Abstract
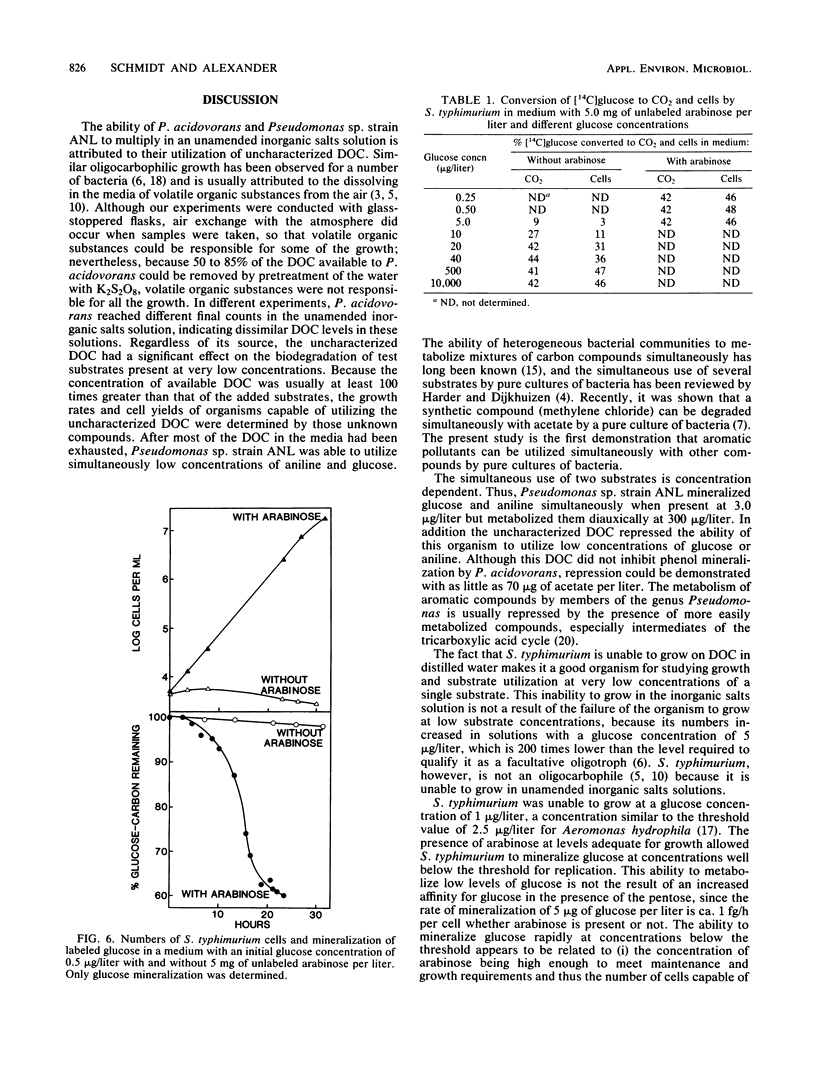
Pseudomonas acidovorans and Pseudomonas sp. strain ANL but not Salmonella typhimurium grew in an inorganic salts solution. The growth of P. acidovorans in this solution was not enhanced by the addition of 2.0 micrograms of phenol per liter, but the phenol was mineralized. Mineralization of 2.0 micrograms of phenol per liter by P. acidovorans was delayed 16 h by 70 micrograms of acetate per liter, and the delay was lengthened by increasing acetate concentrations, whereas phenol and acetate were utilized simultaneously at concentrations of 2.0 and 13 micrograms/liter, respectively. Growth of Pseudomonas sp. in the inorganic salts solution was not affected by the addition of 3.0 micrograms each of glucose and aniline per liter, nor was mineralization of the two compounds detected during the initial period of growth. However, mineralization of both substrates by this organism occurred simultaneously during the latter phases of growth and after growth had ended at the expense of the uncharacterized dissolved organic compounds in the salts solution. In contrast, when Pseudomonas sp. was grown in the salts solution supplemented with 300 micrograms each of glucose and aniline, the sugar was mineralized first, and aniline was mineralized only after much of the glucose carbon was converted to CO2. S. typhimurium failed to multiply in the salts solution with 1.0 micrograms of glucose per liter. It grew slightly but mineralized little of the sugar at 5.0 micrograms/liter, but its population density rose at 10 micrograms of glucose per liter or higher. The hexose could be mineralized at 0.5 micrograms/liter, however, if the solution contained 5.0 mg of arabinose per liter.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF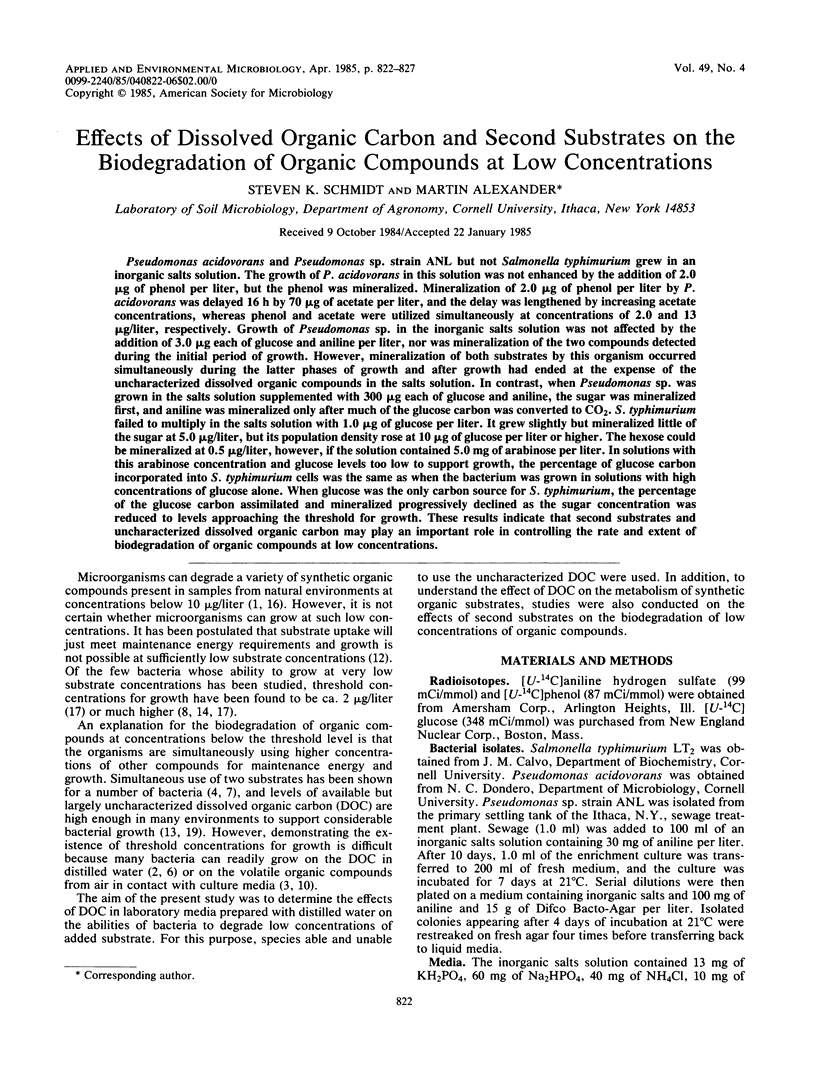




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M. S., Carson L. A., Bond W. W., Petersen N. J. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science. 1971 Aug 27;173(3999):836–838. doi: 10.1126/science.173.3999.836. [DOI] [PubMed] [Google Scholar]
- Geller A. Growth of bacteria in inorganic medium at different levels of airborne organic substances. Appl Environ Microbiol. 1983 Dec;46(6):1258–1262. doi: 10.1128/aem.46.6.1258-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Strategies of mixed substrate utilization in microorganisms. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):459–480. doi: 10.1098/rstb.1982.0055. [DOI] [PubMed] [Google Scholar]
- LaPat-Polasko L. T., McCarty P. L., Zehnder A. J. Secondary substrate utilization of methylene chloride by an isolated strain of Pseudomonas sp. Appl Environ Microbiol. 1984 Apr;47(4):825–830. doi: 10.1128/aem.47.4.825-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata T. E., Marr A. G. Effect of nutrient concentration on the growth of Escherichia coli. J Bacteriol. 1971 Jul;107(1):210–216. doi: 10.1128/jb.107.1.210-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm-Zollinger E. Effects of inhibition and repression on the utilization of substrates by heterogeneous bacterial communities. Appl Microbiol. 1966 Jul;14(4):654–664. doi: 10.1128/am.14.4.654-664.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman P. D., Jones D. Regulation of citrate synthase and microbial taxonomy. Nature. 1968 Jul 20;219(5151):270–272. doi: 10.1038/219270a0. [DOI] [PubMed] [Google Scholar]
- van der Kooij D., Visser A., Hijnen W. A. Growth of Aeromonas hydrophila at Low Concentrations of Substrates Added to Tap Water. Appl Environ Microbiol. 1980 Jun;39(6):1198–1204. doi: 10.1128/aem.39.6.1198-1204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D., Visser A., Oranje J. P. Multiplication of fluorescent pseudomonads at low substrate concentrations in tap water. Antonie Van Leeuwenhoek. 1982;48(3):229–243. doi: 10.1007/BF00400383. [DOI] [PubMed] [Google Scholar]



