons. Air leakage that lasted more than 7 days was accepted as a prolonged air leak. We described the morbidity related to the RPPS and defined the terms “complicated RPPS” and “noncomplicated RPPS,” which might offer a new perspective.
We expressed the data as mean, number or percentage and used the Shapiro–Wilk test to test normal distribution of data. We used Friedman variance to analyze intergroup differences and the Kruskal–Wallis test to analyze between-group differences. The cut-off level for statistical significance was p < 0.05.
Results
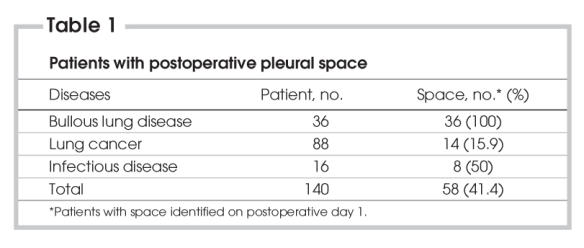
Fifty-eight patients (41.4%) were diagnosed as having RPPS on postoperative day 1 (Table 1). Twenty-two patients' (37.9%) residual pleural spaces were reabsorbed by medical therapy at the end of the first week. The medical means to treat RPPS were negative suction, chest physiotherapy, bronchoscopy, nasotracheal suctioning and bronchodilatator therapy.
Table 1
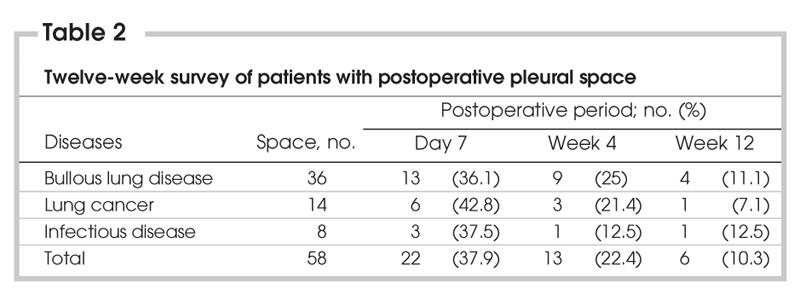
Only 13 (22.4%) and 6 (10.3%) patients had RPPS at the end of postoperative weeks 4 and 12, respectively. Reabsorption time of RPPS is shown in Figure 1 and Table 2.
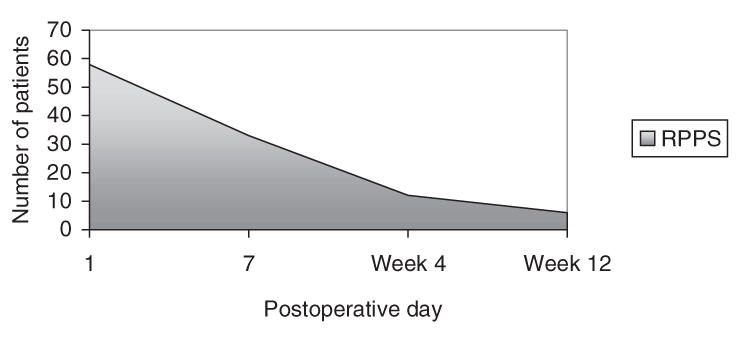
FIG. 1. Graphical depiction of patients with postoperative pleural space.
Table 2
Diseases
Thirty-six patients with bullous disease underwent bulla resection. All patients developed RPPS on the first day after surgery. There were 13, 9 and 4 patients with RPPS on postoperative day 7 and week 4 and 12, respectively. In 88 patients with lung cancer, a lobectomy, bilobectomy or segmentectomy was performed. There were 14 patients (15.9%) with postoperative space on the first day. The number of patients with RPPS were 6, 3 and 1 on postoperative day 7, weeks 4 and week 12, respectively. A lobectomy or a wedge resection was performed in patients with infectious disease (e.g., tuberculosis, bronchiectasis). Of these, 3 patients had RPPS on postoperative day 7. Only 1 patient had RPPS at postoperative weeks 4 and 12. Patients with bullous lung disease were more likely to develop RPPS postoperatively (p < 0.001), compared with patients with infectious disease and lung cancer. The rate of patients with RPPS diminished significantly at postoperative week 12 (p < 0.001).
Operations
We noticed the RPPS in 36 (100%) patients who underwent bullectomy, 5 (17.8%) of 16 patients who underwent wedge resections, 18 (22.5%) of 80 patients after lobectomy operations and 8 (100%) patients after bilobectomy or lobectomy combined with segmentectomy/wedge resection operations on postoperative day 1. Patients who underwent bullectomy and lobectomy or bilobectomy combined with segmentectomy had a higher incidence for RPPS, which was statistically significant (p < 0.001).
Localization
We evaluated the localization of the RPPS in the first week. Lower thoracic RPPSs were detected only after right lower bilobectomy (3/3 patients; 100%), and upper thoracic RPPSs were most commonly seen in patients with bullectomy (8/36 patients; 22%). Upper bilobectomy patients and left upper lobectomy patients also had a higher incidence of RPPS (p < 0.001).
Reoperations and redrainage
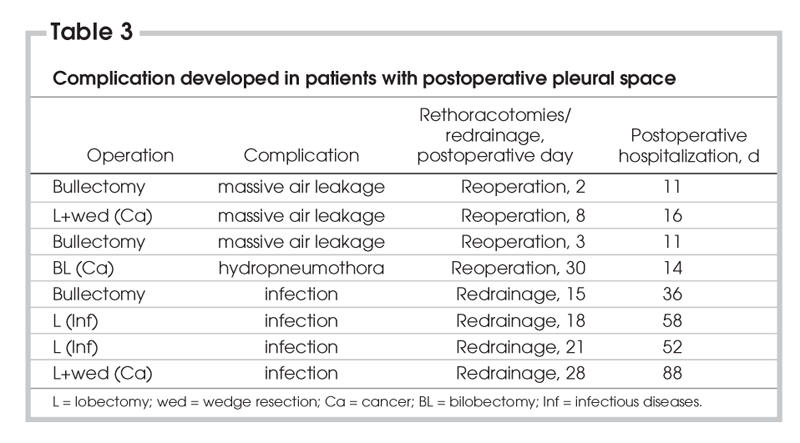
Four patients (6.8%) had reoperations related to RPPS complications. In the first patient, we performed bullectomy with a manual suturing technique, using nonabsorbable suture (Vicry; Ethicon, Edinburg, Scotland). This patient developed a massive air leak and RPPS on postoperative day 2. A reoperation was performed by sutures supported with parietal pleura. He was discharged with RPPS on postoperative day 8. The second patient had reoperation on postoperative day 8 for a prolonged air leak and RPPS after lobectomy and wedge resection for lung cancer. The parenchyma was resutured. He was discharged on postoperative day 7; RPPS was reabsorbed in the fourth week. The third patient had apical wedge resection due to bullous lung disease. He developed a massive air leak on day 3, and the parenchyma was resutured. The patient was discharged on postoperative day 7, and RPPS was reabsorbed in the fourth week. Five patients (8.5%) developed an infection of the RPPS. Their mean chest tube drainage time was 5.5 days (4–14 d). The first patient in this group had right upper bilobectomy for lung cancer and was discharged on postoperative day 7. The patient developed postoperative hydropneumothorax on day 30 and was treated with chest tube drainage. Staphylococcus aureus was positive in the effusion. Because the RPPS was complicated with infection, we performed concomitant pleural tent and pneumoperitoneum to prevent chronic empyema formation. He was discharged on postoperative day 7. All other patients were diagnosed to have empyema during postoperative weeks 3 and 4 (14–30 days). The cavities were drained and sterilized after 37 (range 20–59) days of drainage. All had been discharged with small residual space in the apical region. Pseudomonas aeruginosa was cultured in 2 patients' pleural effusions, and Staphylococcus aureus was present in others. Two of the patients showed complete resorption of the space in the fourth week with pleural drainage and irrigation, whereas others were rehospitalized because of reinfection. In both patients, the cavities were redrained and reirrigated. One patient developed bronchopleural fistula, which was diagnosed after methylene blue injection into the pleural cavity. Both patients were discharged at the end of the second month without further surgical intervention. Patients with complications are summarized in Table 3.
Table 3
Discussion
Residual pleural space was reabsorbed in 80 days.4 Surgical intervention to decrease the reabsorbtion time did not help after 7 days, so any manoeuvre to decrease the pleural space was not suggested.4 Pleural space after tuberculosis surgery was reabsorbed in 80% of the patients in 4 weeks and 90% of the patients in 1 year, without morbid complication.5
The incidence of pleural space was reported to be between 5% and 40%, depending on the type of resection.1–3 Postoperative pleural space was reported mostly (40%) after bilobectomy operations. Nine percent of the RPPS persisted for 1 to 10 years, 5% of which were reported to become infected.5,6
We demonstrated that RPPS was not a static but a dynamic event: The incidence of postoperative pleural space was 41.4% (58/140) on postoperative day 1 and 10.3% (6/58) on postoperative week 12. The medical means to treat RPPS, such as negative suction, chest physiotherapy, bronchoscopy, nasotracheal suctioning and bronchodilatator therapy, decreased the incidence of pleural space to 37.9% (22/58) at the end of the first week. Four patients with atelectasis and RPPS were free of the space after bronchoscopic aspiration. Of the patients with RPPS on postoperative day 1, 13.7% (8/58) developed complications. The infectious complications were seen during postoperative weeks 3 and 4 (14–30 d). Patients with space problems should be observed cautiously during this period. The most common microorganisms were Pseudomonas aeruginosa and Staphylococcus aureus. Patients without any complications until the end of the fourth week were defined as having noncomplicated RPPS (i.e., benign space) patients. Patients with benign space showed reabsorbtion of the air without complication. Therefore, we did not observe these patients after postoperative week 4.
Several processes can help to reduce space after pulmonary resections, such as mediastinal shift, intercostal narrowing, diaphragmatic elevation and fluid accumulation.7 The surgical manoeuvres to prevent RPPS are perioperative pneumoperitoneum, pleural tent, fibrin glue application on rough surfaces and laser.8–13
It was suggested that a chest tube with a persistent air leak at stress but no leak at rest should be challenged by clamping and close observation.14 However, there should be no air leak from the chest tube when the patient is normally breathing at rest or taking a deep breath.
To conclude, we used the term RPPS for patients who had pneumothorax of more than 20% after the postoperative first week, when most RPPS would disappear. The most common complication of RPPS is infection. RPPS infection can be noticed during the third and fourth weeks, thus we observed the patients carefully during this time. Redrainage seems to be effective when infection occurs, but it increases the hospital stay. After stabilizing the patient and controlling the infection, early operation is recommended. Patients with benign spaces do not require follow-up beyond 1 month after surgery.
Accepted for publication June 11, 2005
Competing interests: None declared.
Correspondence to: Dr. Okan Solak, Kocatepe C. Örnekevler M. Dönerkaya Apt. No: 2/3 Merkez–Afyon Turkey; fax +90 272 2172029; okanchest@yahoo.com
References
- 1.Barker WL. Natural history of residual air spaces after pulmonary resection. Chest Surg Clin N Am 1996;6:585-613. [PubMed]
- 2.Wareham EE, Barber H, McGoey JS, et al. The persistent pleural spaces following partial pulmonary resection. J Thorac Surg 1956;31:593-9. [PubMed]
- 3.Bell JW. Management of the postresection space in tuberculosis. III. Role of pre and postresection thoracoplasty. J Thorac Surg 1956;32:580-92. [PubMed]
- 4.Rhea JT, DeLuca SA, Greene RE. Determining the size of pneumothorax in the upright patient. Radiology 1982;144:733-6. [DOI] [PubMed]
- 5.Silver AW, Espinos EE, Byron FW. The fate of postresection spaces. Ann Thorac Surg 1966;2:311-6. [DOI] [PubMed]
- 6.Barker WL, Langston HT, Noffah P. Postresectional thoracic spaces. Ann Thorac Surg 1966;2:299-310. [DOI] [PubMed]
- 7.Cerfolio RJ, Holman WL, Kathali CR. Pneumoperitoneum often concomitant resection of the right middle and lower lobes (Bilobectomy). Ann Thorac Surg 2000;70:942-7. [DOI] [PubMed]
- 8.Rice TW, Kirby TJ. Prolonged airleak. Chest Surg Clin N Am 1992;2:803-11.
- 9.Hiroaki Nomori. Hirotoshi Horio, Keichi Suemasu. Mixing collagen with fibrin glue to strengthen the sealing effect for pulmonary air leakage. Ann Thorac Surg 2000;70:1666-70. [DOI] [PubMed]
- 10.LoCicero J 3rd Hartz RS, Frederickson JW, et al. New application of laser in pulmonary surgery: Hemostasis and sealing of airleaks. Ann Thorac Surg 1985;40:546-53. [DOI] [PubMed]
- 11.Miscall L, Duffy RW, Nolan RB, et al. Pleural tent as a simultaneous tailoring procedure in combination with pulmonary resections. Am Rev Tuber 1956;73:831-9. [DOI] [PubMed]
- 12.Brunelli A, Refai MA, Muti M, et al. Pleural tent after upper lobectomy: A prospective randomized study. Ann Thorac Surg 2000;69:1722-4. [DOI] [PubMed]
- 13.Toker A, Dilege, Bostanc K, et al. Perioperative pneumoperitoneum to prevent space and air leak after lobectomy operations. Turk Respir J 2000;1:9-11.
- 14.Kato R, Kobamashi T, Watanabe M, et al. Can the chest tube draining the pleural cavity with persistent air leakage be removed. Thorac Cardiovasc Surg 1992;40:292-6. [DOI] [PubMed]


