Abstract
T cell receptor ζ (TcRζ)/CD3 ligation initiates a signaling cascade that involves src kinases p56lck and ζ-associated protein 70, leading to the phosphorylation of substrates such as TcRζ, Vav, SH2-domain-containing leukocyte protein 76 (SLP-76), cbl, and p120/130. FYN binding protein (FYB or p120/130) associates with p59fyn, the TcRζ/CD3 complex, and becomes tyrosine-phosphorylated in response to receptor ligation. In this study, we report the cDNA cloning of human and murine FYB and show that it is restricted in expression to T cells and myeloid cells and possesses an overall unique hydrophilic sequence with several tyrosine-based motifs, proline-based type I and type II SH3 domain binding motifs, several putative lysine/glutamic acid-rich nuclear localization motifs, and a SH3-like domain. In addition to binding the src kinase p59fyn, FYB binds specifically to the hematopoietic signaling protein SLP-76, an interaction mediated by the SLP-76 SH2 domain. In keeping with this, expression of FYB augmented interleukin 2 secretion from a T cell hybridoma, DC27.10, in response to TcRζ/CD3 ligation. FYB is therefore a novel hematopoietic protein that acts as a component of the FYN and SLP-76 signaling cascades in T cells.
Ligation of the T cell receptor (TcRζ)/CD3 and the CD4/CD8-p56lck coreceptors activates src protein-tyrosine kinases p56lck and p59fyn (1) and, in turn, the phosphorylation of immunoreceptor tyrosine-based activation motifs within the TcRζ and CD3 chains (2, 3). Phosphorylation of TcR components results in recruitment and activation of ζ-associated protein 70 (ZAP-70) (4, 5), and possibly other proteins such as Shc (6). p56lck also has been noted to phosphorylate CD5 and CD28 (7, 8), and ZAP-70 has been shown to selectively phosphorylate the downstream protein SH2-domain-containing leukocyte protein (SLP-76) (9, 10). These data are consistent with a scenario where p56lck regulates proximal events, while ZAP-70 acts further downstream. TcR ligation also leads to the phosphorylation of phospholipase-Cγ1 (11), the protooncogene Vav (12, 13), p120/130 (14, 15), c-cbl (16, 17), and SLP-76 (18).
Recent studies have implicated hematopoietic proteins Vav and SLP-76 in downstream signaling cascade of T cells. Overexpression of the protein has been reported to augment TcRζ/CD3-induced interleukin 2 (IL-2) transcription (19, 20). Recently, cloned SLP-76 has been reported as a potential downstream link to Vav (19). The protein contains potential tyrosine phosphorylation sites in its N terminus, a C-terminal SH2 domain, and a central proline-rich region that interacts with SH3-domain-containing proteins such as Grb-2. As in the case of Vav, SLP-76 is specifically expressed in hematopoietic cells and undergoes tyrosine phosphorylation upon TcR cross-linking (9, 19–21). The Vav SH2 domain binds SLP-76 (20, 21), specifically two pYESP motifs (Tyr-113 and Tyr-128) (10). ZAP-70 has also been identified as the kinase capable of phosphorylating SLP-76 at one or both of these sites and facilitating complex formation (10). Overexpression of SLP-76 augments TcR/CD3-induced IL-2 promoter activity and may act synergistically with Vav in augmenting IL-2 transcription (19). However, some T cells can produce IL-2 in the absence of Vav/SLP-76 binding (10). Of note, the SLP-76-mediated effects depend on the integrity of its SH2 domain (20), a domain that binds to two substrates of 62 kDa and 120 kDa (20).
Unlike p56lck and ZAP-70, the role of the p59fyn kinase remains to be elucidated. p59fyn can be expressed as two isoforms, one form expressed in lymphoid cells (p59fynT) and another expressed more ubiquitously [p59fyn(B)] (22). p59fyn(T) binds to subunits of the TcRζ/CD3 complex by N-terminal residues (23). Engagement of the TcRζ/CD3 complex with anti-CD3 mAb stimulates the p59fyn(T) kinase activity (24, 25). In transgenic mice, the expression of constitutively active p59fyn(T) potentiates proliferation (26). Recently, we identified p120/130 as a ligand for the SH2 domain of p59fyn(T) (14, 15). p120/130 therefore appears to be well-suited to serve as downstream target of p59fyn(T).
In this paper, we report the cDNA cloning of human and murine p120/130 (renamed FYB for FYN-Binding protein) and show that it possesses a number of unique features and is restricted to T cells and myeloid cells. In addition, we show that FYB also binds to the hematopoietic-signaling protein SLP-76 via its SH2 domain. Consistent with this, expression of FYB altered IL-2 secretion by a T cell hybridoma, DC27.10, in response to TcRζ/CD3 ligation. FYB is therefore a novel hematopoietic protein that acts as a component of the FYN and SLP-76 signaling cascades in T cells.
MATERIALS AND METHODS
Antiserum.
The generation of the anti-FYB serum has been described (15). Briefly, p120 and p130 FYB were initially purified from DC27.10 cells by column affinity chromatography using the glutathione S-transferase (GST)–SH2-FYN domain fusion protein. A gel slice containing approximately 30 μg of protein in complete Freund’s adjuvant was injected into a New Zealand white female rabbit for the first immunization, followed by a booster dose (30 μg in incomplete Freund’s adjuvant) after 5 weeks; two rabbits were immunized. Rabbit serum was collected for further analysis.
Cloning of FYB and DNA Constructs.
A human Jurkat T cell-derived cDNA library constructed into the λEX.lox expression vector (Novagene; a gift from Hamid Band, Harvard Medical School, Boston, MA) was screened with anti-FYB antiserum. Full-length FYB cDNA was obtained by rescreening of a human tonsil-derived cDNA library constructed into λgt11 (GenBank accession no. AF001862). To clone the murine cDNA of FYB, the human cDNA sequence was used as an hybridization probe to screen a mouse T cell-hybridoma-derived cDNA library constructed in λZAP-Express (Stratagene; a gift from Linda Clayton, Dana–Farber Cancer Institute, Boston, MA; GenBank accession no. AF001863). Full-length FYB cDNA was inserted into the pSRα mammalian expression vector carrying the influenza hemagglutinin (HA) epitope tag at the N-terminal end [FYB(Ha)]. For the GST–FYB constructs, a full-length cDNA clone was inserted into the pAc-GHLT vector (PharmMingen) for expression in SF21 insect cells. Generation of GST–SH2/SLP-76 fusion protein has been described elsewhere (18).
Northern Blot Analysis.
The human multiple-tissue Northern blot was obtained from CLONTECH. For the hematopoietic-cell Northern blot, T cell, B-cell, and myeloid cell total RNA samples were extracted from intact cells with guanidine isothyocynate. Fifteen micrograms of total RNA was separated in a 1.2% agarose denaturing RNA gel, and the samples were transferred to nitrocellulose and probed with [32P]dCTP-labeled FYB or GAPDH cDNA probes. Membranes were exposed to autoradiography for 24–36 h.
Protein Precipitations and Immunoblot Analysis.
Triton X-100 lysis buffer (1% in 20 mM Tris⋅HCl, pH 8.1/150 mM NaCl) was used to solubilize cells as described (14). After preclearing, cell lysates were incubated with antibodies or GST fusion proteins for 1 h and then precipitated with protein A-Sepharose or glutathione-Sepharose, respectively. For GST–FYB precipitation, the fusion protein was expressed in insect cells and then coincubated with DC27.10 lysates. Analysis by immunoblotting was carried out with chemoimmunofluorescence and the Renaissance detection kit (DuPont).
IL-2 Bioassays.
HA–FYB cDNA was introduced into T cells (0.1–4.0 μg per 106 cells) by electroporation with a ElectroCell manipulator 600 (BTX, San Diego) set at 250 V and 960 μF. After an overnight incubation, cells were cultured in microtiter plates at 105 cells per well in the presence of 10−10 to 10−9 M 145–2C11 and phorbol 12-myristate 13-acetate (2 ng/ml). After 24 h in culture, supernatants were stored at −70°C and thawed before the IL-2 assay. Multiple dilutions of the supernatants were added to IL-2-dependent CTLL-2 cells at a concentration of 2500 cells per well in a 96-well plate. Cells were cultured for 24 h followed by an 18-h pulse with 1 μCi of [3H]thymidine (1 Ci = 37 GBq) before harvesting and measuring radioactivity. The amount of IL-2 in these supernatants was determined by comparing with the standard curve established with recombinant IL-2. The half-maximum of stimulation is defined as 100 units/ml (27).
RESULTS AND DISCUSSION
We have described (1, 14, 15, 24) a 120/130-kDa protein (termed p120/130 or FYB for Fyn binding protein) that is distinct from the cbl protooncogene, binds to the src kinase p59fyn, and undergoes tyrosine phosphorylation with TcRζ/CD3 ligation. By using an anti-FYB specific rabbit antiserum generated against purified protein, we screened a Jurkat-cell-line-derived expression cDNA library constructed into λEX.lox. Distinct partial cDNA clones were initially obtained and used for rescreening of a tonsil-derived cDNA library, resulting in the isolation of a 2.7-kb FYB cDNA that covered a contiguous open reading frame encoding 783-amino acid residues with a predicted molecular mass of 86 kDa (Fig. 1A). With the human cDNA as a probe to screen a murine T cell-hybridoma-derived cDNA library, we next isolated the murine isoform of FYB (Fig. 1A). The two deduced peptide sequences exhibited overall good degree of homology (76.8%), particularly in stretches that suggest putative functional domains (Fig. 1A and below). A blast homology search showed that FYB possessed an unique overall sequence, except for the presence of a SH3-related domain at the C-terminal end of the protein (residues 705–768 in the human sequence). The amino acid composition of FYB shows a high proportion of prolines (13.5%) and charged residues (12% lysines, 8.2% glutamic acid, and 6.7% aspartic acid). Kyte–Doolittle hydrophobicity analysis shows the protein to be relatively hydrophilic (data not shown). Several proline residues are clustered as a type I SH3 recognition motif (Arg-Xaa-Pro-Xaa-Xaa-Pro) (residues 312–317) or type II (Pro-Xaa-Xaa-Pro-Xaa-Arg) motifs (residues 237–242, 357–362, and 417–422) (28). There are 16 tyrosine residues, one of which is within a YEDI motif that matches the binding site for src SH2 domains (residues 462–465) (29). Of note, two lysine/glutamic acid-rich clusters (29 of 40 residues) are also found with similarity to bipartide nuclear localization motifs KR/RR-X11–12-KK/RK found in nuclear proteins such as p53, c-Rel, c-jun, and RNA polymerase I (30) (data not shown). Interestingly, although several residues in this region differ between human and murine, the substitutions represent conservative changes (i.e., a charged arginine replaces lysine).
Figure 1.
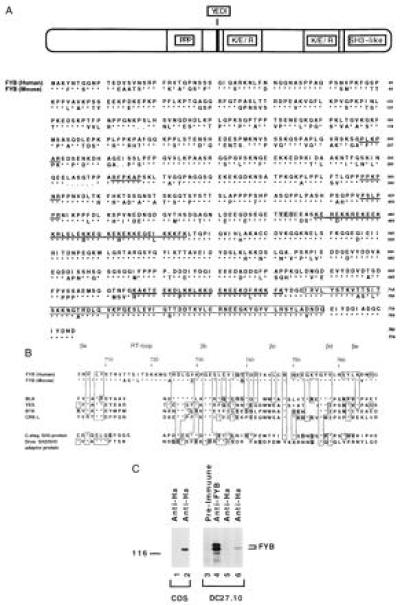
Sequence and structural motifs of FYB. (A) Overall amino acid sequence of human (upper sequence) and murine (lower sequence) FYB cDNAs. Representation depicts overall structure and the location of potential motifs that include a proline-rich region followed by a YEDI motif, two putative lysine/glutamic acid/arginine-rich nuclear localization motifs, and a SH3-like domain. Amino acid sequences of human and murine FYB (783 and 774 residues, respectively) show the position of the type I and type II proline-rich SH3 domain recognition motifs (underlined with bold line), the YEDI motif (indicated within shaded box), putative nuclear localization motifs (underlined with double line), and the C-terminal SH3-like domain (indicated within open box). (B) Schematic comparison of the FYB SH3-like domain with other SH3 domains. SH3-like domain shows particular homology to the SH3 domains of the protein-tyrosine kinases such as B lymphocyte kinase, p60yes, Bruton’s/agammaglobulinemia tyrosine kinase, and CRK-like protein (CRKL). Similarity is also seen with SH3 domain of a C. elegans SH3-carrying-protein and a Drosophila SH2/SH3 adaptor protein. Conserved SH3 domain residues include the βa region valine (residue 707), a RT loop tyrosine (residue 709), aspartic acid (residue 726), and a glycine (residue 732), a βd region glycine-tyrosine (residues 756–757), and a tyrosine (residue 762) proximal to βe region. (C) FYB cDNA clone generates polypeptide recognized by anti-FYB antiserum. HA-tagged FYB cDNA was expressed in COS (lane 2) and DC27.10 (lane 6) cells by using electroporation. Cell lysates were subjected to immunoprecipitation using anti-HA (lanes 1, 2, 5, and 6) or anti-FYB (lane 4) and precipitates were immunoblotted with anti-FYB serum.
The SH3-like domain shows particular homology to the SH3 domains of the protein-tyrosine kinases such as B lymphocyte kinase, p60yes, and Bruton’s/agammaglobulinemia tyrosine kinase and the SH3 domains of CRK-like protein (CRKL) (Fig. 1B). The βb to βe region shows some 40% identity with the B lymphocyte kinase SH3 domain. Similarity is also seen with SH3 domains of an SH3 carrying Caenorhabditis elegans protein and a Drosophila SH2/SH3 adaptor protein. Hallmark residues of SH3 domains such as the βa region valine (residue 707), a RT loop tyrosine (residue 709), aspartic acid (residue 726), and a glycine (residue 732), a βd region glycine-tyrosine (residues 756–757), and a tyrosine (residue 762) proximal to βe region are conserved (28). Interestingly, the FYB SH3-like domain differs from a canonical SH3 domain due to the presence of an extended RT loop that is some six to eight residues larger than that observed in other domains. Further, it is missing a conserved tryptophan within the βc region and a conserved proline in the βd–βe intervening region (therefore termed SH3-like domain). These distinct features could alter the binding characteristics of the domain.
Expression of a HA epitope-tagged full-length FYB cDNA [FYB(Ha)] in COS or DC27.10 cells followed by precipitation by anti-HA mAb and anti-FYB blotting showed that the 120-kDa protein is recognized by the anti-FYB serum (Fig. 1C, lanes 2 and 6, respectively). In both cells, the expressed protein comigrated with the lower band of the doublet precipitated by the anti-FYB serum from cell lysates (lane 4), suggesting that our cDNA encodes the 120-kDa form of FYB. The discrepancy between the predicted molecular mass and its migration on SDS/PAGE is likely due to numerous prolines and charged residues in the protein, as has been noted for other proteins such as SLP-76 and GABA-1 (18, 31). We have shown (15) that the p120 and p130 isoforms are related to one another.
Northern blot analysis revealed hybridization to a 4.5-kb mRNA band that is restricted to T cell and myeloid cells (Fig. 2). High expression was found in thymus and peripheral blood lymphocytes, with no expression in nonhematopoietic cells (Fig. 2 Top). Lower expression was noted in spleen than in thymus or peripheral blood cells. Among lymphoid cells, FYB was expressed in T cells (purified peripheral T cells, Jurkat cells, and HPB-ALL cells) and the myeloid cell line HL-60 (Middle) but, surprisingly, not in purified peripheral B cells or various B cell lines (Ramos and Daudi cells). Reprobing of the same blot with a G3PDH probe revealed equivalent amounts of RNA in all lanes (data not shown). The pattern of expression in lymphoid cells determined by Northern blotting matched that detected by immunoblotting with the antiserum (Fig. 2 Bottom).
Figure 2.
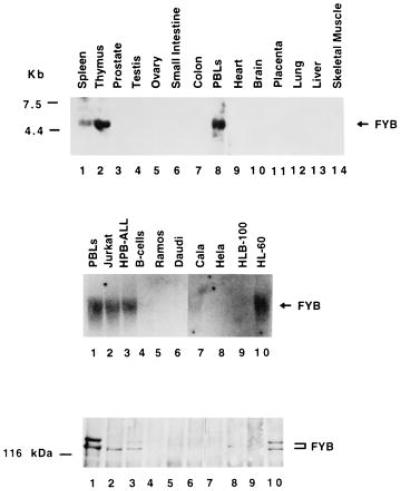
Expression of FYB is restricted to T and myeloid cells. (Top) Northern blot hybridization with the human FYB cDNA clone against CLONTECH poly(A)+ RNA purified from spleen (lane 1), thymus (lane 2), prostate (lane 3), testis (lane 4), ovary (lane 5), small intestine (lane 6), colon (lane 7), peripheral blood lymphocytes (lane 8), heart (lane 9), brain (lane 10), placenta (lane 11), lung (lane 12), liver (lane 13), and skeletal muscle (lane 14). (Middle) Hybridization of FYB cDNA against total RNA purified from human peripheral blood lymphocyte-purified T cells (lane 1), Jurkat cells (lane 2), HPB-ALL cells (lane 3), tonsil-purified B-cells (lane 4), Ramos cells (lane 5), Daudi cells (lane 6), lung cell line Cala (lane 7), uterine cell line Hela (lane 8), breast cell line HLB-100 (lane 9), and myeloid cell line HL-60 (lane 10). (Lower) Parallel immunoblot analysis of cells from Middle with anti-FYB antiserum.
We have reported (14, 15) that FYB associates with the src kinase p59fyn. To confirm that our cDNA clone encoded for a protein that associates with Fyn, FYB and p59fyn(T) were coexpressed in COS cells and assessed for an ability to associate. As seen in Fig. 3, precipitations against FYB and FYN reciprocally coprecipitated each other, as determined by immunoblotting analysis using anti-phosphotyrosine (lanes 4 and 8) and anti-fyn antibodies (lane 16). Further, anti-phosphotyrosine blotting detected FYB only in the presence of the Fyn kinase showing that FYB can act as a substrate for Fyn (lanes 6 and 8 versus 10 and 12). Lck phosphorylated the substrate to a much lesser extent than Fyn (data not shown).
Figure 3.
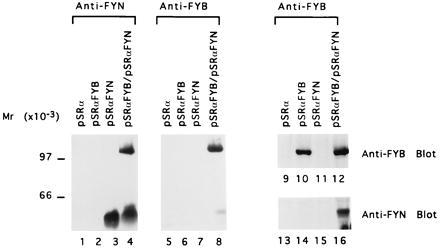
FYB and p59fyn(T) associate in COS cells. COS cells were transfected with pSRα vector control (lanes 1, 5, 9, and 13), pSRα-FYB(Ha) (lanes 2, 6, 10, and 14), pSRαFyn (lanes 3, 7, 11, and 15), or pSRα-FYB(Ha) and pSRαFyn (lanes 4, 8, 12, and 16). Cell lysates were incubated with anti-Ha mAb to precipitate FYB or with anti-fyn as indicated, precipitates were separated by SDS/PAGE, and samples were analyzed with anti-phosphotyrosine (lanes 1–8), anti-FYB (lanes 9–12), or anti-Fyn (lanes 13–16).
The restricted pattern of expression of FYB suggests that it might participate in T cell signaling. Recent studies have defined a TcRζ/CD3-initiated signaling cascade involving proteins ZAP-70, Vav, and SLP-76 (9, 10, 18, 19, 21). Overexpression of these proteins augments IL-2 transcription, an effect that for SLP-76 depends on the integrity of its SH2 domain (20). Interestingly, this domain binds a protein in the 120-kDa range (18, 20). As seen in Fig. 4A, anti-SLP-76 coprecipitated a 120/130-kDa protein doublet from activated T cells that corresponded to FYB, as detected by anti-phosphotyrosine (lane 2) and anti-FYB (lane 4) blotting. In fact, FYB was the principal phosphoprotein coprecipitated with SLP-76. Precipitation with anti-FYB served as a control (lane 5). Conversely, GST–FYB fusion protein preferentially coprecipitated SLP-76 from T cell lysates, as detected by anti-phosphotyrosine (lane 7) and anti-SLP-76 (lane 9) immunoblotting.
Figure 4.
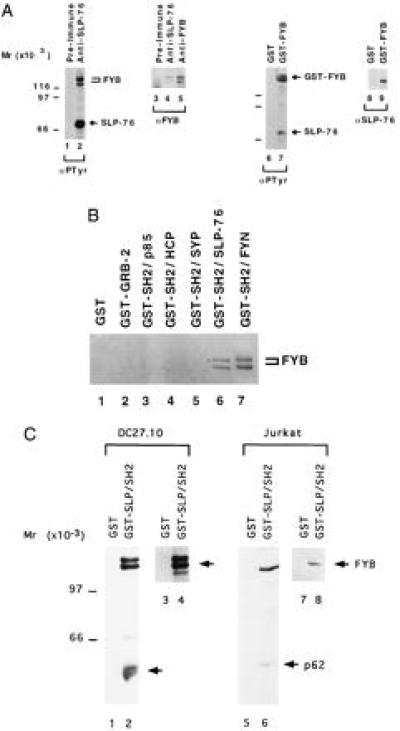
FYB associates with SLP-76 SH2 in T cells. (A) FYB associates with SLP-76. Lysates from DC27.10 cells were subjected to precipitation with preimmune (lanes 1 and 3), anti-SLP-76 (lanes 2 and 4), anti-FYB (lane 5), or GST (lanes 6 and 8) and GST–FYB (lanes 7 and 9) fusion proteins. Precipitates were then analyzed by immunoblotting with anti-phosphotyrosine mAb 4G10 (lanes 1, 2, 6, and 7), anti-FYB (lanes 3–5), or anti-SLP-76 (lanes 8 and 9). (B) SLP-76 SH2 domain specifically recognizes FYB from T cells. DC27.10-derived lysates were incubated with GST fusion proteins as indicated: GST alone (lane 1), GRB-2 (lane 2), SH2-p85 (lane 3), SH2-HCP (lane 4), SH2-SYP (lane 5), SH2-SLP-76 (lane 6), and SH2-FYN (lane 7). Precipitates were separated by SDS/PAGE and analyzed by immunoblotting with anti-FYB. (C) SLP-76 SH2 domain preferentially binds to FYB from T cells. Cell lysates from DC27.10 cells (lanes 1–4) and Jurkat (lanes 5–8) were incubated with GST (lanes 1, 3, 5, and 7) or GST–SH2-SLP-76 fusion protein (lanes 2, 4, 6, and 8) and subjected to immunoblotting analysis with anti-phosphotyrosine (lanes 1, 2, 5, and 6) or anti-FYB (lanes 3, 4, 7, and 8) serum.
SLP-76 SH2 binding to FYB was shown by GST–SH2-SLP-76 fusion protein precipitation of FYB from cell lysates, as detected by anti-FYB blotting (Fig. 4B, lane 6). GST, GST–GRB-2, and the GST–SH2 domains of p85, HCP, and SYP served as negative controls (lanes 1–5), and the SH2 domain of Fyn served as a positive control (lane 7). As shown by others (20), the SLP-76-SH2 domain precipitates several proteins at 120/130, 70, 62, and occasionally 38 kDa. Anti-phosphotyrosine blotting against GST–SLP-76-SH2 domain precipitates showed that FYB is the most prominent phosphoprotein among these ligands in both the DC27.10 murine and the human Jurkat T cell lines (Fig. 4C, lanes 2 and 6, respectively).
We next assessed whether FYB was capable of up-regulating anti-CD3-induced IL-2 production. For this, we used the T cell hybridoma DC27.10, which produces IL-2 in response to anti-CD3 (32). Anti-CD3 ligation (at 10−10 and 10−9 M 2C11 mAb) of FYB-transfected cells induced IL-2 secretion by 3- to 5-fold when compared with mock-transfected cells (Fig. 5). Importantly, the observed increase was dependent on the levels of FYB being expressed, with maximum potentiation at 1.0 μg of DNA. At this level, anti-FYB blotting of cell lysates revealed a 2.0- to 2.5-fold increase in FYB expression from transfection (data not shown). This initial increase in IL-2 secretion was followed by a decrease at higher levels of transfected FYB cDNA (i.e., 4 μg), an observation accompanied by an increase in cell death (data not shown). Potentiation was also observed in response to anti-CD3 plus phorbol 12-myristate 13-acetate (Fig. 5). These data provide the important observation that FYB is functionally linked to TcRζ/CD3 induction of IL-2 secretion in T cells.
Figure 5.
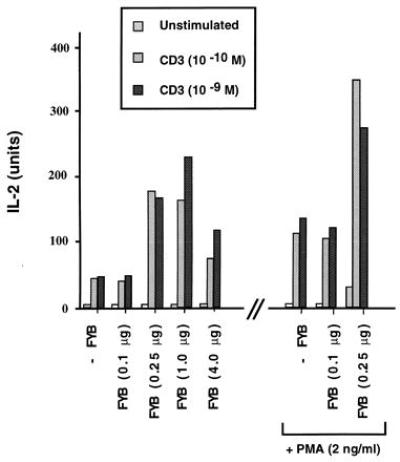
FYB up-regulates TcRζ/CD3-induced IL-2 production. FYB transfected DC27.10 cells showed a 3- to 5-fold augmentation of IL-2 production in response to anti-CD3 alone or anti-CD3 plus phorbol ester. FYB cDNA (at 0.1, 0.25, 1.0, or 4.0 μg per 106 cells) or mock-transfected DC27.10 cells (32) were incubated overnight with medium alone, anti-CD3, or anti-CD3 mAb plus phorbol 12-myristate 13-acetate, as indicated. Cell culture supernatants were assayed for IL-2 by a bioassay using CTLL-2 cells.
The identification of FYB as a novel intracellular signaling protein that is restricted to T and myeloid cells, associates with FYN and SLP-76, and up-regulates IL-2 secretion in T cells points to a role in T cell activation. Recent studies have implicated ZAP-70, Vav, and SLP-76 in TcRζ/CD3 signaling (9, 10, 18, 19, 21). Overexpression of Vav or SLP-76 cooperates in augmenting TcR-mediated IL-2 transcription (19, 20), although binding per se is not required in all T cells (10). SLP-76 potentiation of transcription depends on the integrity of its SH2 domain (20). It is in this regard that FYB may serve as a mediator of SLP-76 function, or vice versa. Consistent with this, both are expressed primarily in T and myeloid cells (Fig. 2 and refs. 9 and 15) and augment IL-2 secretion (Fig. 5). However, unlike with SLP-76, we were unable to show an effect of FYB on NF-AT transcriptional activity (data not shown), suggesting that FYB may influence IL-2 secretion by a different mechanism, possibly by acting on other transcriptional factors, or at a posttranscriptional level. Clearly, further experiments are needed to clarify the interrelationships of Vav, SLP-76, and FYB and their role in the signaling network initiated by the TcRζ/CD3 complex.
In addition to SLP-76, FYB is phosphorylated by and interacts with the SH2 domain of FYN. p59fyn has been implicated in T cell proliferation and cytokine production (33–35). We previously showed that TcR ligation induces FYB phosphorylation, which remains stable for at least 6 h of extended kinetics (15). Further, FYB phosphorylation is reduced in T cells from Fyn−/− mice (15). Hence, one consequence of FYN activation would be the phosphorylation of FYB to create the sites needed for SLP-76 binding. Alternatively, FYB could bring FYN in close proximity with SLP-76, thereby allowing phosphorylation of SLP-76. Although p59fyn does not facilitate Vav/SLP-76 complex formation (10), it could act to regulate some other aspect of SLP-76 and FYB function or provide docking sites for other proteins. In this context, the tyrosine kinase Pyk2 has been reported to be selectively regulated by Fyn (36).
Given its unique structure, the molecular basis of FYB signaling remains to be elucidated. Structurally, human and murine FYB are conserved, with some divergence found mostly near the N-terminal part of the molecule, a region with no apparent homology to other proteins. Conservation was most apparent in the potential functional motifs such as the SH3-like domain and the putative nuclear localization motifs, suggesting that FYB may localize to both the cytoplasm and nucleus. Further work is required to determine the molecular basis of the difference between the 120-kDa and 130-kDa forms and the targets of FYB in the signaling cascade.
Acknowledgments
C.E.R. is a Scholar of the Leukemia Society of America. A.J.D. is the recipient of the Claudia Adams Barr Investigator Award.
ABBREVIATIONS
- FYB
FYN-binding protein
- SLP-76
SH2-domain-containing leukocyte protein-76
- ZAP-70
ζ-associated protein-70
- GST
glutathione S-transferase
- HA
hemagglutinin
- IL-2
interleukin 2
Note Added in Proof
Musci and Koretzky (37) have recently cloned 130-kDa SLP-76 associated protein (SLAP-130), which is virtually identical to our 120-kDa isoform of FYB except for two conserved substitutions at residues 275 (V → L) and 393 (A → P) and a nonconserved substitution at residue 273 (P → L).
Footnotes
References
- 1.Rudd C E, Janssen O, Cai Y C, da Silva A J, Raab M, Prasad K V. Immunol Today. 1994;15:225–234. doi: 10.1016/0167-5699(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 2.Mustelin T. Immunity. 1994;1:351–356. doi: 10.1016/1074-7613(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 3.Wange R L, Samelson L E. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 4.Chan A C, Iwashima M, Turck C W, Weiss A. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 5.Iwashima M, Irving B A, van Oers N S C, Chan A C, Weiss A. Science. 1994;263:1136–1139. [PubMed] [Google Scholar]
- 6.Ravichandran K S, Lee K K, Songyang Z, Cantley L C, Burn P, Burakof S J. Science. 1993;262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- 7.Raab M, Yun-Cai C, Bunnell S C, Heyeck S D, Berg L J, Rudd C E. Proc Natl Acad Sci USA. 1995;92:8891–8895. doi: 10.1073/pnas.92.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raab M, Yamamoto M, Rudd C. Mol Cell Biol. 1994;14:2862–2870. doi: 10.1128/mcb.14.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardenburg J B, Fu C, Jackman J K, Flotow H, Wilkinson S E, Williams D H, Johnson R, Kong G, Chan A C, Findell P R. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 10.Raab M, da Silva A J, Findell P R, Rudd C E. Immunity. 1997;6:1–11. doi: 10.1016/s1074-7613(00)80422-7. [DOI] [PubMed] [Google Scholar]
- 11.Weiss A, Koretzky G, Schatzman R C, Kadlecek T. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulbins E, Coggeshall K M, Baier G, Katzav S, Burn P, Altman A. Science. 1993;260:822–825. doi: 10.1126/science.8484124. [DOI] [PubMed] [Google Scholar]
- 13.Bustelo X R, Ledbetter J A, Barbacid M. Nature (London) 1992;356:68–74. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- 14.da Silva A J, Janssen O J, Rudd C E. J Exp Med. 1993;178:2107–2113. doi: 10.1084/jem.178.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva A J, Rosenfield J M, Mueller I, Bouton A, Hirai H, Rudd C E. J Immunol. 1997;158:2007–2016. [PubMed] [Google Scholar]
- 16.Donovan J A, Wange R L, Langdon W Y, Samelson L E. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 17.Reedquist K A, Fukazawa T, Druker B, Panchamoorthy G, Shoelson S, Band H. Proc Natl Acad Sci USA. 1994;91:4135–4139. doi: 10.1073/pnas.91.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackman J K, Motto D G, Sun Q, Tanemoto M, Turck C W, Peltz G A, Koretzky G A, Findell P R. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Motto D G, Koretzky G A, Weiss A. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 20.Motto D G, Ross S E, Wu J, Hendriks-Taylor L R, Koretzky G A. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onodera H, Motto D G, Koretzky G A, Rothstein D M. J Biol Chem. 1996;271:22225–22230. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- 22.Cooke M P, Perlmutter R M. The New Biologist. 1989;1:66–74. [PubMed] [Google Scholar]
- 23.Gauen L K T, Kong A-N T, Samelson L E, Shaw A S. Mol Cell Biol. 1992;12:5438–5446. doi: 10.1128/mcb.12.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva A J, Yamamoto M, Zalvan C H, Rudd C E. Mol Immunol. 1992;29:1417–1425. doi: 10.1016/0161-5890(92)90215-j. [DOI] [PubMed] [Google Scholar]
- 25.Tsygankov A, Broker B, Fargnoli J, Ledbetter J, Bolen J. J Biol Chem. 1992;267:18259–18262. [PubMed] [Google Scholar]
- 26.Cooke M P, Abraham K M, Forbush K A, Perlmutter R M. Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins M K. Immunity. 1994;1:443–448. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 28.Mayer B J, Eck M J. Curr Biol. 1995;5:364–367. doi: 10.1016/s0960-9822(95)00073-x. [DOI] [PubMed] [Google Scholar]
- 29.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 30.Dingwall C, Laskey R A. Trends Biochem Sci. 1992;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 31.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. Nature (London) 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 32.Cai Y-C, Cefai D, Schneider H, Raab M, Nabavi N, Rudd C E. Immunity. 1995;3:1–10. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 33.Veillette A, Davidson D. Trends Genet. 1992;8:61–66. doi: 10.1016/0168-9525(92)90351-4. [DOI] [PubMed] [Google Scholar]
- 34.Appleby M, Gross J, Cooke M, Levin S, Qian X, Perlmutter R. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 35.Stein P, Lee H, Rich S, Soriano P. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 36.Qian D, Lev S, van Oers N, Dikic I, Schlessinger J, Weiss A. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musci, M. A. & Koretsky, G. A., (1997) J. Biol. Chem., in press.


