Abstract
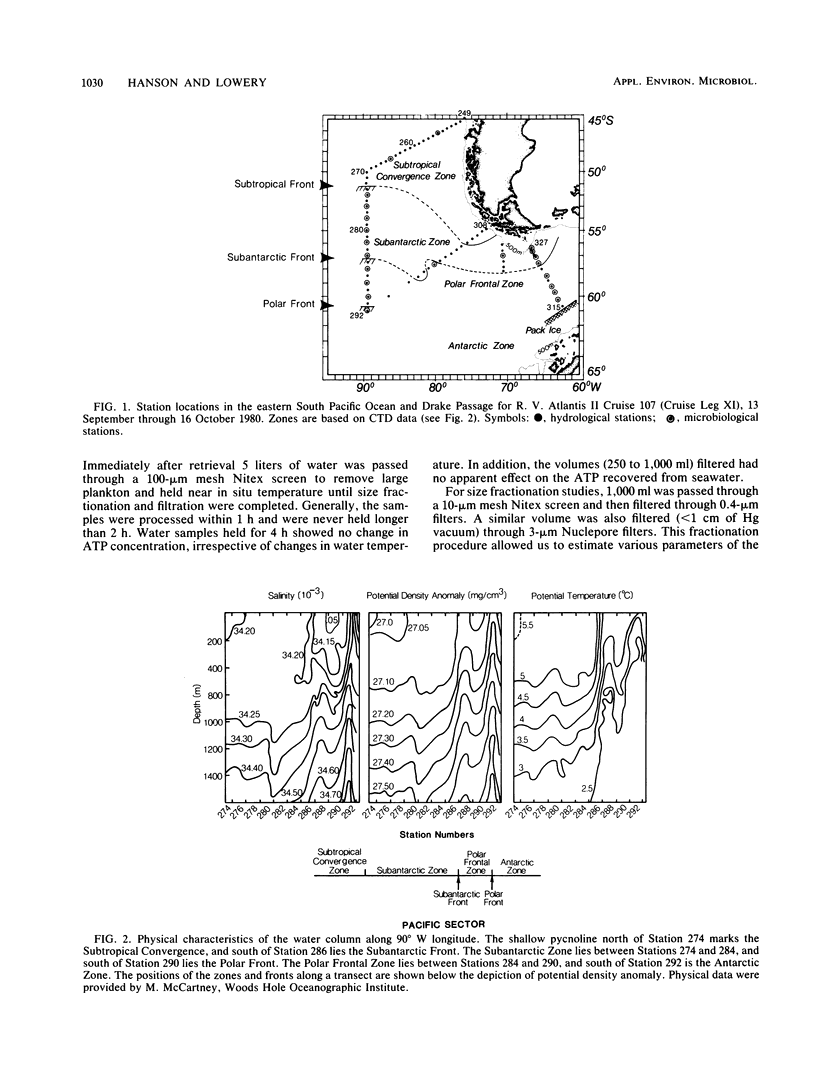
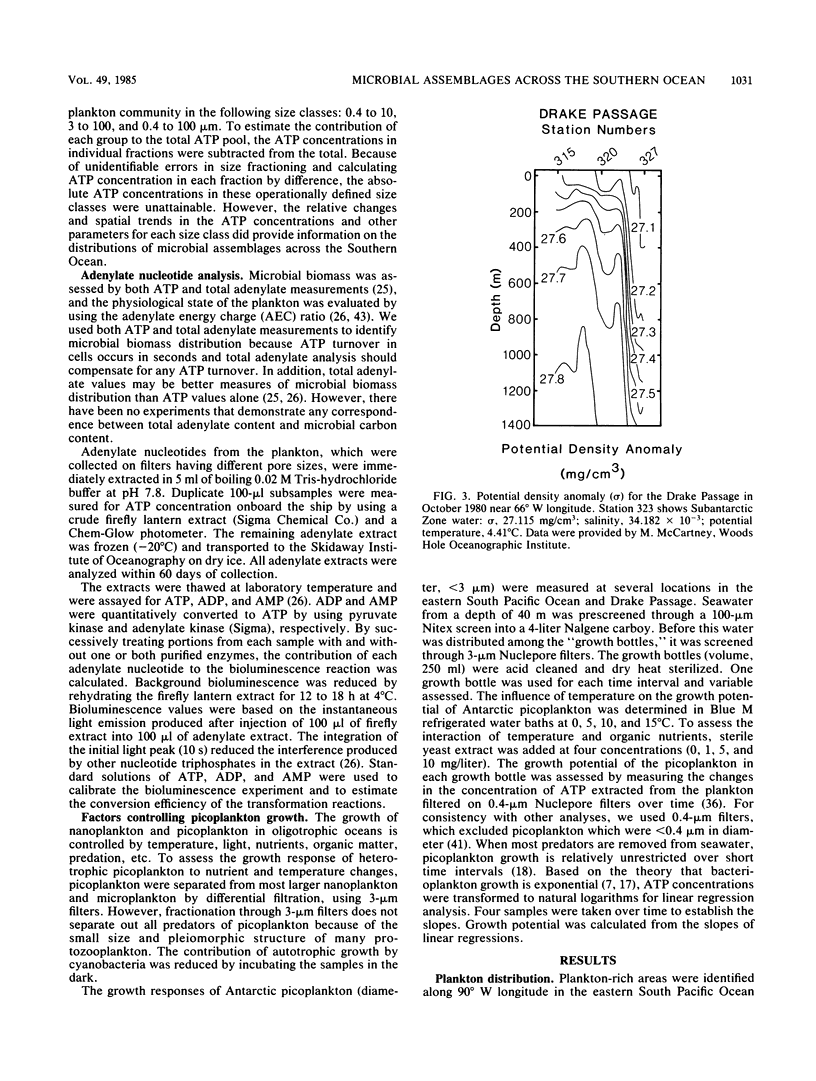
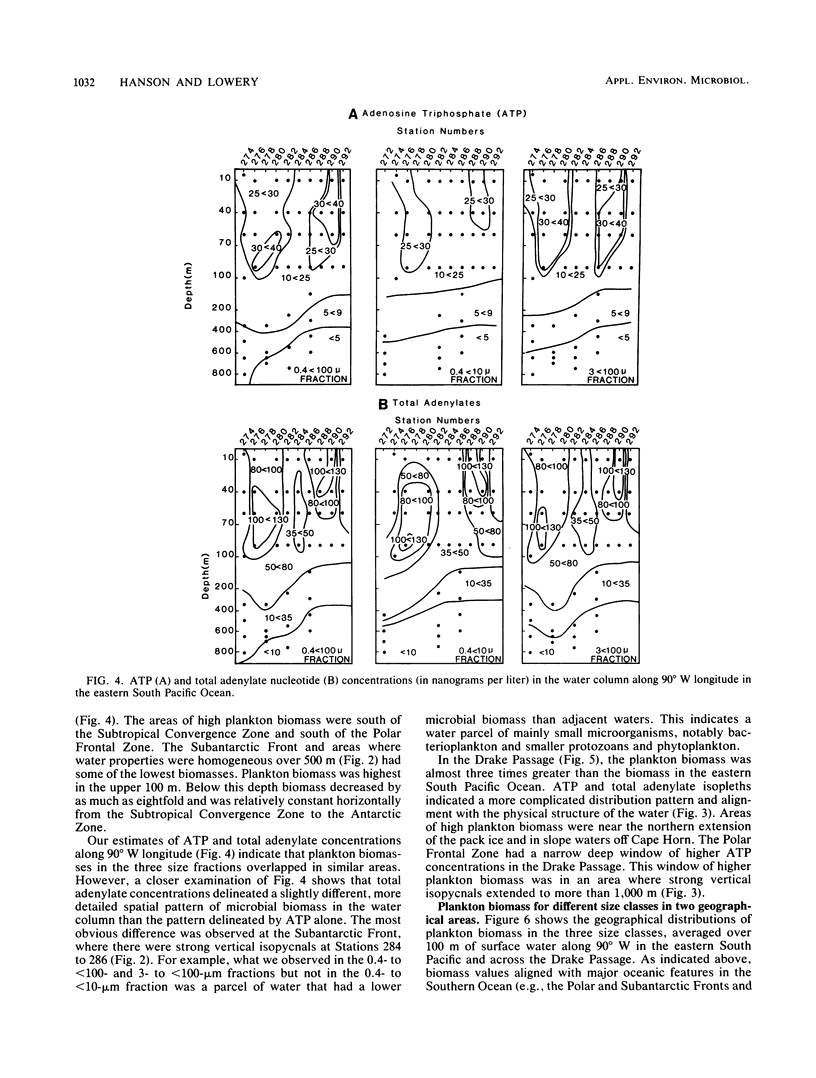
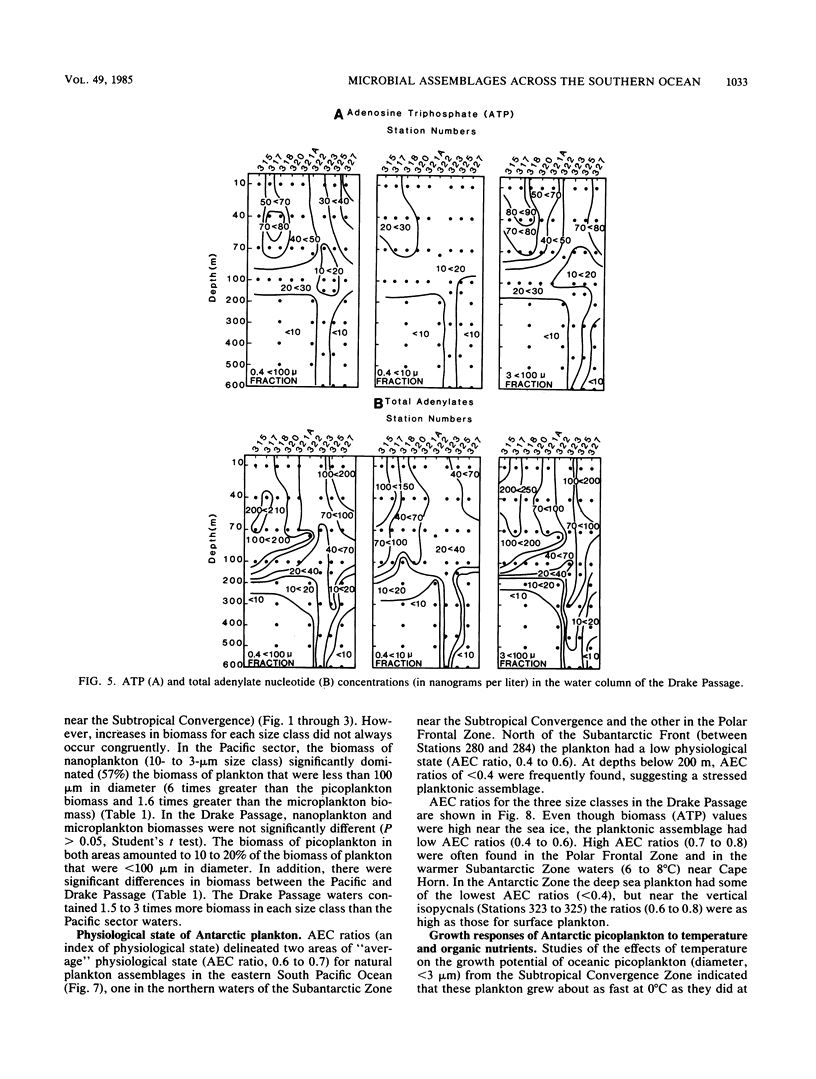
We examined the spatial distributions of picoplankton, nanoplankton, and microplankton biomass and physiological state relative to the hydrography of the Southern Ocean along 90° W longitude and across the Drake Passage in the late austral winter. The eastern South Pacific Ocean showed some large-scale biogeographical differences and size class variability. Microbial ATP biomass was greatest in euphotic surface waters. The horizontal distributions of microbial biomass and physiological state (adenylate energy charge ratio) coincided with internal currents (fronts) of the Antarctic Circumpolar Current. In the Drake Passage, the biological scales in the euphotic and aphotic zones were complex, and ATP, total adenylate, and adenylate energy charge ratio isopleths were compressed due to the extension of the sea ice from Antarctica and constriction of the Circumpolar Current through the narrow passage. The physiological state of microbial assemblages and biomass were much higher in the Drake Passage than in the eastern South Pacific Ocean. The temperature of Antarctic waters, not dissolved organic carbon, was the major variable controlling picoplankton growth. Estimates of picoplankton production based on ATP increments with time suggest that production under reduced predation pressure was 1 to 10 μg of carbon per liter per day. Our results demonstrate the influence of large-scale hydrographic processes on the distribution and structure of microplankton, nanoplankton, and picoplankton across the Southern Ocean.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christian R. R., Hanson R. B., Newell S. Y. Comparison of methods for measurement of bacterial growth rates in mixed batch cultures. Appl Environ Microbiol. 1982 May;43(5):1160–1165. doi: 10.1128/aem.43.5.1160-1165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. B., Shafer D., Ryan T., Pope D. H., Lowery H. K. Bacterioplankton in antarctic ocean waters during late austral winter: abundance, frequency of dividing cells, and estimates of production. Appl Environ Microbiol. 1983 May;45(5):1622–1632. doi: 10.1128/aem.45.5.1622-1632.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe W. J., Bancroft K. Use of the adenylate energy charge ratio to measure growth state of natural microbial communities. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2112–2115. doi: 10.1073/pnas.72.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]


