Abstract
Introduction
Infection after total hip replacement (THR) is a serious medical complication with significant negative ramifications for both the patient and the health care system. The prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) was designed to treat the joint infection while maintaining functional movement in the hip.
Methods
We identified 28 patients treated for infected THR with the PROSTALAC system, by retrospective chart review. Preoperative and intraoperative cultures were taken to identify the causative organism. After PROSTALAC insertion, patients underwent at least 6 weeks of intravenous (IV) antibiotics. Prior to undergoing posttreatment cultures, patients were required to be antibiotic-free for a minimum of 6 weeks, with normal laboratory values. We defined resolution infection as retention of a joint prosthesis for a minimum of 2 years.
Results
Infection was identified in 28 patients in either the joint aspirate or intraoperative cultures. Of these patients, 2 failed to clear infection, requiring repeat PROSTALAC insertion. Two additional patients had positive 48-hour cultures post-second stage, treated with additional IV antibiotics. Retention of the post-PROSTALAC prosthesis is 100% at 2 years.
Conclusion
PROSTALAC has acceptable infection resolution outcomes and appears effective for treating infected THR.
Abstract
Introduction
L'infection consécutive à une arthroplastie totale de la hanche (ATH) est une complication médicale grave qui a d'importantes ramifications négatives tant pour le patient que pour le système de santé. La prothèse de ciment acrylique chargée d'antibiotique (PROSTALAC) a été conçue pour traiter l'infection de l'articulation tout en maintenant le mouvement fonctionnel de la hanche.
Méthodes
Nous avons trouvé, en procédant à une étude rétrospective des dossiers, 28 patients que l'on a traités pour une ATH infectée au moyen du système PROSTALAC. On a prélevé des cultures préopératoires et peropératoire pour déterminer l'organisme pathogène. Après l'insertion de la prothèse PROSTALAC, les patients ont suivi une antibiothérapie intraveineuse (IV) d'au moins six semaines. Avant que l'on procède aux cultures consécutives au traitement, les patients devaient avoir cessé de prendre des antibiotiques depuis au moins six semaines et avoir des résultats de laboratoire normaux. Nous avons défini la résolution de l'infection comme le maintien d'une prothèse articulaire pendant au moins deux ans.
Résultats
On a trouvé une infection chez 28 patients, soit dans la ponction articulaire, soit dans les cultures peropératoires. Parmi ces patients, deux ne se sont pas débarrassés de leur infection, ce qui a obligé à répéter l'insertion de PROSTALAC. Deux autres patients montraient des cultures positives à 48 heures après le deuxième stade et ont été traités au moyen d'autres antibiotiques IV. La rétention de la prothèse après l'administration de PROSTALAC s'établit à 100 % après deux ans.
Conclusion
La prothèse PROSTALAC donne des résultats acceptables sur le plan de la résolution de l'infection et semble efficace pour traiter les ATH infectées.
Deep joint infection after total hip replacement (THR) is a serious complication that requires surgical and prolonged medical management. The costs of treating an infection after THR are reported to be at least US$50 000 per patient.1 Reported infection rates in the literature are currently 1% to 2% for primary THR and are higher after total hip revision.2
Current standards of treatment for infected THR involve thorough débridement, removal of dead and extraneous tissue and exchange of prosthesis. In North America, delayed or 2-stage exchange is considered the standard treatment.3–7 With the 2-stage procedure, the infected prosthesis is removed and the hip undergoes a thorough débridement, leaving it free of any foreign material. The interval period between removing the infected prosthesis and reimplanting a new prosthesis is 6 weeks to 3 months in duration. During this interval, intravenous (IV) antibiotics are administered to help eradicate infection, and a temporary cement spacer laden with antibiotics may be used in the hip joint. Buchholz proposed the use of antibiotics within the cement in the 1970s.3 In vitro and in vivo studies since then have demonstrated that the elution of antibiotics from bone cement is effective in treating infection.6,8
Literature to date suggests an infection resolution rate of 78% to 95% through the use of this 2-stage exchange procedure, with an antibiotic-loaded cement spacer.7 Prosthesis retention for 2 years is the current clinical definition of resolution of infection.4,9–11
The clinical research to date on this system consists of 2 papers, both of which originated from the development centre.4,12 Their results of delayed exchange arthroplasty with the PROSTALAC system reported 97% infection resolution (defined as prosthesis retention of at 2 years).4,9–11
Objectives
At our centre, the PROSTALAC system has been used for over 4 years to treat deep periprosthetic infection. The primary purpose of this portion of the study was to determine the infection resolution rate with use of this system.
Methods
Design
We reviewed the charts of all eligible patients, using a standardized data extraction form. Where charts were lacking, we sought further information from the office charts of the participating surgeons. Data regarding patient demographics (age, sex), date of primary or revision arthroplasty, laboratory values (Elevated Sedimentation Rate (ESR), C-Reactive Protein (CRP), Complete Blood Count (CBC), electrolytes), radiographic information (e.g., bone scan), microbiological results, date of PROSTALAC insertion, antibiotic information, date of second-stage operation and any complications during the treatment interval were collected.
Subjects
Patients were identified through a review of charts from 1998 to 2003 from 3 tertiary hospitals that use the PROSTALAC system for treatment of infection. Ethics approval was received for the study from the Regional Health Ethics Board.
Patients who had 1) undergone previous primary or revision THR, 2) were defined as having a joint infection by a positive culture identifying a causative organism, and 3) were currently being treated or had recently been treated for infection with the PROSTALAC system were eligible for the study. Positive cultures included blood cultures, hip aspirates and tissue cultures at the time of surgery. Patients who had the procedure on a native hip were considered ineligible.
Infection Treatment Protocol
All patients were treated by removing the original prosthesis and with aggressive débridement to remove all foreign material. The use of antibiotics within the cement was variable. Most patients received 1 g of Vancomycin and 2.4 g of Tobramycin per bag of cement embedded around the prosthesis. Three patients received only Vancomycin, and 1 patient received only Tobramycin in their cement mantle.
After surgery, all patients received 6 weeks of IV antibiotics tailored to the sensitivities of intra-operative cultures. Where required, additional antibiotics were administered on an individual basis, as prescribed by the attending infectious diseases physician.
Hip Aspirates Prior to Second Stage
Six to 8 weeks after completing the IV antibiotics, patients underwent a hip aspirate to ensure infection clearance before the second stage. A patient was determined to be free of infection and able to proceed to second-stage arthroplasty when the joint aspirates were negative, and the ESR and CRP were within normal ranges (ESR < 30 mm/h and CRP < 10 mg/L). If patients had positive hip aspirates, they were treated with repeat PROSTALAC.
Analysis
We generated descriptive statistics (means, standard deviations [SDs], proportions and frequencies) for all variables, using Statistics for the Social Sciences (SPSS), version 11.5. Figure 1 shows the surgical states of all patients at the time of the review.
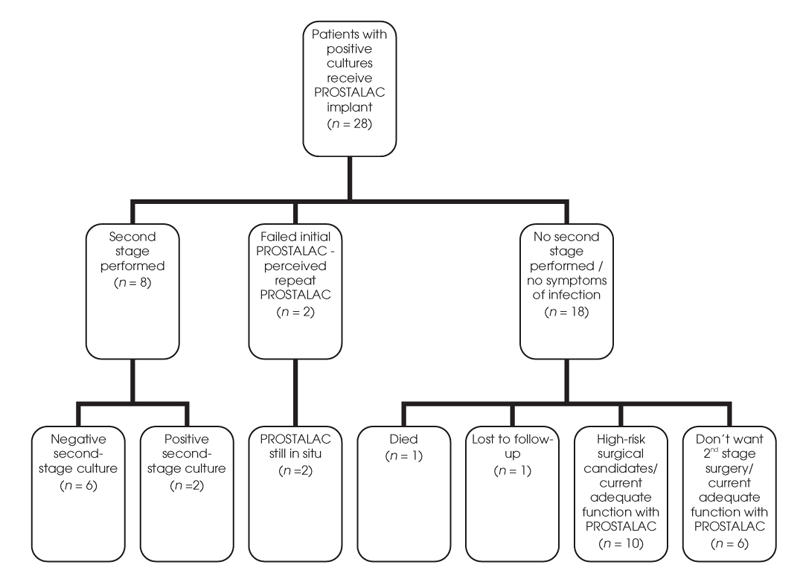
FIG. 1. Surgical states of patients identified to have positive cultures. PROSTALAC = prosthesis with antibiotic-loaded acrylic cement.
Results
Demographics
We identified 28 patients as having undergone PROSTALAC insertion for the treatment of positively identified joint infection. All surgeries were performed by 1 of 3 revision arthroplasty surgeons.
Of the 28 patients, 18 (64%) were women. The average age was 68.2 (SD 15.2, range 29-93) years. Six (21%) patients had more than 2 comorbidities and 17 (57%) patients had 2 or fewer comorbidities. The initial presenting diagnosis for prosthesis insertion was most commonly noninflammatory primary or secondary arthritis (61%), with other diagnoses including inflammatory arthritis (11%), hip fracture (14%), congenital hip dysplasia (3%) and tumour (3%). The remaining diagnoses were not stated in the chart (8%).
Prior to the PROSTALAC insertion, 24 (86%) patients had a primary total hip or hemiarthroplasty, and 4 (14%) had a revision hip replacement. Patients presented with infection at an average of 57.6 (SD 67; range 2–242) months postoperatively.
Microbiological and laboratory results
Of the 28 patients with positive cultures, 6 (21%) had positive joint aspirates, 7 (25%) had positive operative cultures and 15 (54%) had both positive joint and operative cultures (See Table 1). Two (5%) patients were septic on presentation. We collected laboratory investigations that were sensitive for infection (i.e., ESR and CRP) on 24 (86%) patients. Fifteen (65%) ESRs were elevated > 30 millimeter (mm)/hour (h), while the remaining CRP values were elevated > 10 milligram (mg)/litre (L).
Table 1
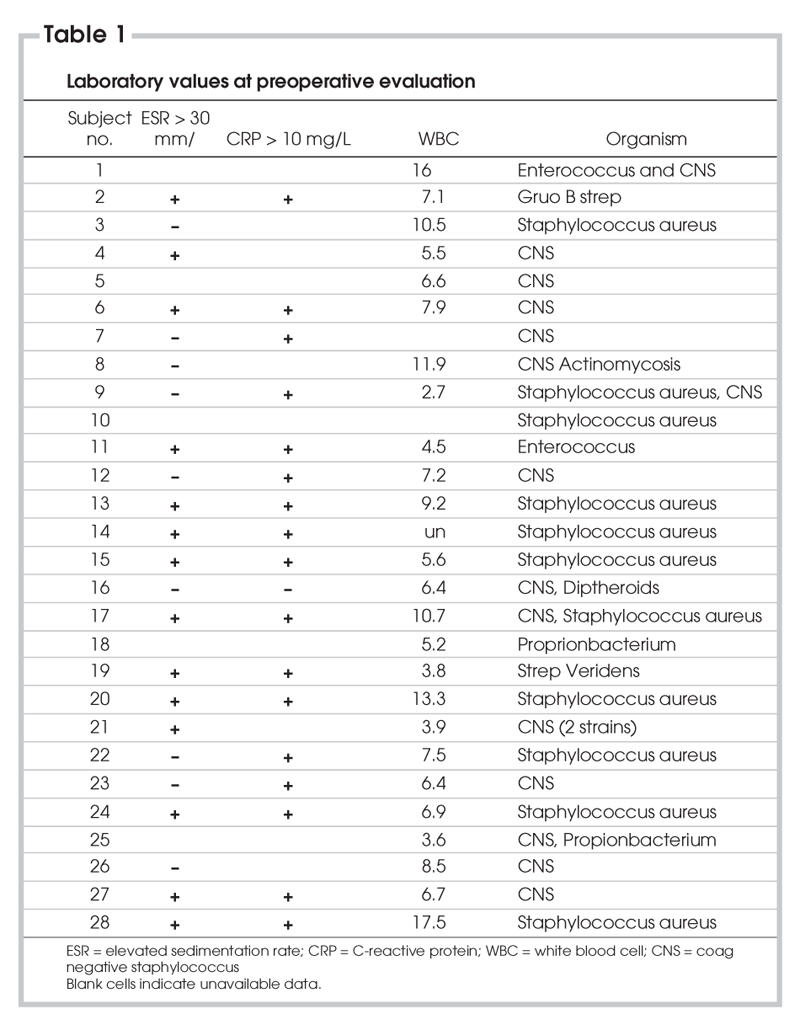
Most organisms identified were Coagulase Negative Staphlococcus (CNS), with Staphalococcus Aureaus being the second most common. There were no incidences of gram-negative organisms. Propionobacterium, Actinomyces, Enterococcus and Diptheroids were also isolated but were typically seen in association with CNS (See Table 1).
Patients completing second-stage surgery (reimplantation of permanent prosthesis)
Eight patients who had a negative hip aspirate and normal ESR and CRP values before the second stage underwent second-stage revision. Of these 8, 2 patients had positive cultures at 48 hours postoperatively from samples taken at second-stage surgery. The intra-operative culture from one patient grew Staph Aureus, the initial infecting organism, in 1 of 2 samples. Intra-operative cultures from the other patient grew CNS, again, the initial infecting organism for this patient. Both patients received an additional 6 weeks of antibiotics after the second stage. Both patients have had no evidence of recurrent infection at the latest follow-up and have retained their post-PROSTALAC hip prosthesis.
One patient required further surgery for loosening of the acetabular component at 2 years postrevision. All cultures taken before and during the surgery were negative, and laboratory work (ESR and CRP) was within normal limits. Thus retention of the post-PROSTALAC prosthesis for the 8 patients is 100% at 2 years.
Failed initial treatment with PROSTALAC
Two patients failed to clear infection based on joint aspirate and elevated ESR and CRP values before the second stage. These 2 patients underwent a repeat PROSTALAC surgery.
Patients not completing second-stage surgery
Eighteen patients (65%), although deemed clear of infection based on aspirates and laboratory values, have not gone on to second-stage surgery. One patient (90 years of age) died in the interim period, and 1 patient was lost to follow-up. Of the remaining 16 patients, 10 were deemed by their surgeons as being high-risk surgical candidates. Two of these patients had severe rheumatoid arthritis and very low functional capacity, owing to their significant systemic disease. These 10 patients remain satisfied with their current function with the PROSTALAC in situ. Another 6 patients have refused further surgery, based on adequate current functioning with the PROSTALAC implant.
Complications
Complications after PROSTALAC insertion included 1 dislocation, requiring a revision of the acetabular cup, 1 episode of Clostridium Difficile secondary to Clindamycin use and 1 episode of neutropenia secondary to Vancomycin use intravenously. There were 2 episodes of periprosthetic fracture; 1 fracture occurred intraoperatively and was handled with a long-stemmed prosthesis and circlage wiring. The second fracture occurred postoperatively, when the patient fell, fracturing at the stem of the prosthesis. The patient returned to the operating room for a long stem prosthesis with circlage wires.
Discussion
Our results for infection resolution, though similar to the developing centre, are not as favourable. Two of 10 patients (20%) in our cohort had positive hip aspirates, an ESR > 30 mm/h and a CRP of > 10 before second stage. These patients did not clear their infection with the PROSTALAC and required a repeat PROSTALAC. An additional 2 of 10 patients (20%) had positive intraoperative cultures at second stage (results at 48 hour postsurgery), despite a negative pre-second–stage hip aspirate and negative ESR and CRP. Both organisms isolated were the same as the identified infecting organism, Staph Aureus and CNS, respectively. The 2 patients treated with an additional 6 weeks of IV Vancomycin have not had a recurrence of infection to date and have retained their prosthesis. Excluding the 2 patients who failed to clear infection after the initial PROSTALAC and using the definition of infection resolution rate as “retention of prosthesis at two years,” our resolution rate is 100%.
Data from the developing centre Younger and colleagues reported negative hip aspirations in all patients before the second stage.12 Five of 30 patients (17%) had positive intraoperative cultures at the second stage. The authors reported these positive cultures at 48 hours as contaminants, and only 1 patient was reported as having recurrent infection. The overall infection resolution reported with retention of prosthesis was 97%.
It is difficult to compare PROSTALAC with simple cement spacers, because most authors do not include information on joint aspirates before the second stage, nor do they include information of deep intraoperative cultures at the second stage. The only comparison that can therefore be made is of prosthesis retention at 2 years as a measure of infection resolution. Literature to date suggests an infection resolution rate of 78% to 95% with this 2-stage exchange procedure, using an antibiotic-loaded cement spacer.3,13 Results of the PROSTALAC are therefore similar to those in the literature.7
In a recent series by Charlton and colleagues, a simple cement spacer resulted in 97.7% of prosthesis re-tention at 2 years.13 Patients did, however, have a high rate of post-second– stage dislocation (11.4%). This high rate of dislocation was not seen in our PROSTALAC cohort, nor in the reports from the developing centre.4,12 It is possible that tissue tension is maintained with PROSTALAC in the interim, allowing easier tissue balancing at second-stage revision.
Unlike the developing centre, a high number of patients in our study have not gone on to second-stage surgery. These patients have been deemed clinically clear of infection and are being observed on an annual basis to monitor their resolution status and the condition of the PROSTALAC implant.
In conclusion, post-second–stage prosthesis retention is currently 100% for up to 4 years after second-stage surgery. Patients who have retained the PROSTALAC implant as a semipermanent implant are also clinically clear of infection. Our results show resolution rates that are comparable with those reported in the literature, suggesting that the PROSTALAC is an excellent alternative to simple cement spacers for the treatment of periprosthetic infection.
Previously presented at the American Academy of Orthopaedic Surgeons Annual Meeting 2004 and the Canadian Orthopaedic Association Annual Meeting 2003.
Research Financial Support was received from the Edmonton Orthopedic Research Committee. None of the authors received benefits or funds in support of this study.
Accepted for publication July 13, 2006
Competing interests: None declared.
Correspondence to: Dr.Lauren Beaupre1F1.52 WMC, 8440-112 Street, Edmonton AB T6G 2B7; fax 780 407-7534; lbeaupre@cha.ab.ca
References
- 1.Masterson EL, Masri BA, Duncan CP. Treatment of infection at the site of total hip replacement. Instr Course Lect 1998;47:297-306. [PubMed]
- 2.Spangehl MJ, Younger AS, Masri BA, et al. Diagnosis of infection following total hip arthroplasty. Instr Course Lect 1998;47: 285-95. [PubMed]
- 3.Buchholz HW, Engelbrecht H. [Depot effects of various antibiotics mixed with Palacos resins]. [Article in German]. Chirurg 1970;41:511-5. [PubMed]
- 4.Kendall RW, Masri BA, Duncan CP, et al. Temporary antibiotic loaded acrylic hip replacement: a novel method for management of the infected THA. Semin Arthroplasty 1994;5:171-7. [PubMed]
- 5.Leunig M, Chosa E, Speck M, et al. A cement spacer for two-stage revision of infected implants of the hip joint. Int Orthop 1998;22:209-14. [DOI] [PMC free article] [PubMed]
- 6.Masri BA, Duncan CP. Chapter 82. In Masri BA, Duncan CP (eds): The Adult Hip. Lippincott-Raven; Philadelphia, 1998.
- 7.Masri BA, Duncan CP. Chapter 81. In Masri BA, Duncan CP (eds): The Adult Hip. Lippincott-Raven; Philadelphia, 1998.
- 8.Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty 1998;13:331-8. [DOI] [PubMed]
- 9.Windsor RE, Insall JN, Urs WK, et al. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am 1990;72:272-8. [PubMed]
- 10.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am 1990;72:878-83. [PubMed]
- 11.Borden LS, Gearen PF. Infected Total Knee Arthroplasty. A Protocol for Management. J Arthroplasty 1987;2:27-36. [DOI] [PubMed]
- 12.Younger AS, Duncan CP, Masri BA. Treatment of infection associated with segmental bone loss in the proximal part of the femur in two stages with use of an antibiotic-loaded interval prosthesis. J Bone Joint Surg Am 1998;80:60-9. [DOI] [PubMed]
- 13.Charlton WP, Hozack WJ, Teloken MA, et al. Complications associated with reimplantation after girdlestone arthroplasty. Clin Orthop Relat Res 2003;407:119-26. [DOI] [PubMed]


