Abstract
The competitiveness of a Rhizobium leguminosarum strain was investigated at two separate locations in field inoculation studies on commercially grown peas. The soil at each location (sites I and II) contained an indigenous R. leguminosarum population of ca. 3 × 104 rhizobia per g of soil. At site I it was necessary to use an inoculum concentration as large as 4 × 107 CFU ml−1 (2 × 106 bacteria seed−1) to establish the inoculum strain in the majority of nodules (73%). However, at site II the inoculum strain formed only 33% of nodules when applied at this (107 CFU ml−1) level. Establishment could not be further improved by increasing the inoculum concentration even as high as 109 CFU ml−1 (9.6 × 107 bacteria seed−1). The inoculum strain could be detected at both sites 19 months after inoculation. Analysis by intrinsic antibiotic resistance patterns and plasmid DNA profiles indicated that a dominant strain(s) and plasmid pool existed among the indigenous population at site II. Competition experiments were carried out under laboratory conditions between a dominant indigenous isolate and the inoculum strain. Both strains were shown to be equally competitive.
Full text
PDF

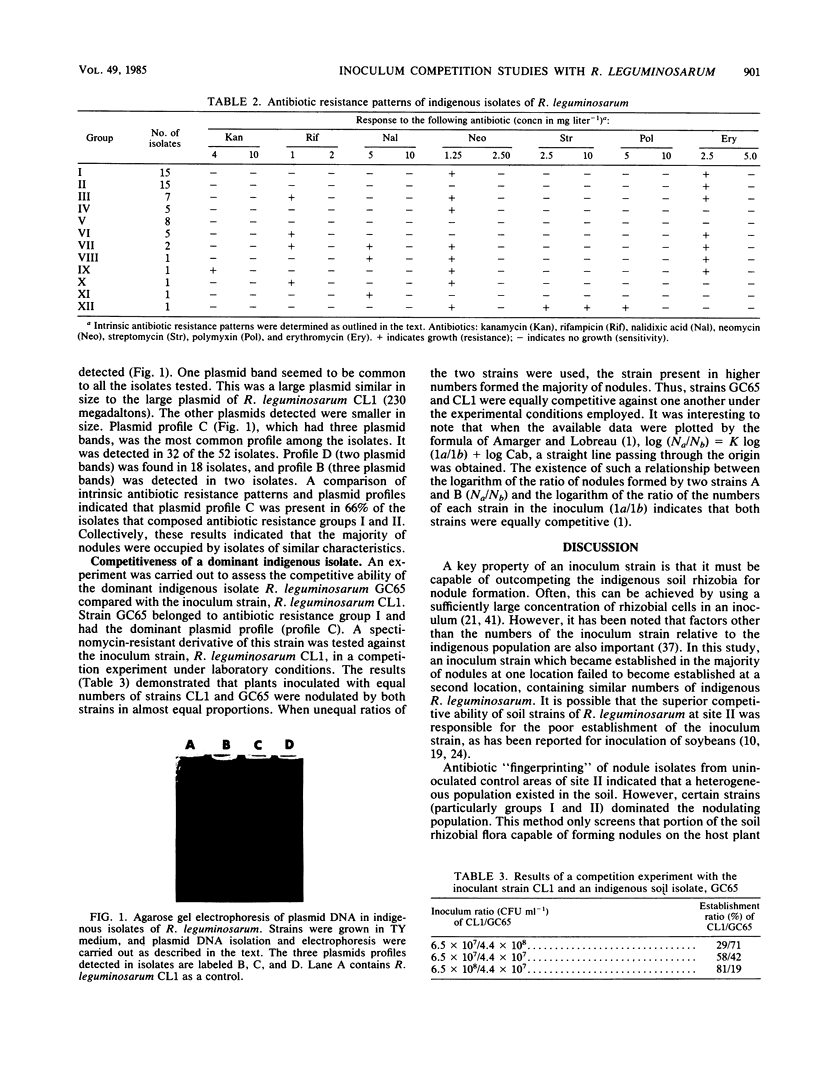


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarger N., Lobreau J. P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982 Sep;44(3):583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedmar E. J., Brewin N. J., Phillips D. A. Effect of Plasmid pIJ1008 from Rhizobium leguminosarum on Symbiotic Function of Rhizobium meliloti. Appl Environ Microbiol. 1984 Apr;47(4):876–878. doi: 10.1128/aem.47.4.876-878.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedmar E. J., Olivares J. Autotransmissible resident plasmid of Rhizobium meliloti. Mol Gen Genet. 1980 Jan;177(2):329–331. doi: 10.1007/BF00267446. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Shine J., Rolfe B. G. Sym plasmid transfer to various symbiotic mutants of Rhizobium trifolii, R. leguminosarum, and R. meliloti. J Bacteriol. 1983 Dec;156(3):1035–1045. doi: 10.1128/jb.156.3.1035-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Kuykendall L. D., Weber D. F. Genetically marked Rhizobium identifiable as inoculum strain in nodules of soybean plants grown in fields populated with Rhizobium japonicum. Appl Environ Microbiol. 1978 Dec;36(6):915–919. doi: 10.1128/aem.36.6.915-919.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Pankhurst C. E. Symbiotic effectiveness of antibiotic-resistant mutants of fast- and slow-growing strains of Rhizobium nodulating Lotus species. Can J Microbiol. 1977 Aug;23(8):1026–1033. doi: 10.1139/m77-152. [DOI] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert F. M., Schmidt E. L. Population Changes and Persistence of Rhizobium phaseoli in Soil and Rhizospheres. Appl Environ Microbiol. 1983 Feb;45(2):550–556. doi: 10.1128/aem.45.2.550-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinghamer E. A., Dudman W. F. Evaluation of spectinomycin resistance as a marker for ecological studies with Rhizobium spp. J Appl Bacteriol. 1973 Jun;36(2):263–272. doi: 10.1111/j.1365-2672.1973.tb04101.x. [DOI] [PubMed] [Google Scholar]
- van Rensburg H. J., Strijdom B. W. Competitive Abilities of Rhizobium meliloti Strains Considered to Have Potential as Inoculants. Appl Environ Microbiol. 1982 Jul;44(1):98–106. doi: 10.1128/aem.44.1.98-106.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



