Abstract
PURPOSE We performed a meta-analysis of randomized placebo-controlled trials of nonergot dopamine agonists (NEDAs) for the treatment of restless legs syndrome.
METHODS A systematic literature search was conducted through July 2007. The primary outcome measures assessed were the percentage of responders to medication as determined by the Clinical Global Impression-Improvement (CGI-I) scale and the adjusted mean change in the International Restless Legs Syndrome Study Group Scale (IRLS) score from baseline compared with placebo. Meta-regression analysis was performed to evaluate the impact of study duration on the primary outcomes. Safety endpoints were also evaluated.
RESULTS A total of 14 trials (n = 3,197 subjects) were included in the meta-analysis. NEDA use resulted in greater response as measured by the CGI-I scale (relative risk [RR] 1.36; 95% CI, 1.24 to 1.49; P <.001), and greater reductions in IRLS scores (weighted mean difference [WMD] −4.93; 95% CI, −6.42 to −3.43; P <.001) from baseline vs placebo. Meta-regression analysis showed an inverse relationship between study duration and reduction in IRLS score. NEDAs were associated with a significant risk of adverse events (including nausea, dizziness, somnolence, and fatigue.)
CONCLUSIONS Use of NEDAs in patients with moderate-to-severe restless legs syndrome results in significant reductions in symptom severity, but a significant portion of patients will discontinue their use as a result of adverse events.
Keywords: Restless legs syndrome, dopamine agonists, nonergot, meta-analysis
INTRODUCTION
Restless legs syndrome is a sensorimotor disorder affecting approximately 12% of the adult population.1,2 Although women and the elderly have a higher prevalence of restless legs syndrome, there is conflicting evidence of the impact ethnicity has on prevalence.2–5 Restless legs syndrome is characterized by an irresistible urge to move the legs, which may begin or worsen during periods of rest or inactivity and often affects sleep.6 Physical activity, such as walking or stretching, often relieve these urges.
The Medical Advisory Board of the Restless Legs Syndrome Foundation developed a treatment algorithm for restless legs syndrome in 2004.7 For patients with daily symptoms, treatment options included dopamine agonists, anticonvulsants (eg, gabapentin), and opioids.7
Dopamine agonists, particularly nonergot dopamine agonists (NEDAs), have become the mainstay of therapy for patients with daily symptoms of restless legs syndrome. NEDAs are generally preferred to ergot dopamine agonists (eg, pergolide, cabergoline), which have been associated with clinically important heart valve damage and resultant regurgitation.8–10
Numerous clinical trials have evaluated the efficacy and safety of NEDAs for restless legs syndrome with conflicting results. Most of these studies lacked adequate power to detect potential NEDA benefits and risks. We therefore performed a meta-analysis and meta-regression analysis to evaluate the effect of NEDAs on efficacy, withdrawal resulting from adverse effects, and overall risk of adverse effects in patients with restless legs syndrome.
METHODS
Study Selection
Included trials had to (1) be randomized trials of a NEDA, (2) be placebo controlled, and (3) report data on either the percentage of responders to medication (defined as “much improved” or “very much improved”) as determined by the Clinical Global Impression-Improvement (CGI-I) scale or the adjusted mean change in International Restless Legs Syndrome Study Group Scale (IRLS)11 score from baseline. The CGI-I scale is a clinician-assessed 7-point score that ranges from 1 (“very much improved”) to 7 (“very much worse”). A response to restless legs syndrome treatment is defined as having a patient who is either “much improved” (score of 2) or “very much improved” (score of 1). The IRLS is a validated scale containing 10 equally weighted questions, each rated from 0 to 4 (with a maximum score of 40), with lower scores signifying less-severe disease. Tolerability was assessed by evaluating study withdrawal rates that were due to adverse events and incidence of commonly reported adverse events (including headache, nausea, dizziness, somnolence, and fatigue).
Using the prespecified inclusion criteria, we conducted a systematic literature search of MEDLINE, EMBASE, CINAHL, and Web of Science, from the earliest possible date through July 2007, for all relevant articles published in English. We used the following Medical Subject Headings (MeSH) and keywords: “pramipexole,” “ropinirole,” “rotigotine,” “sumanirole,” and “dopamine agonist” in combination with “restless legs syndrome” and “RLS.” Results were limited to trials in humans. We manually searched references from reports of clinical trials or review articles to identify additional relevant studies. In addition, we reviewed the Food and Drug Administration (FDA) Web site (http://www.fda.gov; accessed August 7, 2007) and the European Agency for the Evaluation of Medicinal Products (EMEA) Web site (http://www.emea.eu.int; accessed August 7, 2007), as well as the Web sites for the manufacturers of the following agents for additional study data: pramipexole (Mirapex, Boehringer-Ingelheim, http://www.boehringer-ingelheim.com; accessed August 7, 2007), ropinirole (Requip, Glaxo-Smithkline, http://us.gsk.com; accessed August 7, 2007), rotigotine (Neupro, Schwarz Pharma, http://www.schwarzpharma.com; accessed August 7, 2007), and sumanirole (Pfizer, http://www.pfizer.com; accessed August 7, 2007). Two investigators (W.L.B., C.I.C.) reviewed all potentially relevant articles independently.
Validity Assessment
The Jadad scale was calculated by 2 investigators (W.L.B., C.I.C.) and used to assess the methodological quality of included trials.12 This rating scale uses the following quality assessment criteria—use of and methods for generating randomization, use of and methods for double-blinding, and description of patient withdrawals and dropouts—as these are inherent controls of bias. One point was given for each satisfied criterion. An aggregate score between 0 and 5 was calculated for each included trial (0 = weakest, 5 = strongest), with trials scoring less than 3 deemed to have lower methodological quality.
Data Abstraction
Two investigators (W.L.B., C.I.C.) independently abstracted all data using a standardized data abstraction tool, and disagreements were resolved by a third party (C.M.W.). Data related to each efficacy and safety endpoint were sought from the constituent studies. In cases where there was more than 1 published report on the same population or group of patients, the most recent article was selected, although previous articles were reviewed to supplement missing data where applicable. For studies with both an open-label and double-blind treatment portion, only the double-blind portion was used in this meta-analysis, because unblinded or open-labeled trials may exhibit exaggerated treatment effects.
Statistical Analysis
Incidence of patient response, as measured by the CGI-I scale, was treated as a dichotomous variable. Weighted averages were reported as relative risk (RR) with associated 95% confidence intervals (CI). A DerSimonian and Laird random-effects model was used in calculating relative risk and 95% confidence intervals for this and all subsequent analyses.13 Risk difference (as well as number needed to treat) was calculated for response to the CGI-I scale, whereas the adjusted mean change in IRLS score from baseline was calculated as the difference between the adjusted mean IRLS score in the NEDA and placebo groups and reported as a weighted mean difference (WMD) and its 95% confidence interval. In addition, withdrawals caused by adverse events and incidence of prespecified adverse events were reported as relative risk and 95% confidence.
Statistical heterogeneity was addressed using the Q statistic (P <.10 was considered representative of significant statistical heterogeneity) and the I2 statistic, which assesses the degree of inconsistency across studies and ranges from 0% to 100%, with the higher percentage representing a higher likelihood of heterogeneity.14 Whereas categorization of values for I2 may not be appropriate in all situations, I2 values of 25%, 50%, and 75% have been regarded as representative of low, medium, and high statistical heterogeneity, respectively. We used visual inspection of funnel plots, Egger’s weighted regression statistics, and the trim and fill method to assess for publication bias.15 The trim and fill method uses funnel plot symmetry to estimate the number of “missing” studies and the magnitudes of their effects. It reestimates the overall effect size after imputing potentially missing studies into the meta-analysis to determine whether the results of the original analysis were replicated. Statistics were performed using StatsDirect statistical software, version 2.4.6 (StatsDirect Ltd, Cheshire, England), and MIX statistical software (Bax L, Yu LM, Ikeda N, Tsuruta N, Moons KGM. MIX: Comprehensive Free Software for Meta-analysis of Causal Research Data - Version 1.54. 2006; at http://www.mix-for-meta-analysis.info). P <.05 was considered statistically significant, except where otherwise indicated.
To evaluate the effect of heterogeneity between included studies on the meta-analysis’ conclusions, we performed the following subgroup and sensitivity analysis: (1) studies of less than 12 weeks’ follow-up and those with greater than or equal to 12 weeks’ follow-up were analyzed separately; (2) unpublished studies were excluded; (3) studies not using or reporting intention-to-treat principles were excluded; (4) studies with a Jadad score <3 were excluded; and (5) a fixed-effect model (Mantel-Haenszel fixed-effect model)16 was chosen for the primary analyses.
We further evaluated the impact of time on the primary outcomes using random-effects meta-regression estimation via iterative maximum likelihood (REML). Meta-regression analysis is an indirect way to examine the possibility of effect modification by duration of treatment. The logarithmically transformed relative risks in the CGI-I scale and change in IRLS score were examined. Meta-regression analysis was performed with SPSS, version 11.0 (SPSS, Chicago, Illinois).
RESULTS
Study Characteristics
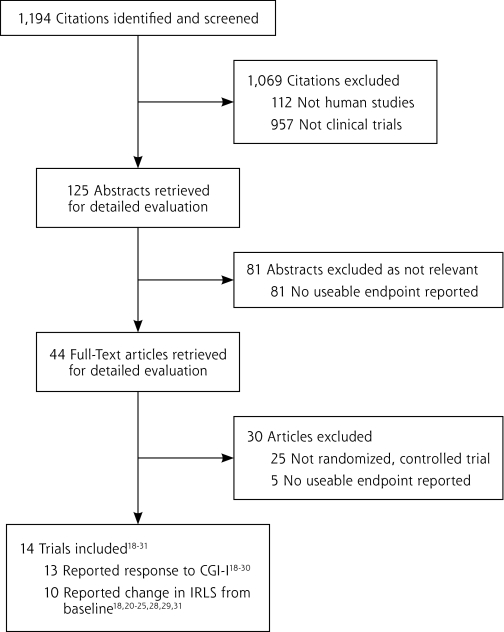
The initial search yielded 1,194 potential literature citations (Figure 1 ▶). Of those, 1,150 were excluded through review of abstracts, leaving 44 articles for full publication review. We found 14 studies (n = 3,197 subjects) that conformed to our inclusion criteria (Table 1 ▶).17–30 We excluded 30 trials from analysis, including those that had an open-label design,31–33 a cross-over design,34–38 or were withdrawal studies (meaning that the effect of continuing treatment vs withdrawing treatment after a double-blind treatment period was assessed).39,40
Figure 1.
Flow diagram of trial identification, inclusion, and exclusion.
IRLS = International Restless Legs Syndrome Study Group Scale score; CGI-I = Clinical Global Impression-Improvement scale.
Table 1.
Clinical Trial Characteristics
| Reference | Design | N | Baseline IRLS Mean+SD | Follow-up (weeks) | Drug/Dose | Adjusted Mean Difference IRLS Mean+SE | Treatment CGI-I Responder Rate (%) | Jadad Score |
| Allen (2004)17 | P, R, DB, PC | 55 | N/A | 12 | Ropinirole 0.25–4.0 mg/d | –1.2±2.1 | Ropinirole=17/32 (53) Placebo=17/33 (52) |
3 |
| Stiasny-Kolster (2004)18 | P, R, DB, PC | 63 | 25.0 ± 5.0 | 1 | Rotigotine 1.125–4.5 mg/24 h | N/A | Rotigotine=34/49 (69) Placebo=6/14 (43) |
4 |
| Trenkwalder (2004)19 | P, R, DB, PC | 284 | 24.4 ± 5.8 | 12 | Ropinirole 0.25–4.0 mg/d | –3.0±1.1 | Ropinirole=78/146 (53) Placebo=56/138 (41) |
4 |
| Walters (2004)20 | P, R, DB, PC | 267 | 23.6 ± 5.9 | 12 | Ropinirole 0.25–4.0 mg/d | –2.5±1.1 | Ropinirole=78/131 (60) Placebo=53/135 (39) |
5 |
| Bogan (2006)21 | P, R, DB, PC | 381 | 22.0 ± 5.0 | 12 | Ropinirole 0.25–4.0 mg/d | –3.7±0.9 | Ropinirole=137/187 (73) Placebo=109/193 (56) |
5 |
| Inoue (2006)22 | P, R, DB, PC | 41 | N/A | 6 | Pramipexole 0.125–0.75 mg/d | –11.5±3.0 | Pramipexole=16/20 (80) Placebo=11/21 (52) |
3 |
| Partinen (2006)23 | P, R, DB, PC | 107 | 22.7 ± 4.1 | 3 | Pramipexole 0.125–0.75 mg/d | –9.2±1.7 | Pramipexole=65/86 (76) Placebo=9/21 (43) |
3 |
| Winkelman (2006)24 | P, R, DB, PC | 339 | 23.4 ± 5.1 | 12 | Pramipexole 0.25–0.75 mg/d | –4.3±1.1 | Pramipexole=183/254 (72) Placebo=44/85 (52) |
4 |
| Garcia-Borreguero (2007)25 | P, R, DB. PC | 270 | 25.4 | 8 | Sumanirole 0.5–4.0 mg/d | N/A | Sumanirole=104/212 (49) Placebo=26/51 (51) |
3 |
| Oertel (2007)26 | P, R, DB. PC | 341 | 28.0 ± 6.3 | 6 | Rotigotine 0.5–4 mg/24 h | N/A | Rotigotine=212/280 (76) Placebo=29/53 (55) |
5 |
| Oertel (2007)27 | P, R, DB. PC | 338 | 24.7 ± 5.2 | 6 | Pramipexole 0.125–0.75 mg/d | –6.6±1.1 | Pramipexole=141/224 (63) Placebo=37/114 (32) |
3 |
| RRL10001328 | P, R, DB, PC | 359 | 26.0 ± 4.4 | 12 | Ropinirole 0.5–6.0 mg/d | –4.1±1.0 | Ropinirole=124/175 (71) Placebo=92/184 (50) |
4 |
| ROP10189229 | P, R, DB, PC | 298 | 23.5 ± 6.2 | 12 | Ropinirole 0.25–4.0 mg/d | N/A | Ropinirole=92/152 (61) Placebo=76/146 (52) |
4 |
| SKF10146830 | P, R, DB, PC | 54 | 26.6 ± 5.3 | 7 | Ropinirole 0.25–4.0 mg/d | –9.9±2.9 | N/A | 3 |
CGI-I = Clinical Global Impression – Improvement scale; DB = double-blind; IRLS = International Restless Legs Syndrome Study Group scale; N/A=not available; P=prospective; PC=placebo control; R=randomized.
In the included trials, patients received ropinirole in 7 trials (n = 1,698 subjects),17,19–21,28–30 pramipexole in 4 trials (n = 825 subjects),22–24,27 rotigotine in 2 trials (n = 404 subjects),18,26 and sumanirole in 1 trial (n = 270 subjects).25 Thirteen trials provided CGI-I scale responder rates,17–29 and 10 provided mean change in IRLS score from baseline.17,19–24,27,28,30 Enrollment ranged from 41 to 381 patients. The mean patient age ranged from 51 to 76 years, and approximately two-thirds were female. Duration of follow-up ranged from 1 to 12 weeks. All trials enrolled patients with similar disease severity (baseline IRLS scores ranged between 22 and 28).
Quantitative Data Synthesis
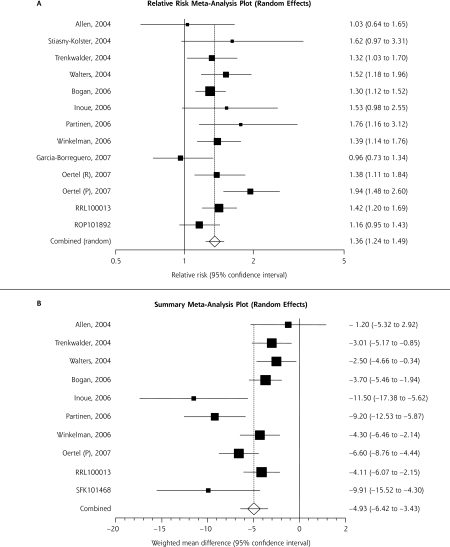
Upon meta-analysis, NEDA use resulted in a statistically significantly greater response to therapy, as measured by the CGI-I scale compared with placebo (Figure 2A ▶). This corresponded to a risk difference of 0.18 (95% CI, 0.13–0.23; P <.001) and a number needed to treat of 6 (95% CI, 5–8). A statistically significant 5-point reduction in the adjusted mean change in the IRLS score from baseline with the NEDAs compared with placebo was also seen (Figure 2B ▶). Statistical heterogeneity was suggested for the adjusted mean change in the IRLS score (Q statistic, P value <.001; I2 = 69%), although it was nonsignificant for the CGI-I scale (Q statistic, P = 0.12; I2 = 33%). Review of the forest plots for each (Figure 2 ▶) shows that included studies were in general agreement on the positive effects of NEDAs, but not the magnitude of benefit.
Figure 2.
Nonergot dopamine agonist’s impact on response to clinical global impression-improvement scale (A) and International Restless Legs Syndrome Study Group Scale score (B).
CI=confidence interval.
Note: The squares represent individual studies and the size of the square represents the weight given to each study in the meta-analysis. Error bars represent 95% CIs. The diamond represents the combined results. The solid vertical line extending upwards from 0 (CGI) or 1 (IRLS) is the null value.
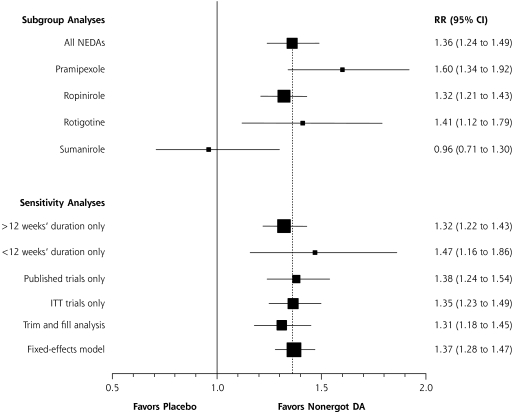
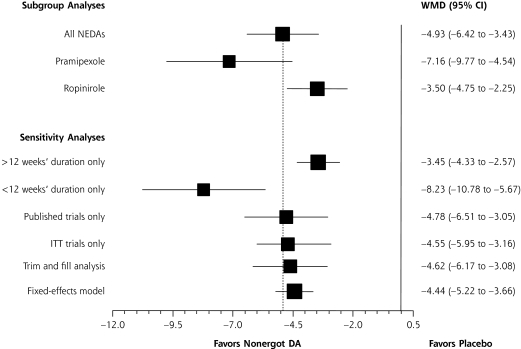
On visual inspection of the funnel plots, little asymmetry was noted for either the CGI-I scale or the IRLS score, suggesting a low risk of publication bias (not shown). The addition of theoretical studies using the trim and fill method did not significantly alter the study results (subgroup analysis, Figures 3 ▶ and 4 ▶). Egger’s weighted regression analysis confirmed the low-likelihood of publication bias for both the CGI-I and IRLS measures (P = .59 and .10, respectively).
Figure 3.
Subgroup and sensitivity analyses of nonergot dopamine agonists evaluating response to Clinical Global Impression-Improvement scale.
CI=confidence interval; DA = dopamine agonists; ITT = intention to treat; NEDA = nonergot dopamine agonist; RR = relative risk.
Note: The dashed vertical line represents the combined treatment effect for the original analysis.
Figure 4.
Subgroup and sensitivity analyses of nonergot dopamine agonists evaluating mean change in International Restless Legs Syndrome Study Group scale.
CI=confidence interval; DA = dopamine agonists; ITT = intention to treat; NEDA = nonergot dopamine agonist; WMD = weighted mean difference.
Note: The dashed vertical line represents the combined treatment effect for the original analysis.
Upon subgroup analysis, pramipexole, ropinirole, and rotigotine use resulted in a greater response than placebo, as measured by CGI-I scale (P <.01 for each), whereas sumanirole did not have a significant effect on CGI-I scale scores (P = .80) (Figure 3 ▶). Both pramipexole and ropinirole showed a statistically significant reduction in the adjusted mean change in the IRLS score from baseline as compared with placebo (P <.001 for each), but no study using either rotigotine or sumanirole was eligible for inclusion in the IRLS analysis (Figure 4 ▶). In sensitivity analysis the conclusions of the meta-analysis remained robust to methodological changes.
Upon meta-analysis of tolerability endpoints, patients receiving NEDAs were more likely to withdraw because of adverse events than those taking placebo (RR 1.35; 95% CI, 1.00–1.81; number needed to harm = 77; P = .048) (Table 2 ▶). When analyzed separately, patients receiving pramipexole, rotigotine, and sumanirole had no significant increase in withdrawal rates, whereas those receiving ropinirole had significantly higher rates of withdrawals caused by adverse events (P = .02). NEDAs as a class were found to significantly increase patients’ risk of nausea, dizziness, somnolence, and fatigue (P <.05 for all). A trend toward a higher risk of headaches was noted for all NEDAs (P = .09). When each drug was analyzed individually, pramipexole significantly increased nausea risk; ropinirole significantly increased nausea, dizziness, somnolence, and fatigue risk; and sumanirole significantly increased headache risk.
Table 2.
Adverse Events
| Group | W/D due to ADEs RR (95% CI) | Headache RR (95% CI) | Nausea RR (95% CI) | Dizziness RR (95% CI) | Somnolence RR (95% CI) | Fatigue RR (95% CI) |
| Dopamine agonists | 1.35 (1.00–1.81)a | 1.20 (0.98–1.47) | 3.25 (2.36–4.48)a | 1.47 (1.02–2.13)a | 1.94 (1.45–2.61)a | 1.37 (1.01–1.86)a |
| Pramipexole | 1.15 (0.49–2.69) | 0.99 (0.64–1.54) | 2.68 (1.51–4.76)a | 1.03 (0.51–2.07) | 1.55 (0.75–3.20) | 1.09 (0.64–1.85) |
| Ropinirole | 1.49 (1.06–2.10)a | 1.21 (0.95–1.54) | 3.95 (2.76–5.66)a | 1.72 (1.13–2.62)a | 2.03 (1.47–2.80)a | 1.72 (1.18–2.68)a |
| Rotigotine | 0.46 (0.08–2.58) | 1.34 (0.54–3.33) | 0.82 (0.16–4.24) | 0.58 (0.19–1.73) | N/A | 0.78 (0.32–1.92) |
| Sumanirole | 1.11 (0.06–19.45) | 2.68 (1.01–7.11)a | 4.77 (0.65–34.69) | 4.53 (0.62–33.04) | N/A | N/A |
ADEs=adverse drug events; CI=confidence interval; N/A = not available; RR = relative risk; W/D = withdrawal.
aP<.05.
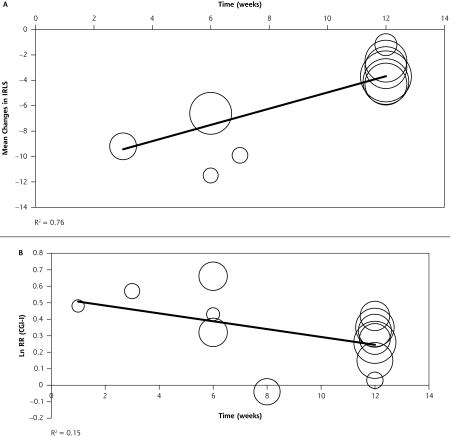
Random-effects meta-regression analysis showed a significant relationship between the study duration and the adjusted mean change in the IRLS score from baseline (P <.001) (Figure 5 ▶). Studies of a shorter duration showed more robust improvements in symptoms than those with longer durations. Approximately a 0.66-point lesser score reduction was seen for each additional study week. A similar relationship was not seen with response to treatment, according to the CGI-I score (P = .27) (Figure 5 ▶).
Figure 5.
Random-effects meta-regression evaluating effect of NEDAs on mean change in IRLS (A) and CGI-I (B) over time.
NEDAs = nonergot dopamine agonists; IRLS = International Restless Legs Syndrome Study Group Scale; CGI-I = Clinical Global Impression-Improvement; RR=relative risk.
Note: The circles represent individual studies, and the area of the circle is proportional to the weight of each study. The dark line represents the regression equation as represented by the following equation: (A) weighted mean difference = −11.3545 + 0.6574 × study duration; (B) LnRR=0.5304–0.0219 × study duration.
DISCUSSION
In this meta-analysis of 14 randomized, controlled trials, patients receiving NEDAs for treatment of restless leg syndrome showed significant improvement in their symptoms and disease severity as evidenced by improvements in CGI-I scale and IRLS scores from baseline compared with placebo. These beneficial effects must be weighed against a statistically significant increase in withdrawals resulting from adverse events, as well as an increased incidence of individual adverse events.
When analyzed separately, all NEDAs, with the exception of sumanirole, significantly improved response to treatment and reduced the IRLS score from baseline. Studies of sumanirole did not seem to find a beneficial effect on the CGI-I scale and did not report IRLS scores in a manner conducive to meta-analysis; however, only 1 study of sumanirole met the criteria for inclusion into our meta-analysis. In contrast, the effects on CGI-I scale and IRLS scores qualitatively seem more robust with pramipexole than the other NEDAs, but we could not determine the direct comparative efficacy between NEDAs in our meta-analysis. The qualitatively greater improvements with pramipexole could potentially be explained by the relatively short duration of pramipexole studies (3 to 6 weeks), because it is during this time the drug’s effects are most prominent.
Meta-regression analysis showed that the beneficial effects of NEDAs, in terms of improvements in IRLS scores from baseline, are most prominent during the first few weeks of therapy (P <.001). These effects appear to diminish somewhat in trials evaluating a 12-week treatment period. Results must be interpreted cautiously, however, given that confounders other than study duration may have an impact on effect size. Thus, any relationsships that are identified may not be causal. Notably, previously conducted longer-term follow-up extension studies (up to 2 years) have shown that NEDAs maintain their beneficial effects, although augmentation is seen in 33% to 50% of patients.39,41,42 Thus, even though the constituent studies were of a relatively short-term nature (up to 12 weeks), the benefits found in our meta-analysis are representative of the overall benefit seen in long-term clinical use.
Although not associated with heart valve problems, as is treatment with ergot dopamine agonists, the NEDA use is somewhat limited by its high rate of adverse events. We found significant increases in the rate of withdrawals caused by adverse events, as well as increased incidence of nausea, dizziness, somnolence, and fatigue, with the NEDAs as a class compared with placebo. When evaluated separately, ropinirole significantly increased the number of withdrawals that were due to adverse effects and significantly increased the risk of nausea, dizziness, somnolence, and fatigue compared with placebo. In contrast, pramipexole did not increase the risk of withdrawals because of adverse effects and only increased nausea risk compared with placebo. Although rotigotine did not increase the risk of withdrawals because of adverse events or of any individual adverse event, and although sumanirole only increased the risk of headache, there was low power to identify adverse effects, as there were only 2 studies for rotigotine and 1 study for sumanirole included in the meta-analysis. A subsequent reevaluation of tolerability will be needed when these drugs are more rigorously studied.
It is possible that the NEDA agents might differ in terms of efficacy and safety as a result of pharmacologic and pharmacokinetic differences. Whereas ropinirole and sumanirole show activity primarily toward the D2 receptor and serotonin receptors, pramipexole predominantly acts on the D3 receptor.43 Rotigotine acts on D1, D2, and D3 receptors, as well as serotonin and α2-adrenergic receptors. Sumanirole has a relatively short half-life of 3 to 5 hours, ropinirole and rotigotine have a modest half-life of approximately 6 hours, and pramipexole has a long half-life that ranges from 8 to 12 hours.43 The direct link between receptor specificity, half-life, and efficacy and safety has not been determined, however, and more work is needed.
There are some limitations to this meta-analysis. First, we included trials that were not published and available only online, making critical evaluation of their methods and results challenging. Even so, our main study results remained robust despite the removal of these trials in sensitivity analysis. Second, as with any meta-analysis, the potential for publication bias is a concern. Publication bias is defined as “the tendency on the parts of investigators, reviewers, and editors to submit or accept manuscripts for publication based on the direction or strength of the study findings.”44 Although visual inspection of our meta-analysis’ funnel plot could not rule out publication bias, the results of the trim and fill analysis showed it is unlikely that publication bias significantly affected our study results. This conclusion is further supported by the nonsignificant Egger’s weighted regression statistic. Finally, limitations of symptom score and disease severity scales should be mentioned. A clinically important difference between treatment options on the IRLS is a point of ongoing debate, which makes interpretation of the differences in the current meta-analysis (approximately 3 to 7 points on a 40-point IRLS score, or a 7.5%-17.5% improvement) difficult to interpret. It should be noted, however, that the benefits of therapy on the IRLS score were above that derived from placebo, which has been shown to be robust in several studies.19,20 Further research correlating differences in the IRLS score to quality-of-life scores or clinical events is necessary to be able to say what magnitude of difference in these scales are clinically significant.
In conclusion, the use of NEDAs (pramipexole, ropinirole, rotigotine) in patients with moderate-to-severe restless legs syndrome results in significant reduction in symptom severity but increases the risk of withdrawal of therapy as a result of adverse effects. There may be qualitative differences in both efficacy and safety between agents, although no definitive comparisons can be made. Meta-regression analysis suggests that these agents are most beneficial in the early stages of treatment. Future studies should be conducted to compare directly the efficacy of individual NEDAs against each other in patients with restless legs syndrome, as well as include a longer period follow-up period to assess the long-term effects of these agents.
Conflicts of interest: none reported
REFERENCES
- 1.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005; 165(11):1286–1292. [DOI] [PubMed] [Google Scholar]
- 2.Berger K, Kurth T. RLS epidemiology—frequencies, risk factors and methods in population studies. Mov Disord 2007. Jun 7; [Epub ahead of print] DOI:10.1002/mds.21589. [DOI] [PubMed]
- 3.Lee HB, Hening WA, Allen RP, et al. Race and restless legs syndrome symptoms in an adult community sample in east Baltimore. Sleep Med. 2006; 7(8):642–645. [DOI] [PubMed] [Google Scholar]
- 4.Dinwiddie LC. Restless legs syndrome: not just a problem for dialysis patients. ANNA J. 1997; 24(6):655–662. [PubMed] [Google Scholar]
- 5.Berger K, Luedemann J, Trenkwalder C, et al. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004; 164(2):196–202. [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, Pichietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003; 4(2):101–119. [DOI] [PubMed] [Google Scholar]
- 7.Silber MH, Ehrenberg BL, Allen RP, et al. For The Medical Advisory Board of the Restless Legs Syndrome Foundation. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004; 79(7):916–922. [DOI] [PubMed] [Google Scholar]
- 8.Horvath J, Fross RD, Kleiner-Fisman G, et al. Severe multivalvular heart disease: A new complication of the ergot derivative dopamine agonists. Mov Disord. 2004; 19(6):656–662. [DOI] [PubMed] [Google Scholar]
- 9.Peralta C, Wolf E, Alber H, et al. Valvular heart disease in Parkinson’s Disease vs controls: An echocardiographic study. Mov Disord. 2006; 21(8):1109–1113. [DOI] [PubMed] [Google Scholar]
- 10.Zanettini R, Antonini A, Gatto G, et al. Valvular heart disease and the use of dopamine agonists for Parkinson’s Disease. N Engl J Med. 2007; 356(1):39–46. [DOI] [PubMed] [Google Scholar]
- 11.The International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003; 4(2):121–132. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996; 17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompsom SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 22(4):719–748. [PubMed] [Google Scholar]
- 17.Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004; 27(5):907–914. [DOI] [PubMed] [Google Scholar]
- 18.Stiasny-Kolster K, Kohnen R, Schollmayer E, Moller JC, Oertel WH. Patch application of the dopamine agonist rotigotine to patients with moderate to advances stages of restless legs syndrome: A double-blind, placebo-controlled pilot study. Mov Disord. 2004; 19(12):1432–1438. [DOI] [PubMed] [Google Scholar]
- 19.Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomized, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004; 75(1):92–97. [PMC free article] [PubMed] [Google Scholar]
- 20.Walters AS, Ondo WG, Dreykluft T, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004; 19(12):1414–1423. [DOI] [PubMed] [Google Scholar]
- 21.Bogan RK, Fry JM, Schmidt MH, et al. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006; 81(1):17–27. [DOI] [PubMed] [Google Scholar]
- 22.Inoue Y, Fujita M, Shimizu T, et al. Efficacy and safety of pramipexole in Japanese patients with restless legs syndrome. Mov Disord. 2006; 21:S442. [Google Scholar]
- 23.Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: A polysomnographic dose-finding study—the PRELUDE study. Sleep Med. 2006; 7(5): 407–417. [DOI] [PubMed] [Google Scholar]
- 24.Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006; 67(6): 1034–1039. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Borreguero D, Winkelman J, Adams A, et al. Efficacy and tolerability of sumanirole in restless legs syndrome: a phase II, randomized, double-blind, placebo-controlled, dose-response study. Sleep Med. 2007; 8(2):119–127. [DOI] [PubMed] [Google Scholar]
- 26.Oertel WH, Benes H, Garcia-Borreguero D, et al. Efficacy of rotigotine transdermal system in severe restless legs syndrome: A randomized, double-blind, placebo-controlled, six-week dose-finding trial in Europe. Sleep Med. 2007; 10.1016/j.sleep.2007.04.010. [DOI] [PubMed]
- 27.Oertel WH, Stiasny-Kolster K, Bergtholdt B, et al. Efficacy of pramipexole in restless legs syndrome: a six-week, multicenter, randomized, double-blind study (Effect-RLS Study). Mov Disord. 2007; 22(2):213–219. [DOI] [PubMed] [Google Scholar]
- 28.A 12-week, double-blind, placebo-controlled, twice-daily dosing study to assess the efficacy and safety of Ropinirole in patients suffering from restless legs syndrome (RLS) requiring extended treatment coverage (III_RRL100013.pdf). http://ctr.gsk.co.uk/summary/ropinirole/studylist.asp. Accessed Mar 19, 2007.
- 29.A 12-week, double-blind, placebo-controlled, parallel-group study to assess the effectiveness of Ropinirole in patients willing to take regular medication for their restless legs syndrome in a primary care setting (III_ROP101892.pdf). http://ctr.gsk.co.uk/summary/ropinirole/studylist.asp. Accessed Mar 19, 2007.
- 30.A double-blind, randomized, placebo-controlled, parallel-group study to investigate the tolerability of a dose-escalating regimen of ropinirole in patients suffering from restless legs syndrome (RLS) (II_101468_207.pdf). http://ctr.gsk.co.uk/summary/ropinirole/studylist.asp. Accessed Mar 19, 2007.
- 31.Becker PM, Ondo W, Sharon D. Encouraging initial response of restless legs syndrome to pramipexole. Neurology. 1998; 51(4):1221–1223. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed I. Ropinirole in restless legs syndrome. Mo Med. 2002; 99(9):500–501. [PubMed] [Google Scholar]
- 33.Stiasny-Kolster K, Oertel WH. Low-dose pramipexole in the management of restless legs syndrome: An open-label trial. Neuropsychobiology. 2004; 50(1):65–70. [DOI] [PubMed] [Google Scholar]
- 34.Saletu M, Anderer P, Saletu-Zyhlarz, et al. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002; 252(4):185–194. [DOI] [PubMed] [Google Scholar]
- 35.Saletu M, Anderer P, Saletu B, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. Neurosychobiology. 2004; 41(4):190–199. [DOI] [PubMed] [Google Scholar]
- 36.Adler CH, Hauser RA, Sethi K, et al. Ropinirole for restless legs syndrome: A placebo-controlled crossover trial. Neurology. 2004; 62(8):1405–1407. [DOI] [PubMed] [Google Scholar]
- 37.Pellecchia MT, Vitale C, Sabatini M, et al. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis: an open randomized crossover trial versus levodopa sustained release. Clin Neuropharmacol. 2004; 27(4):178–181. [DOI] [PubMed] [Google Scholar]
- 38.Montplaisir J, Nicolas A, Denesle R. Gomez-Mancilla. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999; 52(5):938–943. [DOI] [PubMed] [Google Scholar]
- 39.Montplaisir J, Karrasch J, Haan J, Volc D. Ropinirole is effective in the long-term management of restless legs syndrome: a randomized controlled trial. Mov Disord. 2006; 21(10):1627–1635. [DOI] [PubMed] [Google Scholar]
- 40.Bliwise DL, Freeman A, Ingram CD, et al. Randomized, double-blind, placebo-controlled, short-term trial of ropinirole in restless legs syndrome. Sleep Med. 2005; 6(2):141–147. [DOI] [PubMed] [Google Scholar]
- 41.Montplaisir J, Denesle R, Petit D. Pramipexole in the treatment of restless legs syndrome: a follow-up study. Eur J Neurol. 2000; 7(Suppl 1):27–31. [DOI] [PubMed] [Google Scholar]
- 42.Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003; 26(7): 819–821. [DOI] [PubMed] [Google Scholar]
- 43.Allen PR, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001; 18(2):128–147. [DOI] [PubMed] [Google Scholar]
- 44.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990; 263(10):1385–1389. [PubMed] [Google Scholar]