Abstract
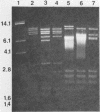
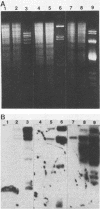
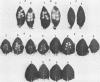
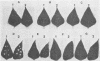
Indigenous plasmids isolated from Pseudomonas tabaci ATCC 11528(pJP1), Pseudomonas angulata 45(pJP30), and P. tabaci BR2(pBPW1) (M. Obukowicz and P. D. Shaw, J. Bacteriol. 155:438-442, 1983) were labeled with Tn3, and the strains were subsequently cured of their respective plasmids. Plasmid-containing and cured isolates caused plant symptoms that were nearly indistinguishable, and the same amount of tabtoxin was produced by P. tabaci strains ATCC 11528 and BR2.
Full text
PDF
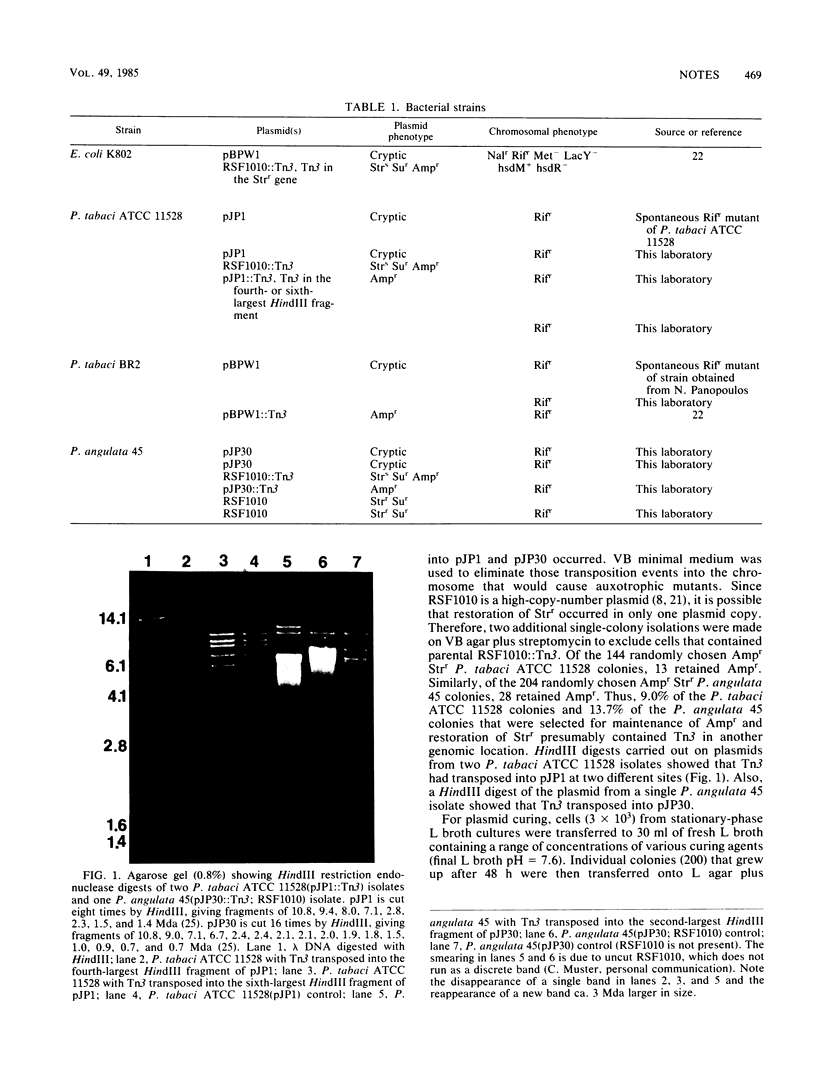

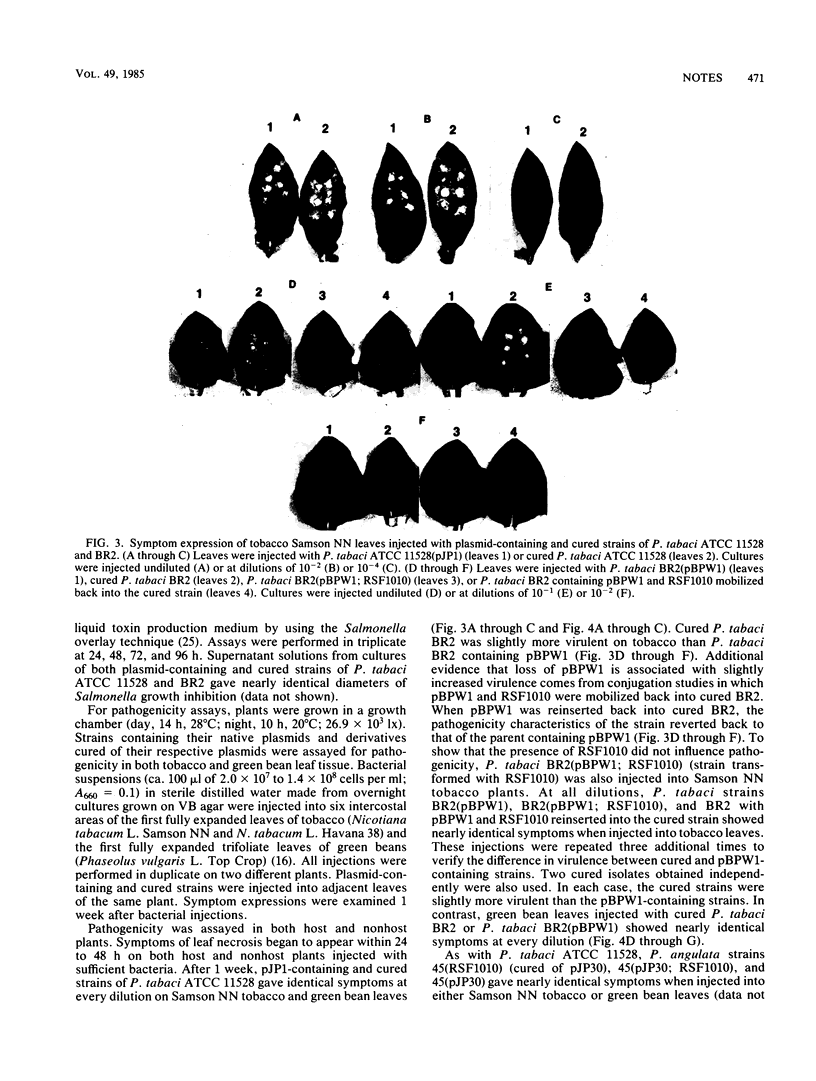


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A., Berg D. E., Botstein D., Lederberg E. M., Novick R. P., Starlinger P., Szybalski W. Nomenclature of transposable elements in prokaryotes. Gene. 1979 Mar;5(3):197–206. doi: 10.1016/0378-1119(79)90078-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Gene transmission among strains of Erwinia amylovora. J Bacteriol. 1973 Dec;116(3):1100–1106. doi: 10.1128/jb.116.3.1100-1106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Cloning characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J Bacteriol. 1982 Jan;149(1):40–46. doi: 10.1128/jb.149.1.40-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacteriol. 1980 Aug;143(2):950–957. doi: 10.1128/jb.143.2.950-957.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiale M. S., Mills D. Detection and characterization of plasmids in Pseudomonas glycinea. J Bacteriol. 1977 Jul;131(1):224–228. doi: 10.1128/jb.131.1.224-228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantotti B. V., Patil S. S., Mandel M. Apparent Involvement of a Plasmid in Phaseotoxin Production by Pseudomonas phaseolicola. Appl Environ Microbiol. 1979 Mar;37(3):511–516. doi: 10.1128/aem.37.3.511-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. C., Vidaver A. K. Transformation of Pseudomonas syringae with nonconjugative R plasmids. Can J Microbiol. 1981 Aug;27(8):759–765. doi: 10.1139/m81-118. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. B., Gunsalus I. C. Isolation of metabolic plasmid DNA from Pseudomonas putida. Biochem Biophys Res Commun. 1977 Mar 7;75(1):13–19. doi: 10.1016/0006-291x(77)91282-7. [DOI] [PubMed] [Google Scholar]
- KLEMENT Z. RAPID DETECTION OF THE PATHOGENICITY OF PHYTOPATHOGENIC PSEUDOMONADS. Nature. 1963 Jul 20;199:299–300. doi: 10.1038/199299b0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahari K., Sakaguchi K. RSF1010 plasmid as a potentially useful vector in Pseudomonas species. J Bacteriol. 1978 Mar;133(3):1527–1529. doi: 10.1128/jb.133.3.1527-1529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukowicz M., Shaw P. D. Tn3 labeling of a cryptic plasmid found in the plant pathogenic bacterium Pseudomonas tabaci and mobilization of RSF1010 by donation. J Bacteriol. 1983 Jul;155(1):438–442. doi: 10.1128/jb.155.1.438-442.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarski J. M., Shaw P. D. Characterization of plasmids from plant pathogenic pseudomonads. Plasmid. 1982 Jan;7(1):85–94. doi: 10.1016/0147-619x(82)90030-0. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Isolation and proof of structure of wildfire toxin. Nature. 1971 Jan 15;229(5281):174–178. doi: 10.1038/229174a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WOOLLEY D. W., PRINGLE R. B., BRAUN A. C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952 May;197(1):409–417. [PubMed] [Google Scholar]
- de Graaff J., Crosa J. H., Heffron F., Falkow S. Replication of the nonconjugative plasmid RSF1010 in Escherichia coli K-12. J Bacteriol. 1978 Jun;134(3):1117–1122. doi: 10.1128/jb.134.3.1117-1122.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]