Abstract
Introduction
Infection after total hip replacement (THR) adversely affects patients' function and health-related quality of life (HRQL). A prosthesis with antibiotic-loaded acrylic cement (PROSTALAC) was designed to improve the function and quality of life of patients undergoing treatment for infected THR.
Methods
We assessed 23 patients with the PROSTALAC implant in situ for treatment of an infected THR for function and HRQL, using standardized outcome measures. These patients were compared with a referent cohort of patients who had undergone assessment of function and HRQL before and 6 months after primary THR in the same tertiary health centres.
Results
The mean (standard deviation) Western Ontario MacMaster (WOMAC) scores for PROSTALAC patients were 70.0 (21.1), 65.8 (20.4) and 63.0 (21.1) for pain, stiffness and function, respectively. The median Harris Hip score was 62.3 (minimum 20.4, maximum 86.3) and median global hip range of motion was 100.0 (minimum 80.0, maximum 140.0) degrees.
Conclusion
The mean WOMAC scores for pain, stiffness and function were better than they were for patients awaiting THR but not as good as 6 months after primary THR. The PROSTALAC implant allows patients to have reasonable function and quality of life during the interim treatment for deep joint infection.
Abstract
Introduction
L'infection consécutive à une arthroplastie totale de la hanche (ATH) a un effet indésirable sur la capacité de fonctionnement des patients et sur la qualité de vie liée à la santé (QVLS). On a conçu une prothèse en ciment acrylique chargée d'antibiotique (PROSTALAC) pour améliorer le fonctionnement et la qualité de vie des patients traités pour une ATH infectée.
Méthodes
Nous avons évalué, au moyen de mesures de résultat normalisées, le fonctionnement et la QVLS de 23 patients ayant reçu la prothèse PROSTALAC pour traiter une ATH infectée. Nous avons comparé ces patients à une cohorte de référence de patients qui avaient subi une évaluation de fonction et de QVLS avant une ATH primaire et six mois après dans les mêmes centres de soins tertiaires.
Résultats
Les résultats moyens (écart type) Western Ontario MacMaster (WOMAC) pour les patients qui avaient une prothèse PROSTALAC se sont établis à 70,0 (21,1), 65,8 (20,4) et 63,0 (21,1) pour la douleur, la raideur et la fonction respectivement. Le score médian de Harris pour la hanche s'est établi à 62,3 (minimum de 20,4 et maximum de 86,3) et l'amplitude globale médiane du mouvement de la hanche s'est établie à 100,0 (minimum de 80,0 et maximum de 140,0) degrés.
Conclusion
Les résultats WOMAC moyens pour la hanche, la raideur et la fonction étaient meilleurs que chez les patients en attente d'une ATH, mais moins bons six mois après une ATH primaire. L'implant PROSTALAC permet aux patients de jouir d'une fonction et d'une qualité de vie raisonnables au cours du traitement consécutif à une infection profonde de l'articulation.
Infection after total hip replacement (THR) is a potentially life-threatening complication that adversely affects patients' lives and function. In the 2-stage procedure commonly used in North America, the infected prosthesis is removed, and there is an interval period wherein the patient does not have a permanent prosthesis in place.1–5
In the past, the hip was left as a Girdlestone resection during the interim period. The Girdlestone procedure results in a shortened limb with contractures of the soft tissues around the hip, which complicates re-implementation of the prosthesis. During the re-implantation procedure, soft tissue balance is difficult, owing to problems with abnormal tissue tension.6 Further, patients with a Girdlestone have a flail limb in the interim period and may be extremely limited in their functional capacity.
The prosthesis of antibiotic loaded acrylic cement (PROSTALAC) system was developed to allow functional hip movement by creating a temporary joint prosthesis surrounded by antibiotic-loaded cement.2,4,5,7
There has been no work published to date on patients' quality of life and function during the infection treatment interval with the PROSTALAC implant in situ. All work has focused on the effectiveness of PROSTALAC for eradicating infection. In a previous article,8 we indicated that, at our centre, infection resolution with the PROSTALAC system appears similar to that reported by the developing centre.2,7
Objectives
The primary purpose of this portion of the study was, first, to report the function and health-related quality of life (HRQL) of patients with the PROSTALAC in situ and, second, to provide reference points for the observed function and HRQL in the PROSTALAC patient group. To do this, we compared our results with a population-based cohort of patients from the same health region who underwent primary THR.
Methods
Design
We identified PROSTALAC patients from 1998–2003 from the 3 tertiary hospitals in our health region that use the PROSTALAC system. Patients with the PROSTALAC implant in situ, who agreed to participate in the review, underwent assessment with standardized HRQL questionnaires and clinical evaluation. We received ethics approval for the study from the Regional Health Ethics Board.
Currently, there are no data in the literature describing the function or HRQL of patients between the first and second stage of hip surgery for infection, regardless of treatment protocol. The only current reference group is patients with hip osteoarthritis who have undergone primary THR. For our purposes, we used the data obtained for another population-based prospective study, which included patients who received perioperative care similar similar to that of the PROSTALAC group. Specifically, the comparison cohort comprised 228 patients from the same health region who received a primary THR between 1995 and 1997. The comparison study evaluated the function and HRQL, using the same outcome measures before and 6 months after their THR.9
Subjects
All patients who had undergone previous primary or revision THR, who were being treated for infection with the PROSTALAC system by one of 3 revision surgeons and who had PROSTALAC in situ were eligible for the study. To be eligible for the HRQL portion of the study, patients were not required to have a positively identified organism; the presence of the PROSTALAC implant in situ was adequate for inclusion, because we were performing clinical and HRQL assessments only. Thus, this cohort is not identical to the cohort reported in Part One of the study.8 The patients in the previous report had positively identified organisms at the time of PROSTALAC insertion and might have already proceeded to second-stage surgery at the time of our review. We excluded patients who could not give informed consent or who had the procedure as part of a primary operation on a native hip.
Method
We contacted eligible patients by telephone and explained the study to them. For those interested in participating, we booked an appointment to assess their hip. We obtained signed informed consent at the time of clinical evaluation. These patients underwent assessment by a physical therapist, which included administration of standardized HRQL questionnaires and a clinical evaluation with the Harris Hip Score (HHS). Patients unable to attend the clinic completed the HRQL questionnaires by telephone.
Outcome measures
The Western Ontario MacMaster (WOMAC) Osteoarthritis Index,10–13 the Short-Form (SF)-3612,14,15 and the HHS16–18 have been proven to be reliable, valid and responsive in other total joint arthroplasty populations. Each WOMAC subscale score represented a range from 0–100 points, with a score of 100 indicating no pain or dysfunction, in the method described by Bombardier, so that the SF-36 and WOMAC scores were unidirectional.11
Analysis
We generated descriptive statistics (means, standard deviations and frequencies) for all variables. We evaluated baseline demographics (age and sex) between the PROSTALAC cohort and the population-based cohort to ensure that the 2 groups were similar, before we compared outcomes. We assessed age with an independent sample t test, and we analyzed sex distribution, using a chi-square, comparing the PROSTALAC cohort with the reference population.
We also analyzed WOMAC and SF-36 outcomes with an independent sample t test, comparing the PROSTALAC cohort with the population-based reference cohort. All data analyses were done with Statistics for the Social Sciences (SPSS), version 11.5.
Results
Demographics
We identified 25 patients as currently having the PROSTALAC in situ. Of these, 13 (57%) were women, similar to the population-based cohort, which had 138 (60%) women (p = 0.93). The average age was 70.1 (standard deviation [SD] 17.3) years (range 29.0 to 93.4 years), which was comparable with the population-based cohort (mean 68.2 SD 11.1 years; p = 0.46).
Three (13%) patients had more than 2 comorbidities, and 16 (70%) patients had 1 or 2 comorbidities. Before the PROSTALAC insertion, 20 (87%) patients had a primary total hip or hemiarthroplasty, and 5 (13%) had a revision hip replacement. Patients presented with infection at a median time of 36.4 months (range 1.5 to 242.2 months), postoperatively.
Only 2 patients refused to participate in the study; the remaining 23 (92%) patients completed the HRQL questionnaires. Thirteen (57%) of these patients underwent clinical evaluations, including a range of motion (ROM) assessment. For the 23 patients who participated in the review, the PROSTALAC had been in situ for 13.2 (SD 10.9) months (range 2.5 to 50.5 months). For 14 (61%) patients, the PROSTALAC implant had been in situ for less than 1 year. For 9 patients (39%), the PROSTALAC implant had been retained for longer than 1 year.
WOMAC Osteoarthritis Index
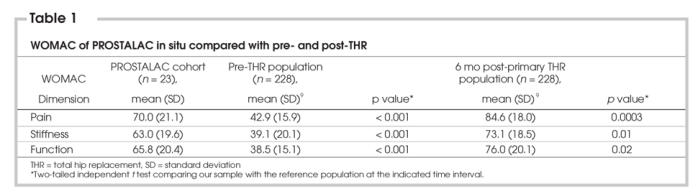
The WOMAC scores for the patients with the PROSTALAC in situ were significantly better than those reported by patients with osteoarthritis waiting to undergo primary THR (See Table 1). However, the PROSTALAC patients reported more pain and stiffness and lower function than patients who were 6 months postprimary THR (See Table 1).
Table 1
SF-36
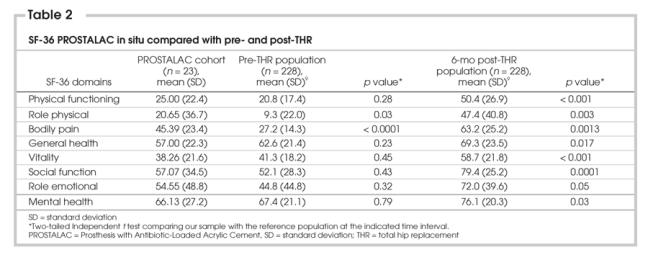
The PROSTALAC patients demonstrated that their HRQL was worse than that of patients who had undergone THA. However, although the PROSTALAC patients' scores were universally lower than scores reported by patients who were 6 months post-THR, bodily pain was significantly lower for our study population than that reported by patients who were waiting to undergo THR (See Table 2).
Table 2
HHS scores
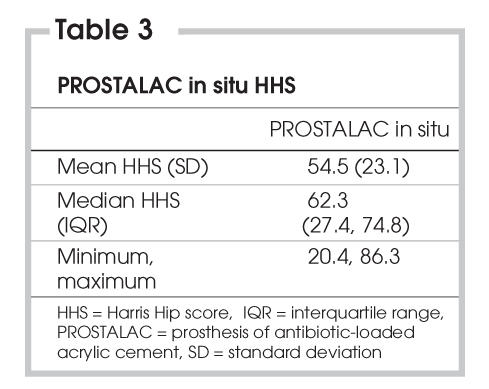
The median HHS score was 62.3 (See Table 3). Ten (44%) patients could walk at least 2 blocks, with the remainder limited to indoor ambulation. Sixteen (70%) patients used walking aids, and 8 (35%) had a severe limp. Only 4 (17%) patients could not climb stairs. Fifteen (65%) patients could put their shoes on, and 10 (44%) could sit in any chair for at least 1 hour. Sixteen patients (70%) reported no more than mild pain.
Table 3
ROM scores
Hip ROM was reported as a composite measure of all planes of motion. The median hip ROM was 100.0 degrees (range 80–140 degrees). Most patients had limited flexion, but none complained about functional difficulties related to limited ROM.
Complications
Functional complications after PROSTALAC insertion included one dislocation requiring a revision of the acetabular cup. Two episodes of periprosthetic fracture occurred, one intra-operatively, and the second postoperatively, when the patient fell and fractured the prosthesis just distal to the prosthesis stem. Both patients received long-stem prosthesis with circlage wires.
Discussion
Published results of deep periprosthetic infection treatment in THR with the PROSTALAC system are currently limited to papers that examine infection resolution.2,7 To date, there are no published data on the function or HRQL status of patients with the PROSTALAC in situ. Our study is the first to examine these issues for patients being treated for deep joint infection with the PROSTALAC implant.
Because no other reference group was available, in situ WOMAC and SF-36 scores were compared with the population data of patients with osteoarthritis before and after uncomplicated THR surgery (See Table 1, Table 2). Our referent group comprised individuals who participated in another prospective observational study that examined outcomes after THR and who were similar in age and sex distribution to our study population.9 Because these patients were also treated in the same hospitals and came from the same referral area, they were an appropriate comparison group and provided a benchmark as to how the PROSTALAC patients were performing. The average WOMAC scores for joint pain, function and stiffness were significantly better than those reported by patients with OA awaiting hip surgery. However, joint pain, stiffness, function and general health status scores were not as favourable as those of patients who had undergone uncomplicated hip arthroplasty.
In a study by Leunig and colleagues,3 22 patients were treated during the interval period with a homemade antibiotic-impregnated cement spacer in a shape similar to a hemiarthroplasty. Although no formal assessment of patient function was done in the interval period in this study, the authors stated that patients were mobile on crutches. In one-half the patients, the cement spacers “failed” either through fracture or extrusion from the joint space. One patient had significant acetabular bone loss secondary to ambulation on the cement spacer. Thus the complication rate with a simple cement spacer appears to be higher than what was seen with the PROSTALAC in situ; we had only 1 dislocation of the temporary prosthesis and 2 periprosthetic fractures, 1 which resulted from the patient falling, after surgery.
Charlton and colleagues6 treated 44 patients with a Girdlestone procedure in the interim treatment period for deep periprosthetic infection and reported an average HHS of 40, which was lower than our reported score of 55. This would suggest that the function of our PROSTALAC patients was superior to that of patients who received treatment with the Girdlestone procedure. Further work is required to compare these 2 treatment approaches for joint infection after THR.
Charlton and colleagues also reported a dislocation rate of 11% after post-second–stage reimplantation. The authors suggested this high dislocation rate was secondary to soft-tissue tension problems related to the Girdlestone procedure.6 Although our patients had not progressed to the second stage, we have not seen dislocation as a common problem with the temporary implant in place. Again, further work is required to determine whether our promising interim results are maintained after reimplantation surgery.
A high number of patients in our study have not gone on to second-stage surgery. Although the surgeons intended to take each patient to the completion of the second stage, 9 patients were considered high-risk surgical candidates and are functioning well with the PROSTALAC in situ; 6 patients refused further surgery on the basis of adequate current function. Thus some of our cohort is using the PROSTALAC as a permanent prosthesis. These patients are being observed on an annual basis to monitor their function and the condition of the PROSTALAC implant.
Our current study is a descriptive analysis of patients who underwent treatment for an infected hip. Ideally, sequential evaluations of the patients would have added significant information about how patients managed after they converted back to a THR or how they managed with long-term PROSTALAC use. Further research is required to determine the long-term effects on function and quality of life of treatment with the PROSTALAC implant.
In conclusion, patients being treated for deep joint infection with the PROSTALAC in situ have lower pain and better joint function than patients with OA awaiting hip arthroplasty. Our HHS is higher than a group of patients undergoing a Girdlestone procedure for the treatment of joint infection. Our results suggest the PROSTALAC is an excellent alternative to simple cement spacers for maintaining acceptable function and pain levels during treatment of periprosthetic infection.
Previously presented at the American Academy of Orthopaedic Surgeons Annual Meeting 2004 and the Canadian Orthopaedic Association Annual Meeting 2003.
Research Financial Support was received from the Edmonton Orthopedic Research Committee. None of the authors received benefits or funds in support of this study.
Accepted for publication July 13, 2006
Competing interests: None declared.
Correspondence to: Dr. Lauren Beaupre, 1F1.52 WMC, 8440-112 Street, Edmonton AB T6G 2B7; fax 780 407-7534; lbeaupre@cha.ab.ca
References
- 1.Buchholz HW, Engelbrecht H. [Depot effects of various antibiotics mixed with Palacos resins]. [Article in German]. Chirurg 1970;41:511-5. [PubMed]
- 2.Kendall RW, Masri BA, Duncan CP, et al. Temporary antibiotic loaded acrylic hip replacement: a novel method for management of the infected THA. Semin Arthroplasty 1994;5:171-7. [PubMed]
- 3.Leunig M, Chosa E, Speck M, et al. A cement spacer for two-stage revision of infected implants of the hip joint. Int Orthop 1998;22:209-14. [DOI] [PMC free article] [PubMed]
- 4.Masri BA, Duncan CP. Chapter 82. In Masri BA, Duncan CP (eds): The Adult Hip. Lippincott-Raven; Philadelphia, 1998.
- 5.Masri BA, Duncan CP. Chapter 81. In Masri BA, Duncan CP (eds): The Adult Hip. Lippincott-Raven; Philadelphia, 1998.
- 6.Charlton WP, Hozack WJ, Teloken MA, et al. Complications associated with reimplantation after girdlestone arthroplasty. Clin Orthop Relat Res 2003;407:119-26. [DOI] [PubMed]
- 7.Younger AS, Duncan CP, Masri BA. Treatment of infection associated with segmental bone loss in the proximal part of the femur in two stages with use of an antibiotic-loaded interval prosthesis. J Bone Joint Surg Am 1998;80:60-9. [DOI] [PubMed]
- 8.Scharfenberger A, Clark M, Lavoie GJ, et al. Treatment of an infected total hip replacement utilizing the PROSTALAC system. Part 1: infection resolution. Can J Surg 2007;50:24-8. [PMC free article] [PubMed]
- 9.Jones CA, Voaklander DC, Johnston DW, et al. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol 2000;27:1745-52. [PubMed]
- 10.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40. [PubMed]
- 11.Bombardier C, Melfi CA, Paul J, et al. Comparison of a generic and a disease-specific measure of pain and physical function after knee replacement surgery. Med Care 1995;33:AS131-44. [PubMed]
- 12.Hawker G, Melfi C, Paul J, et al. Comparison of a generic (sf-36) and a disease specific (womac) (western ontario and mcmaster universities osteoarthritis index) instrument in the measurement of outcomes after knee replacement surgery. J Rheumatol 1995;22:1193-6. [PubMed]
- 13.Stucki G, Sangha O, Stucki S, et al. Comparison of the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index and a self-report format of the self-administered Lequesne-Algofunctional index in patients with knee and hip osteoarthritis. Osteoarthritis Cartilage 1998;6:79-86. [DOI] [PubMed]
- 14.Hayes V, Morris J, Wolfe C, et al. The sf-36 health survey questionnaire: is it suitable for use with older adults? Age Ageing 1995;24:120-5. [DOI] [PubMed]
- 15.Parker SG, Peet SM, Jagger C, et al. Measuring health status in older patients. The SF-36 in practice. Age Ageing 1998;27:13-8. [DOI] [PubMed]
- 16.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 1969;51:737-55. [PubMed]
- 17.Soderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res 2001;384:189-97. [DOI] [PubMed]
- 18.Soderman P, Malchau H, Herberts P. Outcome of total hip replacement: a comparison of different measurement methods. Clin Orthop Relat Res 2001;390:163-72. [DOI] [PubMed]


