Abstract
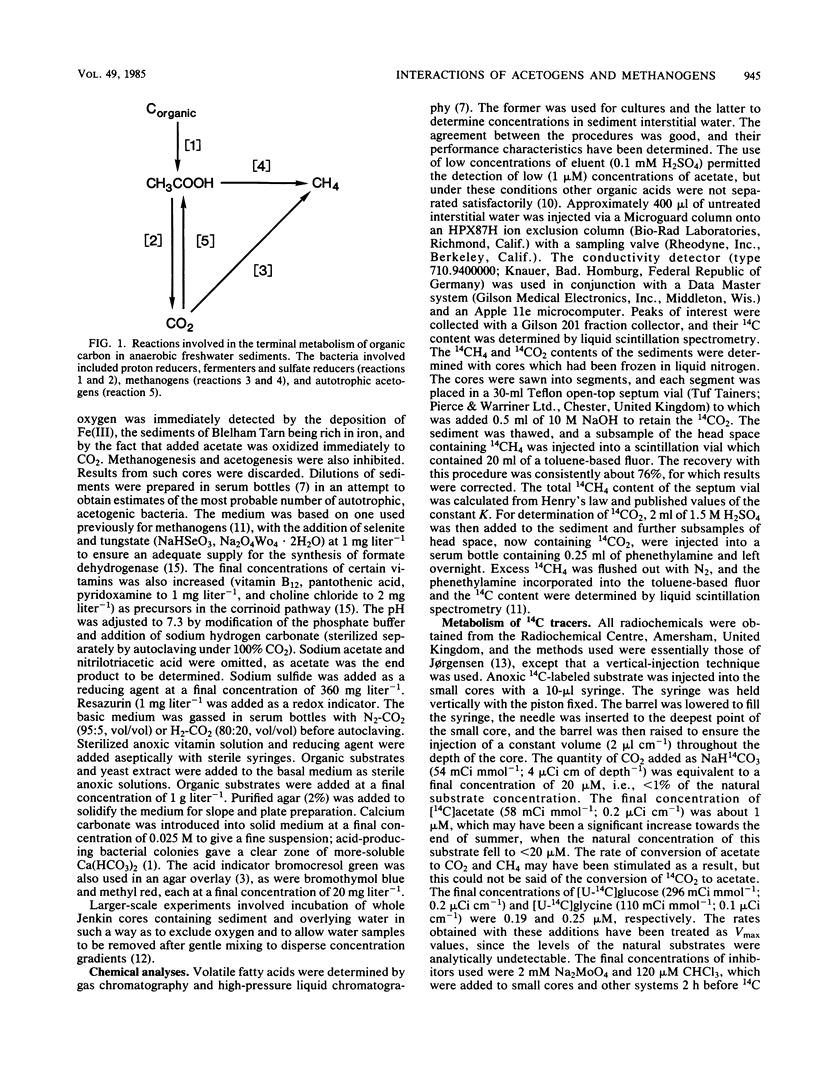
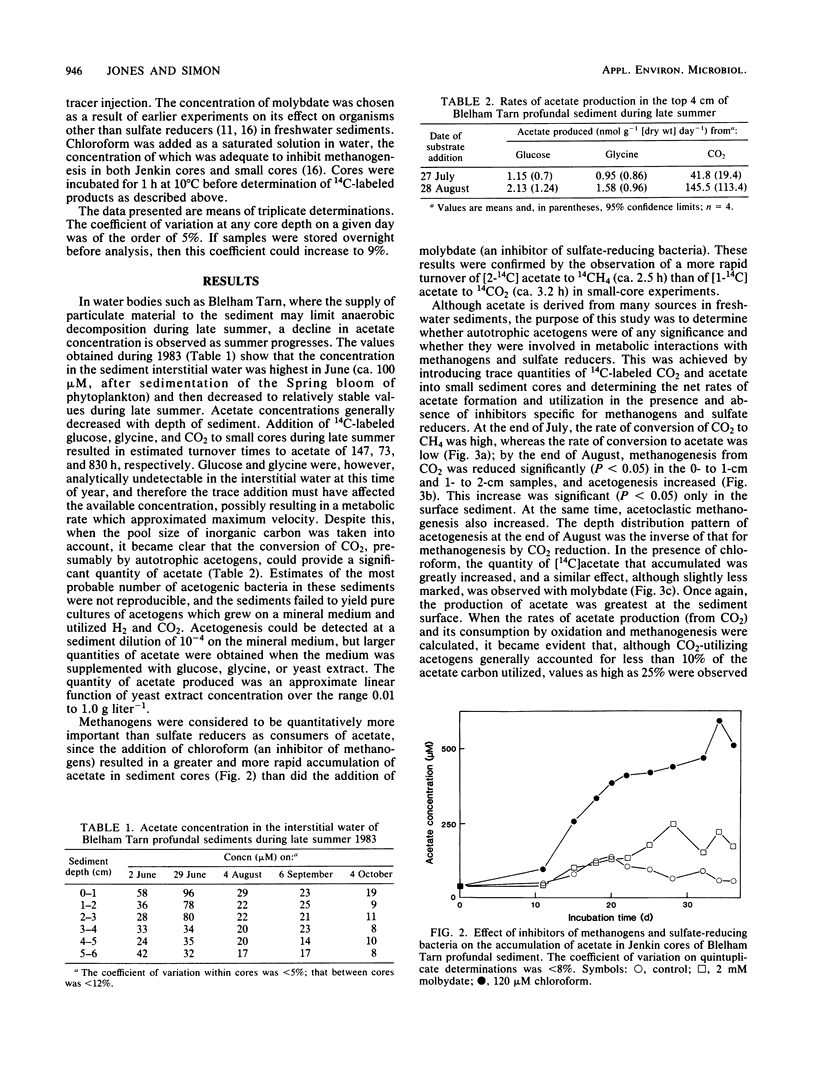
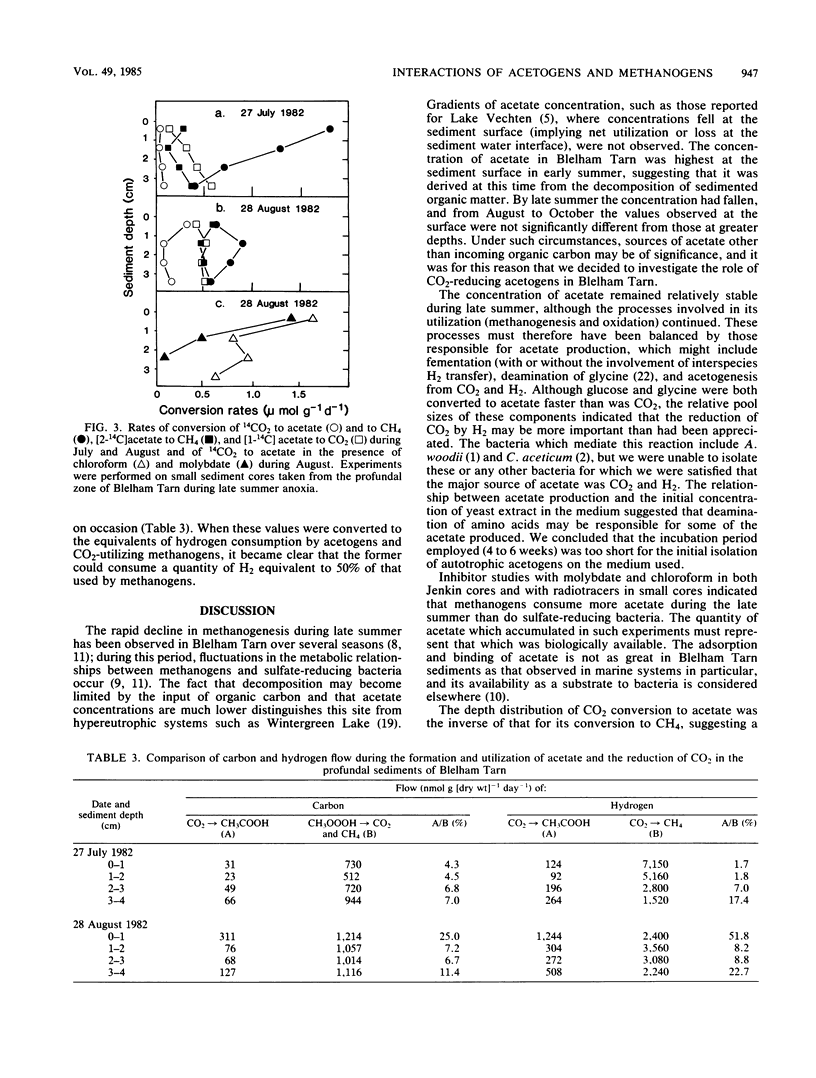
Anaerobic decomposition processes in the profundal sediments of Blelham Tarn (English Lake District) are often limited during late summer by the input of organic carbon. The concentration of acetate in the interstitial water fell from about 100 microM (immediately after sedimentation of the spring diatom bloom) to a relatively constant value of about 20 microM in late summer, during which acetate utilization appeared to be balanced by production. Addition of chloroform and molybdate caused an accumulation of cold acetate in large sediment cores and of [14C]acetate in small cores to which [14C]bicarbonate had been added. In both cases chloroform caused the greater accumulation, implying that acetoclastic methanogens were the more active consumers. The conversion of 14CO2 to [14C]acetate was inversely related, with depth, to its conversion to 14CH4. Methanogenesis from CO2 decreased during late summer, whereas acetogenesis and acetoclastic methanogenesis increased over the same time period. The production of acetate from CO2 was generally equivalent to less than 10% of the acetate carbon utilized but could be as high as 25% of that value. Hydrogen consumption by acetogens could be as high as 50% of that utilized in methanogenesis. The role of acetogenic bacteria in anaerobic processes may therefore be of greater significance in lakes such as Blelham Tarn than in more eutrophic systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun M., Mayer F., Gottschalk G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol. 1981 Jan;128(3):288–293. doi: 10.1007/BF00422532. [DOI] [PubMed] [Google Scholar]
- Braun M., Schoberth S., Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979 Mar 12;120(3):201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Methanogenesis from methanol and methylamines and acetogenesis from hydrogen and carbon dioxide in the sediments of a eutrophic lake. Appl Environ Microbiol. 1983 Apr;45(4):1310–1315. doi: 10.1128/aem.45.4.1310-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


