Abstract
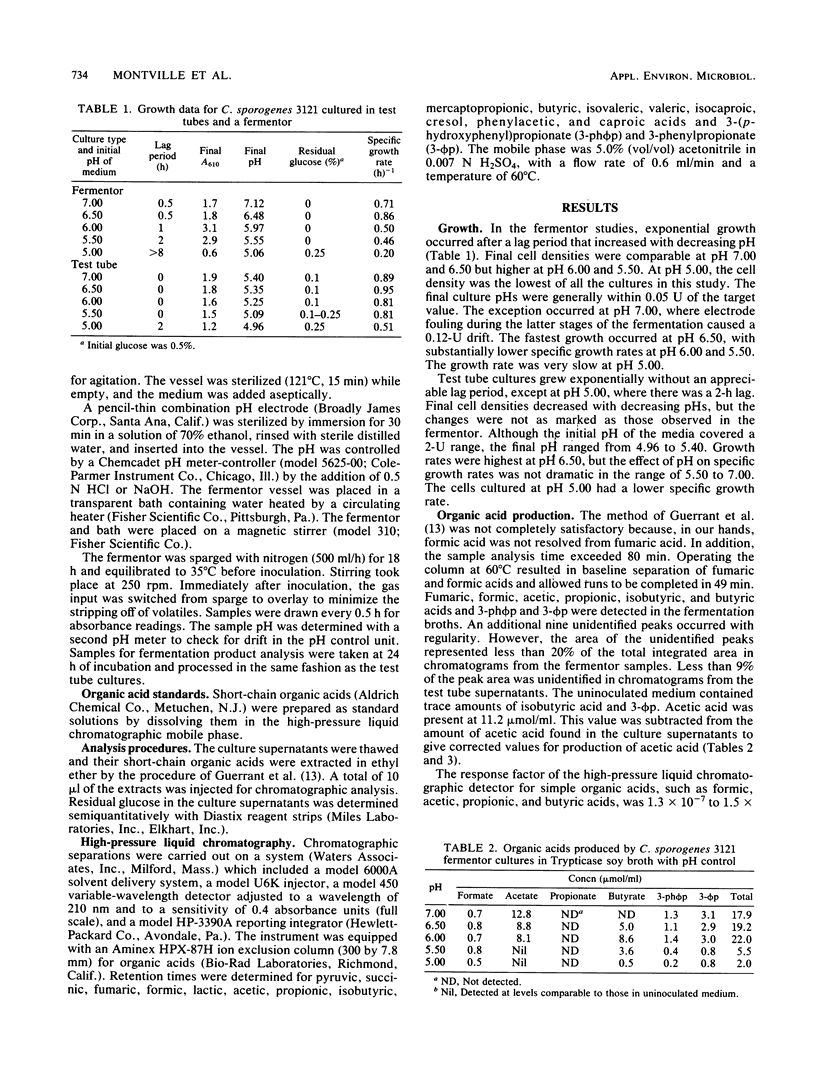
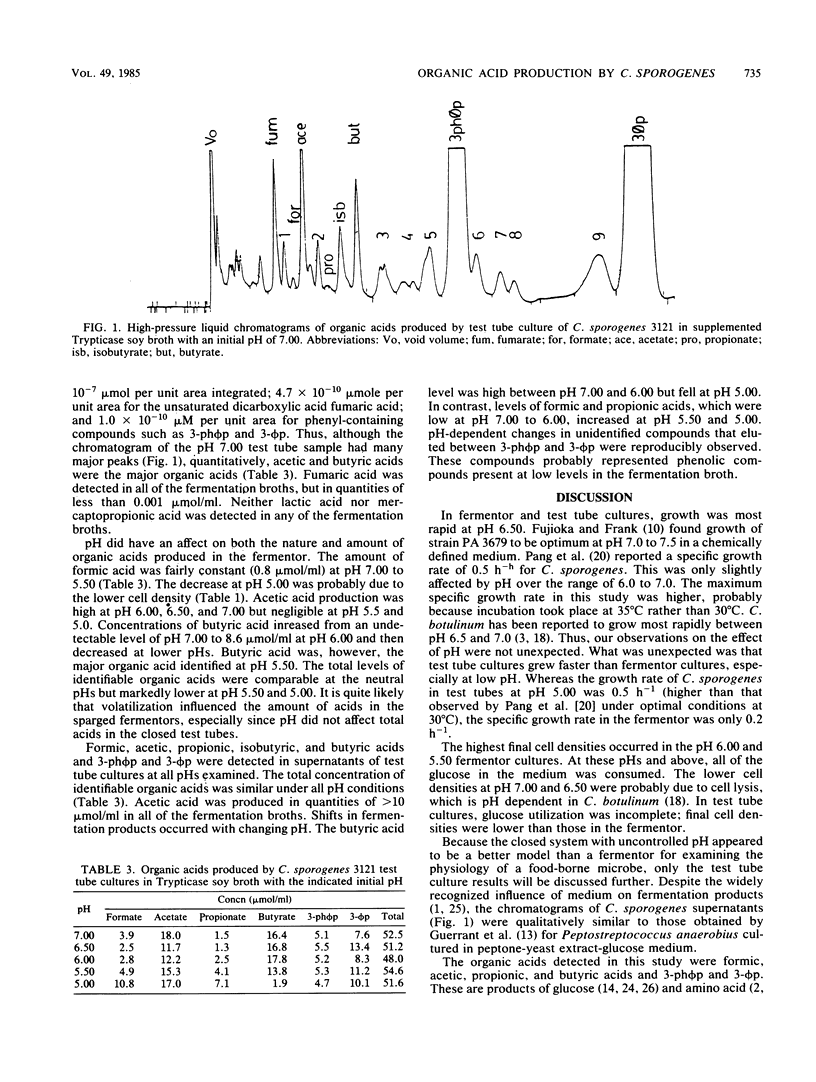
The influence of pH on the growth parameters of and the organic acids produced by Clostridium sporogenes 3121 cultured in test tubes and fermentors at 35 degrees C was examined. Specific growth rates in the fermentor maintained at a constant pH ranged from 0.20 h-1 at pH 5.00 to 0.86 h-1 at pH 6.50. Acetic acid was the primary organic acid in supernatants of 24-h cultures; total organic acid levels were 2.0 to 22.0 mumol/ml. Supernatants from pH 5.00 and 5.50 cultures had total organic acid levels less than one-third of those found at pH 6.00 to 7.00. The specific growth rates of the test tube cultures ranged from 0.51 h-1 at pH 5.00 to 0.95 h-1 at pH 6.50. The pH of the medium did not affect the average total organic acid content (51.5 mumol/ml) but did affect the distribution of the organic acids, which included formic, acetic, propionic, butyric, 3-(p-hydroxyphenyl)propionic, and 3-phenylpropionic acids. Butyric acid levels were lower, but formic and propionic acid levels were higher, at pH 5.00 than at other pHs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anema P. J., Kooiman W. J., Geers J. M. Volatile acid production by Clostridium sporogenes under controlled culture conditions. J Appl Bacteriol. 1973 Dec;36(4):683–687. doi: 10.1111/j.1365-2672.1973.tb04153.x. [DOI] [PubMed] [Google Scholar]
- BONVENTRE P. F., KEMPE L. L. Physiology of toxin production by Clostridium botulinum types A and B. III. Effect of pH and temperature during incubation on growth, autolysis. and toxin production. Appl Microbiol. 1959 Nov;7:374–377. doi: 10.1128/am.7.6.374-377.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50:23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- Clifton C. E. The Utilization of Amino Acids and of Glucose by Clostridium botulinum. J Bacteriol. 1940 May;39(5):485–497. doi: 10.1128/jb.39.5.485-497.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY L. E., COSTILOW R. N. PHYSIOLOGY OF THE SPORULATION PROCESS IN CLOSTRIDIUM BOTULINUM. I. CORRELATION OF MORPHOLOGICAL CHANGES WITH CATABOLIC ACTIVITIES, SYNTHESIS OF DIPICOLINIC ACID, AND DEVELOPMENT OF HEAT RESISTANCE. J Bacteriol. 1964 Sep;88:690–694. doi: 10.1128/jb.88.3.690-694.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY L. E., COSTILOW R. N. PHYSIOLOGY OF THE SPORULATION PROCESS IN CLOSTRIDIUM BOTULINUM. II. MATURATION OF FORESPORES. J Bacteriol. 1964 Sep;88:695–701. doi: 10.1128/jb.88.3.695-701.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R. S., Frank H. A. Nutritional requirements for germination, outgrowth, and vegetative growth of putrefactive anaerobe 3679 in a chemically defined medium. J Bacteriol. 1966 Nov;92(5):1515–1520. doi: 10.1128/jb.92.5.1515-1520.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant G. O., Lambert M. A., Moss C. W. Analysis of short-chain acids from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol. 1982 Aug;16(2):355–360. doi: 10.1128/jcm.16.2.355-360.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmanis M. G., Gatenbeck S. Intermediary Metabolism in Clostridium acetobutylicum: Levels of Enzymes Involved in the Formation of Acetate and Butyrate. Appl Environ Microbiol. 1984 Jun;47(6):1277–1283. doi: 10.1128/aem.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew J. W., Gorbach S. L. Rapid gas chromatographic technique for presumptive detection of Clostridium botulinum in contaminated food. Appl Microbiol. 1975 Feb;29(2):297–299. doi: 10.1128/am.29.2.297-299.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitruka B. M., Costilow R. N. Arginine and ornithine catabolism by Clostridium botulinum. J Bacteriol. 1967 Jan;93(1):295–301. doi: 10.1128/jb.93.1.295-301.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J. Dependence of Clostridium botulinum gas and protease production on culture conditions. Appl Environ Microbiol. 1983 Feb;45(2):571–575. doi: 10.1128/aem.45.2.571-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J. Interaction of pH and NaCl on culture density of Clostridium botulinum 62A. Appl Environ Microbiol. 1983 Oct;46(4):961–963. doi: 10.1128/aem.46.4.961-963.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISMAN B. The Stickland reaction. Bacteriol Rev. 1954 Mar;18(1):16–42. doi: 10.1128/br.18.1.16-42.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland L. H. Studies in the metabolism of the strict anaerobes (genus Clostridium): The chemical reactions by which Cl. sporogenes obtains its energy. Biochem J. 1934;28(5):1746–1759. doi: 10.1042/bj0281746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Stadtman T. C. Selenium-dependent clostridial glycine reductase. Purification and characterization of the two membrane-associated protein components. J Biol Chem. 1979 Jan 25;254(2):447–452. [PubMed] [Google Scholar]
- Turton L. J., Drucker D. B., Ganguli L. A. Effect of glucose concentration in the growth medium upon neutral and acidic fermentation end-products of Clostridium bifermentans, Clostridium sporogenes and peptostreptococcus anaerobius. J Med Microbiol. 1983 Feb;16(1):61–67. doi: 10.1099/00222615-16-1-61. [DOI] [PubMed] [Google Scholar]
- Turton L. J., Drucker D. B., Hillier V. F., Ganguli L. A. Effect of eight growth media upon fermentation profiles of ten anaerobic bacteria. J Appl Bacteriol. 1983 Apr;54(2):295–304. doi: 10.1111/j.1365-2672.1983.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Woods L. F., Wood J. M., Gibbs P. A. the involvement of Nitric Oxide in the inhibition of the phosphoroclastic system in Clostridium sporogenes by sodium nitrite. J Gen Microbiol. 1981 Aug;125(2):399–406. doi: 10.1099/00221287-125-2-399. [DOI] [PubMed] [Google Scholar]



