Abstract
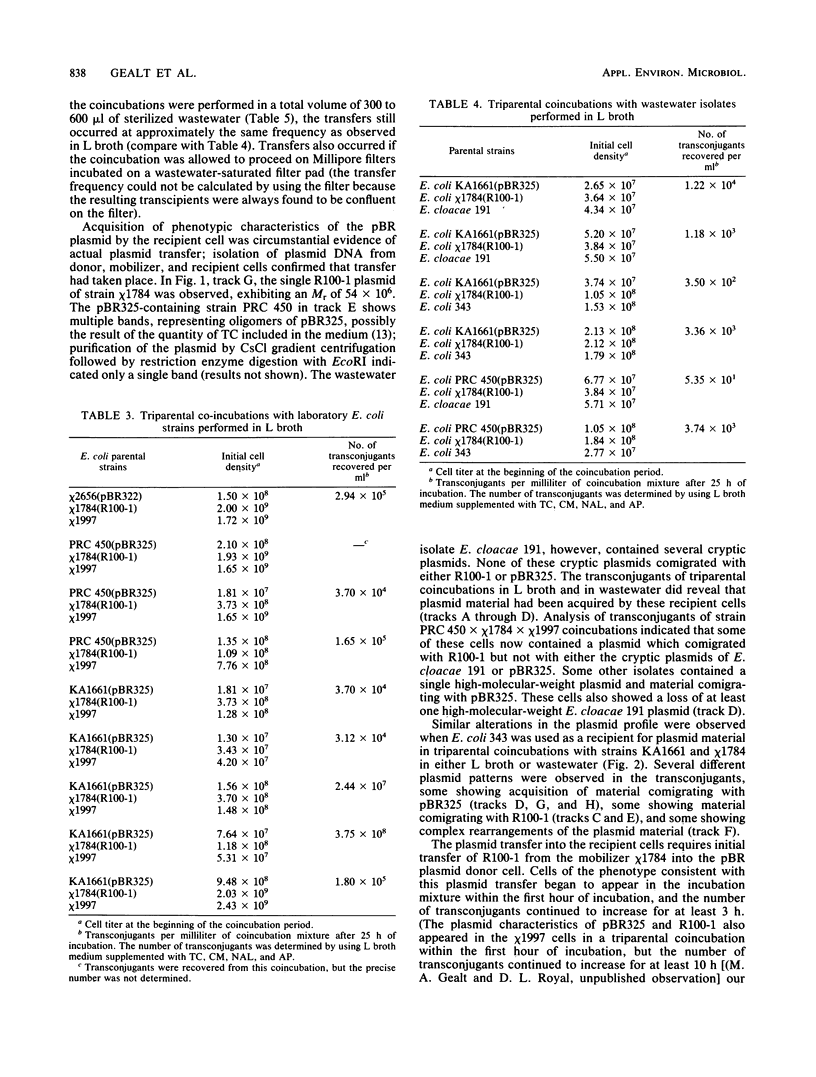
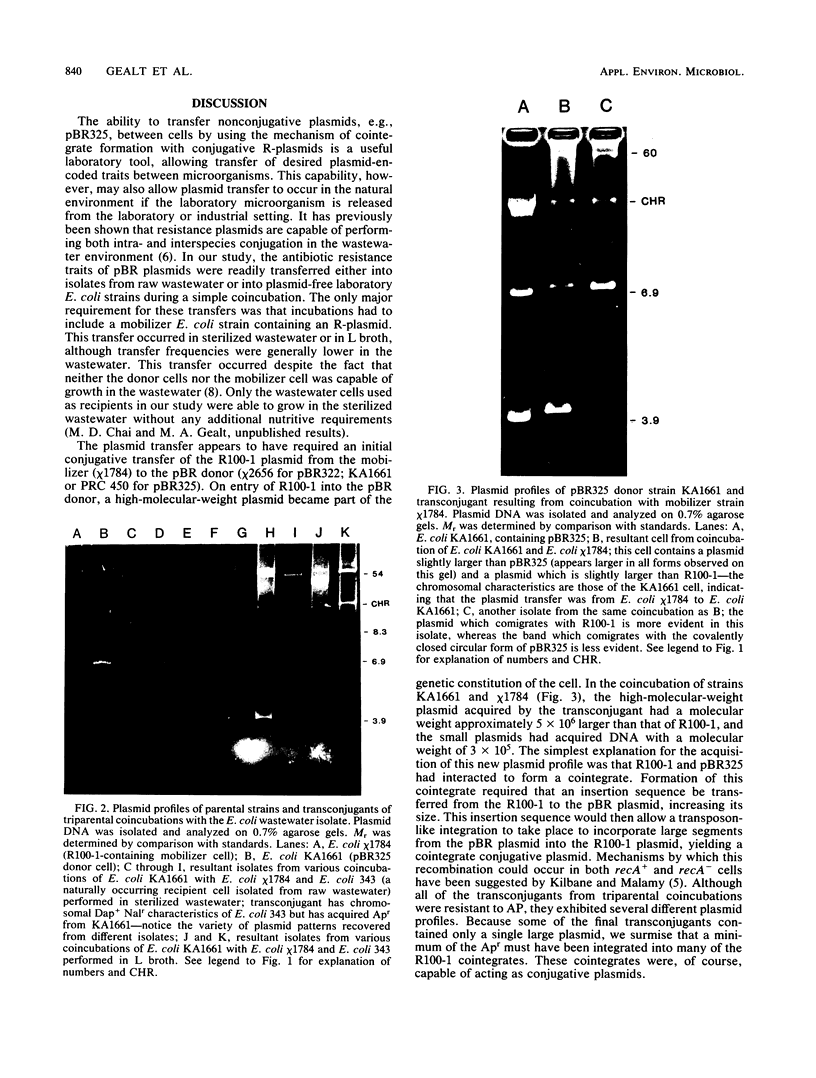
Laboratory strains of Escherichia coli containing plasmid pBR325 (or pBR322) were coincubated with a mobilizer strain of E. coli (containing the conjugative plasmid R100-1) and a recipient strain of bacteria. Bacterial strains isolated from raw wastewater or a plasmid-free E. coli laboratory strain served as recipients. Transfer of the pBR plasmid into the recipient strain occurred during a 25-h coincubation in either L broth or sterilized wastewater; transfer frequencies were several orders of magnitude lower in wastewater. After the coincubation, recipients exhibited both plasmid-encoded phenotypic characteristics and an altered plasmid profile, as shown by agarose gel electrophoresis of purified plasmid DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finnegan J., Sherratt D. Plasmid ColE1 conjugal mobility: the nature of bom, a region required in cis for transfer. Mol Gen Genet. 1982;185(2):344–351. doi: 10.1007/BF00330810. [DOI] [PubMed] [Google Scholar]
- Kilbane J. J., Malamy M. H. F factor mobilization of non-conjugative chimeric plasmids in Escherichia coli: general mechanisms and a role for site-specific recA-independent recombination at orV1. J Mol Biol. 1980 Oct 15;143(1):73–93. doi: 10.1016/0022-2836(80)90125-4. [DOI] [PubMed] [Google Scholar]
- Mach P. A., Grimes D. J. R-plasmid transfer in a wastewater treatment plant. Appl Environ Microbiol. 1982 Dec;44(6):1395–1403. doi: 10.1128/aem.44.6.1395-1403.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehle W., Hecker M., Reichstein B., Mach F. Bildung von Oligomeren des Plasmids pBR322 in Abhängigkeit von der Tetracyklinkonzentration. Z Allg Mikrobiol. 1984;24(2):119–124. [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]






